Feed-induced Variation in the Carotenoid Composition of Brine Shrimp
- DOI
- 10.2991/efood.k.200522.001How to use a DOI?
- Keywords
- Brine shrimp; lycopene; β-carotene; lutein; astaxanthin; bio-encapsulation
- Abstract
Brine shrimp is a genus of aquatic crustacean, and is considered a dietary carotenoid resource by local residents from eastern China. In the present study, harvested brine shrimp were fed with the dried powders of tomato fruit (Solanum lycopersicum, as a lycopene source), marigold flower (Tagetes erecta L., as a lutein ester source), halophile green algae (Dunaliella salina, as free lutein and β-carotene sources) and freshwater green algae (Haematococcus pluvialis, as an astaxanthin ester source). After 30 min of free feeding, the total carotenoid amount and CC of brine shrimp were assessed by UV–VIS and C18-HPLC (high performance liquid chromatography) every hour, respectively. Data from those investigations suggested that: (1) Both carotenes and xanthophylls from feeds were able to be accumulated in brine shrimp. (2) Hydrolysis took place with lutein and astaxanthin esters to form their free forms, (3) β-Carotene was converted into a keto-carotenoid, canthaxanthin. Those observations confirmed that a number of carotenoid molecular modifications had taken place in vivo. Although it was observed that a significant amount of carotenoids were lost during their accumulations in vivo, brine shrimp would still be considered as a species of bio-encapsulated free carotenoid carrier, and therefore as a possible dietary carotenoid resource.
- Copyright
- © 2020 International Association of Dietetic Nutrition and Safety. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Artemia, commonly known as brine shrimp, is a genus of aquatic crustaceans and belongs to the family Artemiidae, order Anostraca, class Brachiopoda, subphylum Crustacea, phylum Arthropoda and kingdom Animalia. While more than eight species have been identified [1], much more geographic-specific strains have been recorded and described [1–5]. Brine shrimp populations are distributed worldwide including China. A few species, such as Artemia sinica, and over 70 geographical strains were found and harvested from many places in both inland salt lakes and salt fields along the seaside of China [6–9]. Brine shrimp is widely used as live feed for marine fish and crustacean [10]. Brine shrimp are known for its cyst, which can be used as feed for aquaculture. The fresh and frozen brine shrimp biomass (adult) are usually supplied to the local shrimp farm for shrimp brood stock maturation. Additionally, it is often used as a model organism in food and pharmaceutical toxicological assessments [11]. However, local consumers from the Gulfs of Chihli, Laizhou and Dalian Bay in China consume brine shrimp as well. Harvested brine shrimp are traditionally ground and then fermented with salts to produce shrimp paste. As a dietary resource, it was imperative to inspect the safety of brine shrimp and the influence of pollution on brine shrimp [12–14].
Carotenoids are a group of natural products. Some of them, such as lycopene, β-carotene, lutein and astaxanthin, were proven to have health-improving functions [15]. Some carotenoids have been isolated and identified from brine shrimp for decades. Carotenoids were proved to improve the stress tolerance of brine shrimp during its diapause and quiescence [16]. Brine shrimp is therefore likely to be a source of natural carotenoids.
Carotenoids from A. salina were isolated and studied. The algal cells consisted exclusively of keto-carotenoids such as canthaxanthin and echinenone. The metabolism of carotenoids, especially β-carotene in A. salina was further recorded. The conversion of β-carotene into canthaxanthin by brine shrimp, A. salina, was then demonstrated [17]. This conversion was also shown in vivo with the aid of [C14] β-carotene tracer technique [7]. Additionally, the Z-isomers of canthaxanthins were proven to have importance in vivo [18]. More recently, keto-carotenoids, including astaxanthin and canthaxanthin, were directly fed to enrich the carotenoid amount of brine shrimp [19]. Currently, various carotenoids, especially xanthophylls, are legally used in foods and feeds [20]. It would therefore be expected to use more species of carotenoids, such as carotenes [21] in the feeds of brine shrimp.
In this study, four food-grade feeds were fed to brine shrimp collected from the Gulf of Chihli, P. R. China. The Total Carotenoid Amount (TCA) and Carotenoid Composition (CC) of brine shrimp were then assessed by UV–VIS spectroscopy and C30-HPLC (High Performance Liquid Chromatography). Variations in carotenoid composition and concentration were observed. Under these conditions, brine shrimp became a carrier of dietary carotenoids. In other words, this process can be called the biological encapsulation of carotenoids. The brine shrimp containing carotenoids can be considered as a raw material to produce high quality shrimp paste. Data from this investigation suggested that a hydrolysis process of carotenoid esters, including astaxanthin and lutein esters was performed in A. sinica. In view of literatures, this observation has not been reported yet.
