Retinopathy of Prematurity—A Brief Review
- DOI
- 10.2991/dsahmj.k.191214.001How to use a DOI?
- Keywords
- Retinopathy of prematurity; risk factors; laser photocoagulation; screening; prevention; blindness; review
- Abstract
Retinopathy of Prematurity (ROP) is a proliferative retinal vascular disease affecting the retina of premature infants and is considered the major cause of blindness in children and the most common cause of retinal vasculopathy in premature and in low-birth-weight infants. The clinical spectrum of ROP varies from spontaneous regression to bilateral retinal detachment and total blindness. Between these two extremes lies the form of ROP, which mandates treatment with laser photocoagulation, or surgery. Increasing rates of preterm births together with better survival rates but lack of efficient screening and delays in the diagnosis causes an increase in the ROP rate. In developing countries, the atypical form of aggressive posterior ROP was reported in the use of unblended oxygen of more mature infants. Prevention of ROP by following efficient protocols of supplemental oxygen, prevention of sepsis, timely screening, and laser treatment requiring coordinated teamwork between neonatologists and trained ophthalmologists are mandatory to prevent ROP.
- Copyright
- © 2019 Dr. Sulaiman Al Habib Medical Group. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Retinopathy of Prematurity (ROP) was first described in 1940 as retrolental fibroplasia and was characterized by a complete retinal detachment behind the lens [1]. Since then, it has been considered an important cause of childhood blindness worldwide [2,3].
The advent of neonatal intensive care units has led to an increase in the survival rate of premature babies; global estimates showed that nearly 15 million babies (10% of births) are born preterm (<37 weeks of completed gestational age) each year [4]. ROP is considered a major public health concern. Absence or delayed screening and lack of trained ophthalmologists to screen and treat ROP are major contributing factors for ROP blindness.
2. INCIDENCE
The incidence of ROP based on population studies was uncertain because of substantial variability in the study designs, gestational ages, survival rates, and treatments used in the studied infants. The reported incidence of ROP-associated blindness was varied and mainly linked to the socioeconomic developments as well as the quality and accessibility of healthcare facilities. In high-income countries, the rate was lower than 10% but was reported to be 40% or higher in low- and middle-income countries [5].
Blencowe et al. [6] reported that in 2010, a total of 184,700 (uncertainty range, 169,600–214,500) preterm babies developed any stage of ROP. They further stated that 20,000 (15,500–27,200) became blind or severely visually impaired from ROP, and 12,300 (8300–18,400) developed mild/moderate visual impairment. In terms of geographical location, they report that 65% of those visually impaired from ROP were born in middle-income regions. In terms of gestation, not only preterm babies were affected as the authors also reported that 6.2% (4.3–8.9%) of all ROP visually impaired infants were born at >32 weeks’ gestation.
In Saudi Arabia, several studies have reported on the incidence of ROP in preterm babies and linked the incidence with gestational age and birth weight [7–9]. A study of ROP in Riyadh [7] reported that 56% of all premature births developed ROP, and 15% of these patients had Stage 3 disease (severe ROP). The mean gestational age of ROP patients was 30 weeks. A prospective study in Jeddah and Riyadh [8] showed that the incidence of ROP was 33.7%, and the risk factors were Patent Ductus Arteiorus (PDA) and intraventricular hemorrhage.
3. PATHOPHYSIOLOGY
Retinopathy of prematurity is considered a multifactorial disease, and its pathogenesis has been extensively studied in humans and in several animal models. The physiological process by which the retina is vascularized in human embryos is divided into two stages: vasculogenesis and angiogenesis. Vasculogenesis is the process that occurs during the early stage of retinal vascularization, in which endothelial progenitor cells differentiate into endothelial cells to form blood vessels. This process begins at 12 weeks and ends at 21 weeks of gestational age. Angiogenesis is a process that occurs in the advanced stage of retinal vascularization by which the blood vessels gradually grow to surround the retina from the optic disc starting at 16 weeks of gestational age, reaching the nasal retina at 32 weeks, and the temporal retina at 36–40 weeks [10–12]. In premature infants, the development of ROP proceeds with an initial phase of retinal microvascular degeneration [11,12] associated with an arrest in progressive vascularization of the peripheral retina. These vascular changes result in retinal ischemia which predisposes to abnormal intravitreal neovascularization, leading to its most significant sequelae retinal detachment and permanent visual loss [13,14].
4. RISK FACTORS
The degree of prematurity is the most consistent risk factor for developing ROP. The lower the birth weight and the gestational age, the higher is the risk for ROP. Other risk factors also studied include use of supplemental oxygen, intraventricular hemorrhage, apnea, mechanical ventilation, sepsis, surfactant therapy, anemia, thrombocytopenia, administration of blood products, double volume exchange transfusions, and low postnatal weight gain proportion (i.e., weight gain <50% of the birth weight) by the 6th week of life [15–21].
Genetic factors might also contribute to the risk of ROP. A study showed that ROP occurs more often in white than in black infants and in boys than in girls [22]. Genetic polymorphisms might change gene function, which could affect the disease; however, no genetic factor identified thus far accounts for a substantial number of patients with the disease. Future studies that make use of genomics and proteomics could be helpful in identification of relevant genetic factors [23].
The link of ROP with other neonatal morbidities such as neurological dysfunction, poor brain growth, necrotizing enterocolitis, intraventricular hemorrhage, and bronchopulmonary dysplasia has been highlighted [24].
In extremely preterm infants, severe ROP predicts risk of death or major disability at age 11 years [25]. Therefore, addressing poor postnatal growth, hyperoxia, infection, and inflammation to reduce risk of ROP might also reduce the risk of these comorbidities.
5. CLASSIFICATION
The ROP International Classification was first published in 1985 [26] and was revised in 2005 [27]. The classification was based on the severity (stage), anterior–posterior location (circumferential extent), and presence or absence of plus disease. The zones of ROP were defined as illustrated in Figure 1a. Zone I was a circle centered on the optic nerve with a radius equal to twice the distance between the optic nerve and the fovea; Zone II was a circle centered on the optic nerve with a radius equal to the distance between the optic nerve and nasal ora serrata; and Zone III was the temporal crescent remaining.