2. MATERIALS AND METHODS
2.1. Chemicals and Reagents
Rock salt (Grade A) was purchased from Qingdao Haidatongyong Co., China. NaHCO3, acetone, hexane, Dichloromethane (DCM) and petroleum ether (BP: 60–90°C) were AR reagents from Beijing Chemical Co., China. Acetonitrile and ethyl acetate were of HPLC grade and purchased from Dikma Technologies Inc., USA. Nitrogen was purchased from Beijing gas Co., China with a purity of more than 99.96% (V/V).
2.2. Reference Samples
Reference samples used in this study for fraction identification and quantification were purchased from CaroteNature GmbH, Gerbestrasse 12, 3072 Ostermundigen, Switzerland. This included: Lycopene (No. 0031; HPLC 95%; Synth.; Cryst.; 1 mg/pack), β-carotene (No. 0003; HPLC 96%; Synth.; Cryst.; 1 mg/pack), (3R,3′R,6′R)-lutein (No. 0133; HPLC 96%; Isolated; Cryst.; 1 mg/pack), canthaxanthin (No. 0380; HPLC 98%; Synth.; Cryst.; 1 mg/pack) and (3S,3′S)-astaxanthin (No. 0406; HPLC 96%; Synth.; Cryst.; 1 mg/pack). The purities of all samples were checked by UV–VIS spectroscopy with published absorption coefficients prior to use. The molecular structures of each fraction are shown in Figure 1.
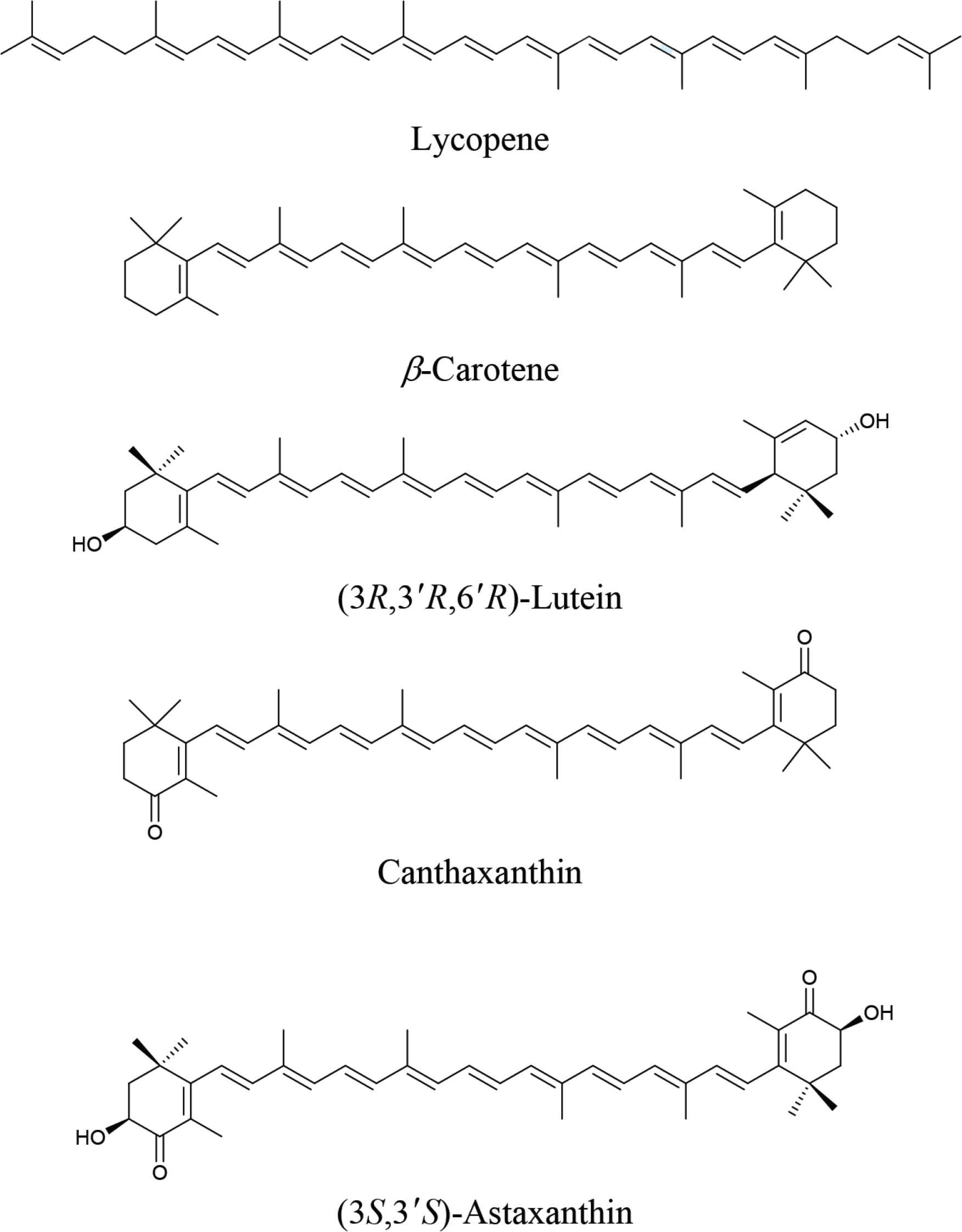
Molecular structures of reference samples.
2.3. Artemia Cysts
Artemia cysts were collected from a salt field in Weifang, Shandong province, China. This type of brine shrimp is considered a geographic-specific strain. The mature shrimp is usually processed to make shrimp paste consumed by local natives. Its cyst is often used as aquaculture feed.
2.4. Feeds
Yeast was used as carotenoid-free feed, and was supplied as a dried powder. This was purchased from a local supplier and passed through a 100-mesh. Carotenoid-containing feeds included the dried powdered (passed through 100 mesh of tomato (Lycopersicon esculentum) fruits, alga (Dunaliella salina and Haematococcus pluvialis) and marigold (Tagetas erecta) flowers collected from local suppliers. Dried tomato fruit powder is a food-grade product (China State Standard No.: NY/T 957-2006). The dried powders of D. salina and H. pluvialis cells are legal novel foods proven by the China Food and Drug Administration. The powder of marigold flower is a raw material to produce food additives (China State Standard: GB26405-2011). Their carotenoid compositions are given in Table 1.
| Feeds | TCA [mg/g DW]a | Carotenoids | RA [% W/W]b |
|---|---|---|---|
| Tomato | 1.35 | Lycopene | 84.53 |
| β-Carotene | 7.12 | ||
| Others | 8.35 | ||
| Dunaliella salina | 23.16 | β-Carotene | 14.66 |
| Free luteinc | 73.33 | ||
| Others | 12.01 | ||
| Marigold | 168.72 | Lutein esters | 97.23 |
| Others | 2.77 | ||
| Haematococcus pluvialis | 15.13 | Astaxanthin esters | 93.24 |
| Others | 6.76 |
TCA: total carotenoid amount (UV–VIS): Conditions: as follow.
RA: relative amount (C18-HPLC). Chromatographic conditions: as follow.
Algae dried powder was prepared from Dunaliella salina cultured in the logarithmic growth phase in which photosynthesis remarkably took place in vivo. A large amount of chlorophylls and free lutein were therefore observed. β-Carotene, in this phase, was not major carotenoid in quantity.
Carotenoid composition of brine shrimp from different feeds
2.5. Instruments and Facilities
HPLC used in this study consisted of Waters 1525 solvent delivery system and a PDA-996 detector, provided by Waters, USA. Diamonsil™ C18 column (5 μm, 4.6 mm × 25 cm) was purchased from Dikma Technologies Inc, USA. MultiSpec-1501 spectrophotometer was from Shimadzu Corporation, Japan.
2.6. Cyst Hatching
Artemia cysts were hatched under the conditions of 28–30°C. A 3% (w/v) saline prepared by dissolving rock salt in distilled water (with a pH = 8), adjusted by adding 2 g NaHCO3 to a liter of the saline, 4000 Lux illumination and continuous air stream delivered by an air pump, with the hatching density of 3 g/L. After the 24–30 h hatching process, nauplii were born.
2.7. Nauplii Cultivation
Nauplii were cultivated under the same conditions as those of their hatching but with 4% (w/v) saline. Dried yeast was selected as feed, and given two times a day and 1 g/L at one time. After 15–20 days, the brine shrimp considered to be sexually mature.
2.8. Carotenoid-containing Feed Consumption
After sexual maturation, the brine shrimp were fed with four carotenoid-containing feeds and yeast as control. In practice, 1000 brine shrimp were selected and cultivated in 1 L saline. After a 12-h fast, 2 g feeds were supplied. After 30-min free feeding, all shrimp were harvested while feed residuals were collected by filtration.
2.9. Dried Weight
A total of 500 harvested shrimp and all residual feeds were fully dried in a vacuum dryer at 70°C. The dried weight of shrimp and feed residuals were then measured. Dried weight of each shrimp was calculated.
2.10. Carotenoid Extraction
The remaining harvested brine shrimp (500 shrimp) from each culture were well grounded with a mortar and pestle with 10 mL of acetone for 5–6 min. After the extracting solution was collected by pipette, the residuals were repeatedly extracted by acetone until the extraction solution was colorless. On average, the extraction needed to be repeated five to six times. In other words, carotenoids were completely extracted under such conditions. All the extracting solutions were collected together in a 250-mL separating funnel. About 50 mL of hexane and distilled water was then added. After the separation phase, the lower phase was removed and the upper phase was collected. The solvent (about 50 mL) was removed from the upper phase with a stream of nitrogen at room temperature. The dried extract was stored in nitrogen at −20°C until analysis. Acetone was collected from the lower phase of the rotary evaporator, which was removed under the conditions of 56°C, −0.06 MPa and 30 rpm and reused, for the extraction.
2.11. Total Carotenoid Amount Assessment by UV–VIS Spectroscopy
1 mg of each reference sample, lycopene, β-carotene, (3R,3′R,6′R)-lutein, canthaxanthin and (3S,3′S)-astaxanthin, were dissolved in 25 mL of petroleum ether, DCM, hexane, DCM, and DCM, respectively as stock solutions (40 μg/mL). The working solutions with concentrations of 0.000, 0.039, 0.078, 0.156, 0.313, 0.625, 1.250, and 2.500 g/mL were then prepared. The absorbance of the working solutions from each reference sample was recorded at wavelengths of 470, 450, 445, 482, and 485 nm, respectively. An equation was regressed between the absorbance and carotenoid amount of each reference sample (R2 > 0.996).
Extracts from tomato-, algae tomato-, algae (D. salina)-, marigold-, algae (H. pluvialis)- and yeast-fed brine shrimp were re-dissolved in 10 mL of petroleum ether, DCM, hexane, DCM, and DCM, respectively. Absorbance of each sample solution was measured under the same conditions as above. The TCA of each sample was calculated according to the regression equations.
The TCA of each sample was assessed by UV–VIS spectroscopy with external references.
2.12. Carotenoid Composition Assessment by C18-HPLC
After filtration with a 0.22-μm filter, the extract solutions were directly injected into C18-HPLC for composition assessment. All samples were assessed under identical chromatographic conditions which were as follows: Column: Diamonsil™ (5 μm, 4.6 mm × 25 cm, Dikma Technologies Inc., USA); mobile phase A: acetonitrile–water (9:1, v/v); mobile phase B: ethyl acetate; linear gradient: B increased from 0% to 100% in 20 min and then kept constant for another 5 min; flow rate: 1.0 mL/min; monitoring wavelength: 470, 450, 445, 485, and 450 nm for extracts from tomato-, D. salina–, marigold-, H. pluvialis– and yeast-fed shrimp, respectively; PDA wavelength range: 250–600 nm; sample injection volume: 20 μL. Through a comparison of the reference samples’ chromatographic behavior e.g. retention time, and electronic absorption spectral characteristics e.g. maximum absorption wavelength, each carotenoid fraction was identified. The peak area of each fraction was integrated. The relative amount (in %) of each carotenoid fraction was then calculated according to its peak area and the equation shown below:
The amount of each carotenoid fraction was calculated based on the following equation:
2.13. Data Processing
Each feeding experiment was repeated more than three times until its analytical result was reproducible i.e. relative standard deviation (RSD) was <7%. The average of the data from the three repeats were calculated and expressed.
3. RESULTS
3.1. Brine Shrimp Cultivation
On average, after a 20-day period of cultivation, most nauplii grew to sexual maturity. In contrast to shrimp fed with D. salina, as usual in Salt Lake, inland and salt pan, seaside, shrimp fed with yeast exhibited a lighter color. Data from the TCA assay suggested that no carotenoids existed in brine shrimp extract before carotenoid-containing feeds were administrated, because yeast, as feed, did not provide any carotenoid to the shrimp. In other words, this is evidence that the carotenoids found in the brine shrimp in the following investigation were not biosynthesized in vivo but supplemented from their feeds. Besides, after 30 min carotenoid-containing feed administration, a rapid variation in body color was seen, which demonstrated that carotenoid could be absorbed by the brine shrimp.
3.2. Feed Consumption Determination
Brine shrimp is a filter feeder with a large amount of feed consumption [22]. In this investigation, 2 g of feed was administrated to 1000 shrimp in 30 min. The particle size of each feed was identical. Normally, about half of the feed was consumed as shown in Figure 2. Data from this investigation suggested that variation in consumption was limited between feeds. In other words, apart from particle size, the species, smell, and color of the feeds did not have an influence on the amount consumed.
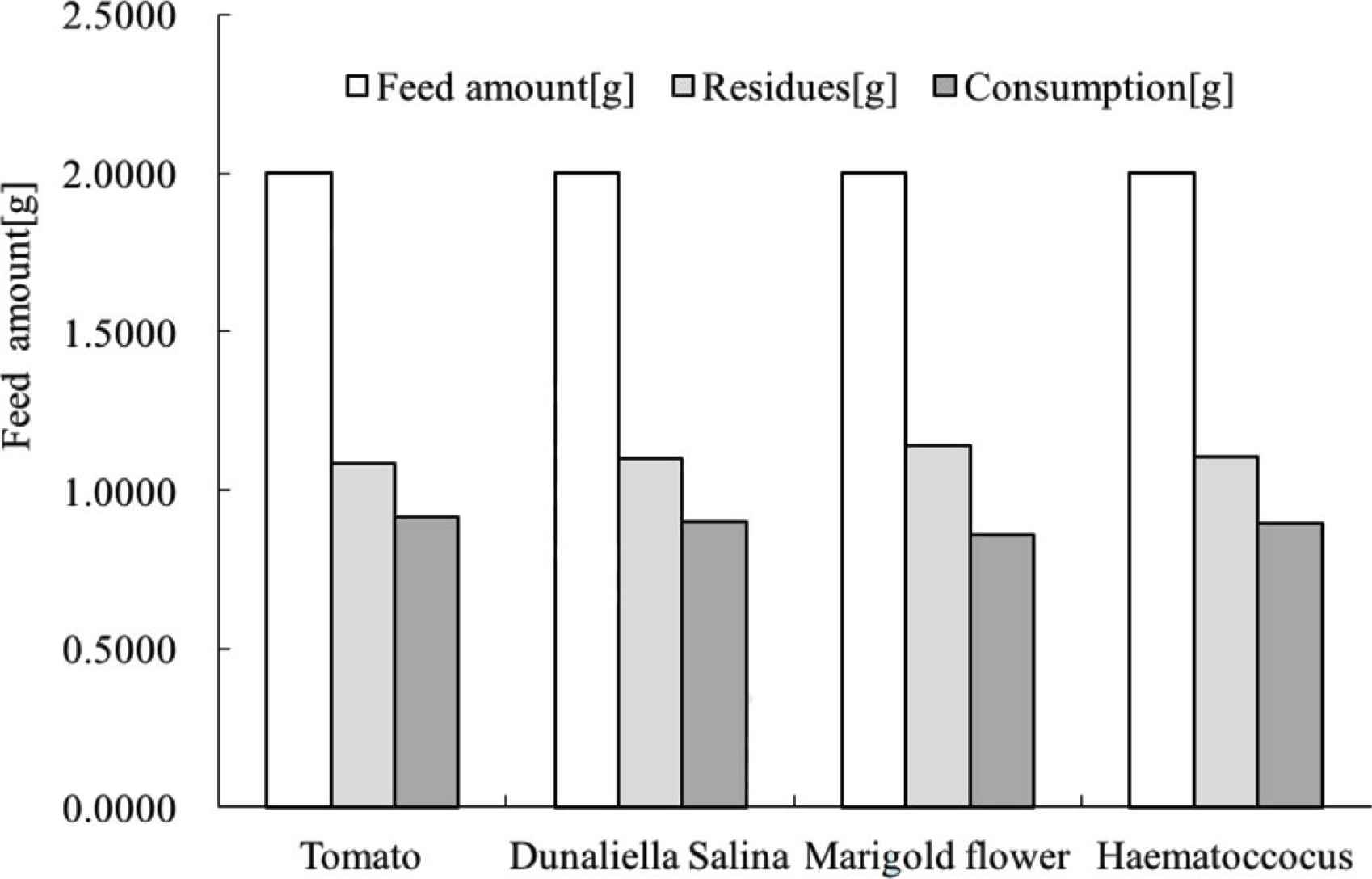
Feed consumption of brine shrimp. Cultivation conditions: see above.
3.3. Fraction Identification from Feeds and Fed Brine Shrimp Extracts
Figure 3a shows the C18-HPLC profiles of extracts from tomato feed (i) and tomato-fed brine shrimp (ii). Figure 3a(i) provides the carotenoid composition of tomato powder extract. The majority of carotenoids from tomato powder extract were lycopene, while a minor amount of β-carotene was seen. As shown in Figure 3a(ii), a fraction at 17.71 min appeared from tomato-fed brine shrimp extract. In comparison with the retention time and electronic absorption spectral characteristics of the fraction with those of the reference sample, the fraction was identified as lycopene. A small amount of β-carotenoids was also detected from tomato-fed brine shrimp extract, as shown in Figure 3a(ii). Additionally, a fraction at 11.82 min indicated the presence of canthaxanthin.
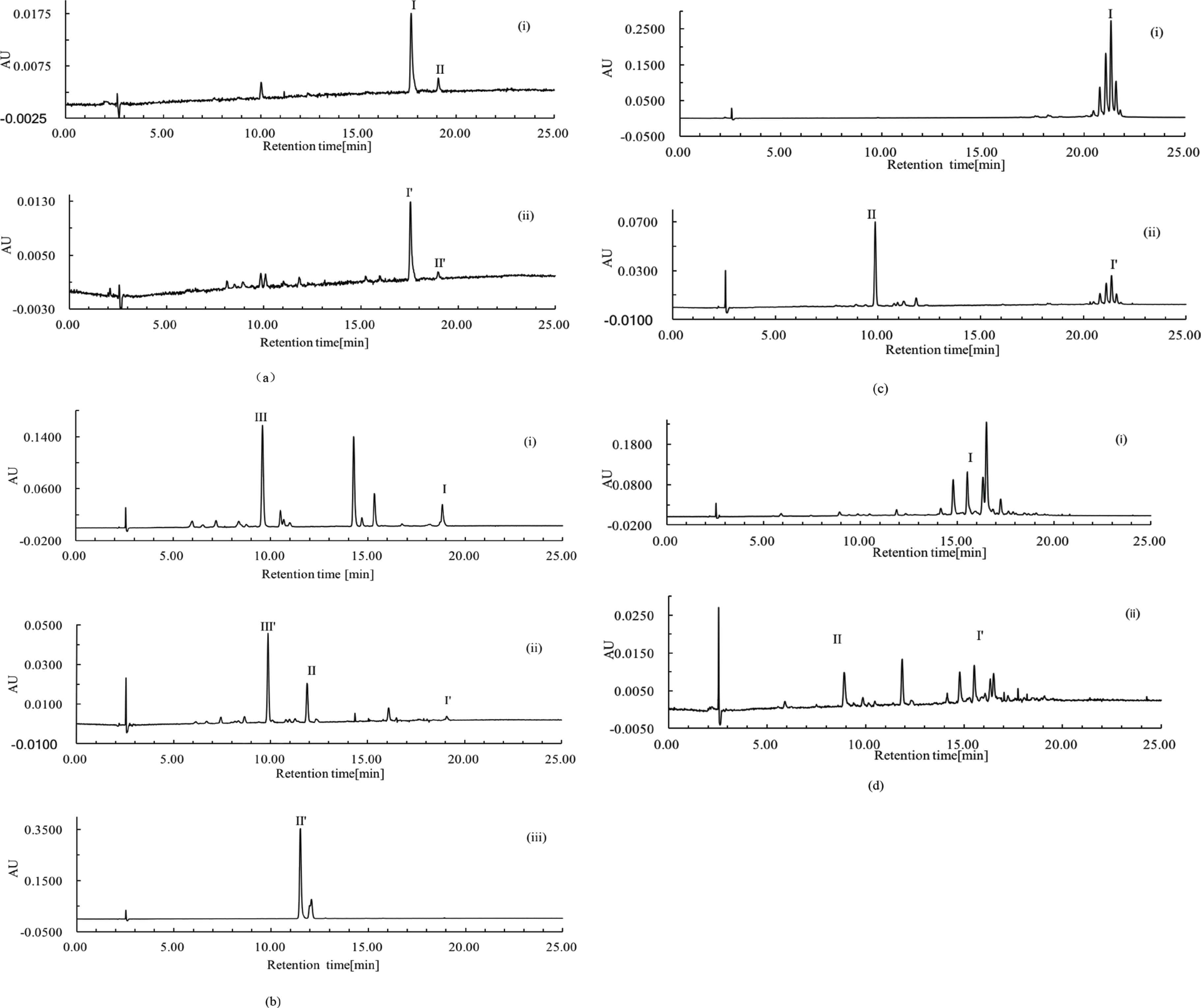
C18-HPLC profiles of extracts. Chromatographic conditions: see above. Fraction identification: see Table 2. (a) Tomato feed (i) and fed brine shrimp (ii). (b) Dunaliella salina feed (i) and fed brine shrimp (ii) and the reference sample of canthaxanthin (iii). (c) Marigold flower feed (i) and fed brine shrimp (i). (d) Haematococcus pluvialis feed (i) and fed brine shrimp (ii). The symbols I, II, III and I′, II′, III′ are fraction number which indicate individual fraction identification. An identical fraction from different samples is labeled by the same stem number, such as I and I′. ‘AU’ is used as the ordinate unit to indicate the ‘Auto-unit’ of absorbance.
Data from Figure 3a(i) and (ii) suggested that no structure modification was undertaken when a decomposition was likely to commence during the absorption and accumulation of lycopene fraction from tomato feed. In contrast to lycopene fraction, a structure conversion of β-carotene fraction into canthaxanthin was observed as reported by Davies et al. [17] and Gilchrist [7].
Dunaliella salina powder is a typical photosynthetic organism and has the abundance of β-carotene and lutein, as shown in Figure 3a(i). Comparing the retention time and electronic absorption spectral characteristics of the reference samples with those of the fraction at 9.61 min, the fraction was identified as free lutein. Additionally, a new fraction that did not occur in the control feed was clearly detected at 11.94 min, as shown in Figure 3b(ii). Figure 3b(iii) is the C18-HPLC profile of the canthaxanthin reference sample. Comparing the retention time and electronic absorption spectral characteristics of the fraction at 11.94 min between (ii) and (iii), it would be identified as canthaxanthin [23].
Dunaliella salina has been reported to be rich in chlorophylls a and b [24]. It was observed from Figure 3b(i) that certain amounts of chlorophylls a and b, identified according to their electronic absorption spectral characteristics, occurred in control feed. Data from Figure 3b(ii) suggested that algae-fed brine shrimp did not accumulate chlorophylls significantly in vivo. In other words, chlorophylls were decomposed efficiently in vivo. This observation has not been reported.
Marigold flower powders are rich in lutein esters which have two hydroxyl groups esterified by fatty acids, including dimyristate, dipalmitate, myristate, palmitate, stearate, distearate etc. [25]. In Figure 3c(i), a group of lutein esters are marked as fraction I. No free lutein fraction was detected from marigold flower powders. Figure 3c(ii) is the C18-HPLC profile of extract from marigold flower-fed brine shrimp. A new fraction at 9.87 min was detected and marked as II. Comparing its retention time and electronic absorption spectral characteristics with those of the reference sample, fraction II was identified as free lutein. A limited amount of lutein esters was seen on the chromatogram.
This observation suggested that a portion of fed lutein esters was hydrolyzed to form free lutein. In other words, a conversion of lutein ester into free lutein took place in vivo. Although there is no evidence to prove that the conversion was catalyzed by an enzyme, an investigation into the isolation and purification of an enzyme that could catalyze this hydrolysis reaction could be done in future studies.
It was reported that a large amount, up to 3% (w/w) of astaxanthin monoesters are in H. pluvialis [26]. Observations shown in Figure 3d(i) agree with published data. Data from this investigation suggested that various astaxanthin monoesters are found in algae powders used in this experiment. Similar to the results from the marigold flower-fed study, a new fraction, at 8.92 min and marked as II, was detected on the chromatogram of algae-fed brine shrimp extract as shown in Figure 3d(ii). Comparing retention time and electronic absorption spectral characteristics with the reference sample, the fraction was identified as free astaxanthin. Furthermore, the astaxanthin monoester amount was reduced. Once again, this observation suggested that a conversion of carotenoid esters into free carotenoid occurred in vivo.
Summarized fraction identifications on chromatograms as shown above are given in Table 2.
| Feeds | Peak No. | Fraction identification |
|---|---|---|
| Tomato (Lycopersicon esculentum) | I/I′ (Figure 3a) | Lycopene |
| II/II′ (Figure 3a) | β-Carotene | |
| Algae (Dunaliella salina) | I/I′ (Figure 3b) | β-Carotene |
| II/II′ (Figure 3b) | Canthaxanthin | |
| III/III′ (Figure 3b) | Free lutein | |
| Marigold (Tagetas erecta) | I/I′ (Figure 3c) | A group of lutein esters |
| II (Figure 3c) | Free lutein | |
| Algae (Haematococcus pluvialis) | I/I′ (Figure 3d) | A group of astaxanthin esters |
| II (Figure 3d) | Free astaxanthin |
Carotenoid identification from feeds and brine shrimp
3.4. Carotenoid Accumulation in Brine Shrimp
Figure 4 illustrates TCA variation and individual carotenoid accumulations of fed brine shrimp. The TCA of brine shrimps from each carotenoid feeding experiment was determined prior to the start of feeding carotenoids. It was observed that the TCA of brine shrimps from all four groups was 0 mg/g due to the feeding of carotenoid-free feeds before the experiments. The maximum TCA of fed brine shrimp from all four groups was seen at 30 min after feeding, followed by a rapid reduction. In the D. salina and H. pluvialis-fed studies, a certain amount of total carotenoid was still seen in all shrimp while no carotenoid could be measured in tomato and marigold flower studies at 5 h after feeding, as shown in Figure 4a and 4c, respectively.
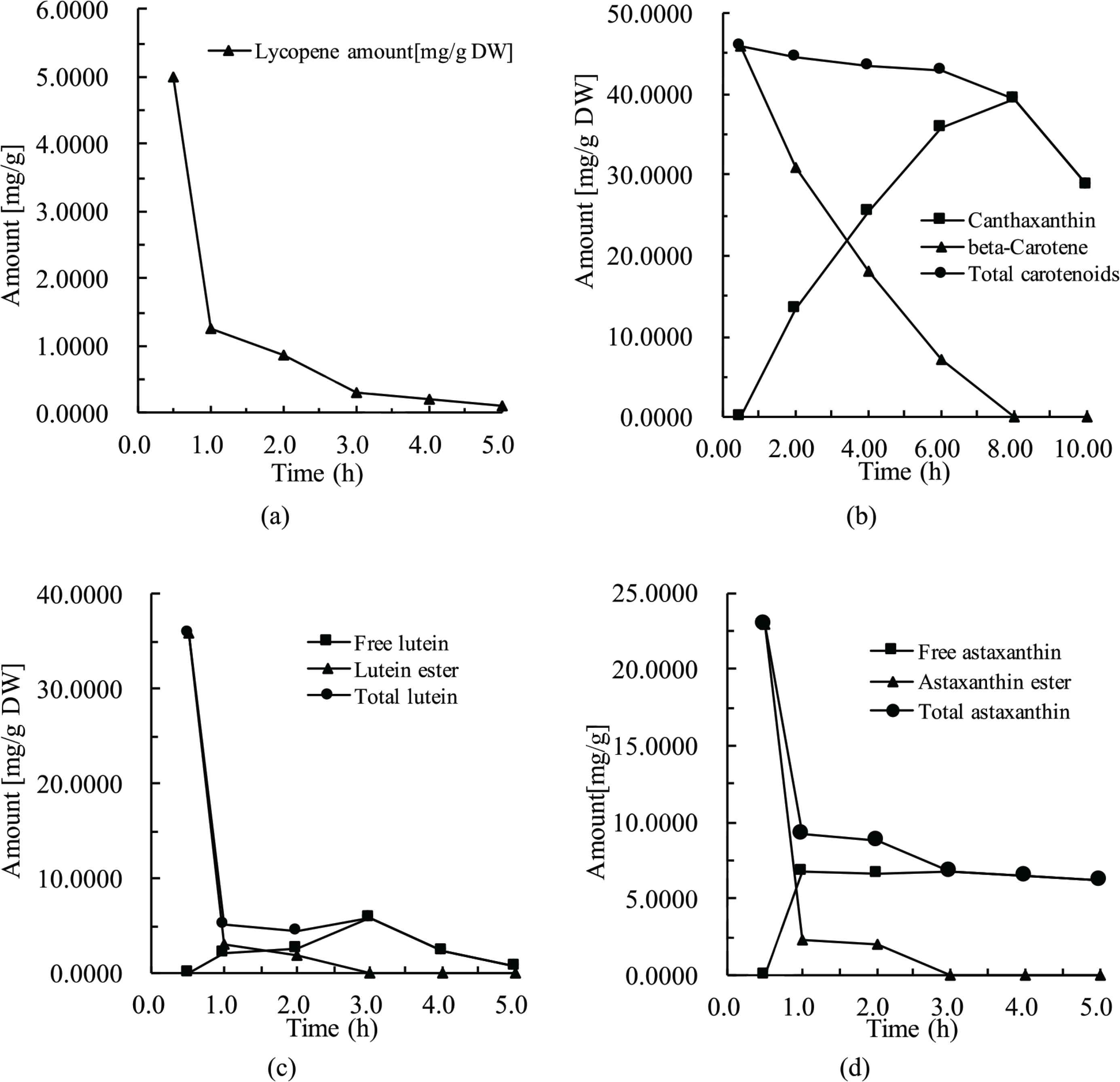
Carotenoid accumulation in brine shrimp. (a) Fed with tomato. (b) Fed with Dunaliella salina. (c) Fed with marigold flower. (d) Fed with Haematococcus pluvialis.
A conversion of β-carotene into canthaxanthin was clearly demonstrated in brine shrimp as shown in Figure 4b. Additionally, data from Figure 4b suggest that the keto-carotenoid is likely to be the most stable carotenoid administrated in this study in vivo.
Similarly, a conversion (or hydrolysis) of carotenoid esters into free compounds was clearly observed in vivo from marigold flower- and H. pluvialis-fed investigations shown in Figure 4c and 4d. Once again, data shown in Figure 4d suggests that the keto-carotenoid is likely to be the most stable in this study in vivo.
4. CONCLUSION
As mentioned above, all carotenoid-containing feeds used in this study, including tomato, marigold flower, D. salina and H. pluvialis powders, were legal food-grade materials. Brine shrimp paste is a traditional food of local residents in the eastern part of China. Observations from this study suggest that carotenoids accumulated in vivo 30 min after feeding. Those observations form a basis to develop a group of novel food products, including brine shrimp pastes, with varied carotenoid composition.
Data from this study also suggests that a carotenoid ester hydrolysis took place in vivo. If this reaction is enzyme-catalyzed, an attempt to isolate and identify the enzyme that may catalyze this reaction is warranted. Cloning of the related gene is expected. This effort is very likely to result in a novel lipase production that may be applied in the hydrolysis of carotenoid esters in vitro.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
BH conceived of the experiment. JH collected data. BH and JH analysed data. JH wrote the manuscript and both authors contributed to editing the manuscript.
ACKNOWLEDGMENT
The study was supported by the financial support from
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Jin Huang AU - Bodi Hui PY - 2020 DA - 2020/06/05 TI - Feed-induced Variation in the Carotenoid Composition of Brine Shrimp JO - eFood SP - 247 EP - 253 VL - 1 IS - 3 SN - 2666-3066 UR - https://doi.org/10.2991/efood.k.200522.001 DO - 10.2991/efood.k.200522.001 ID - Huang2020 ER -
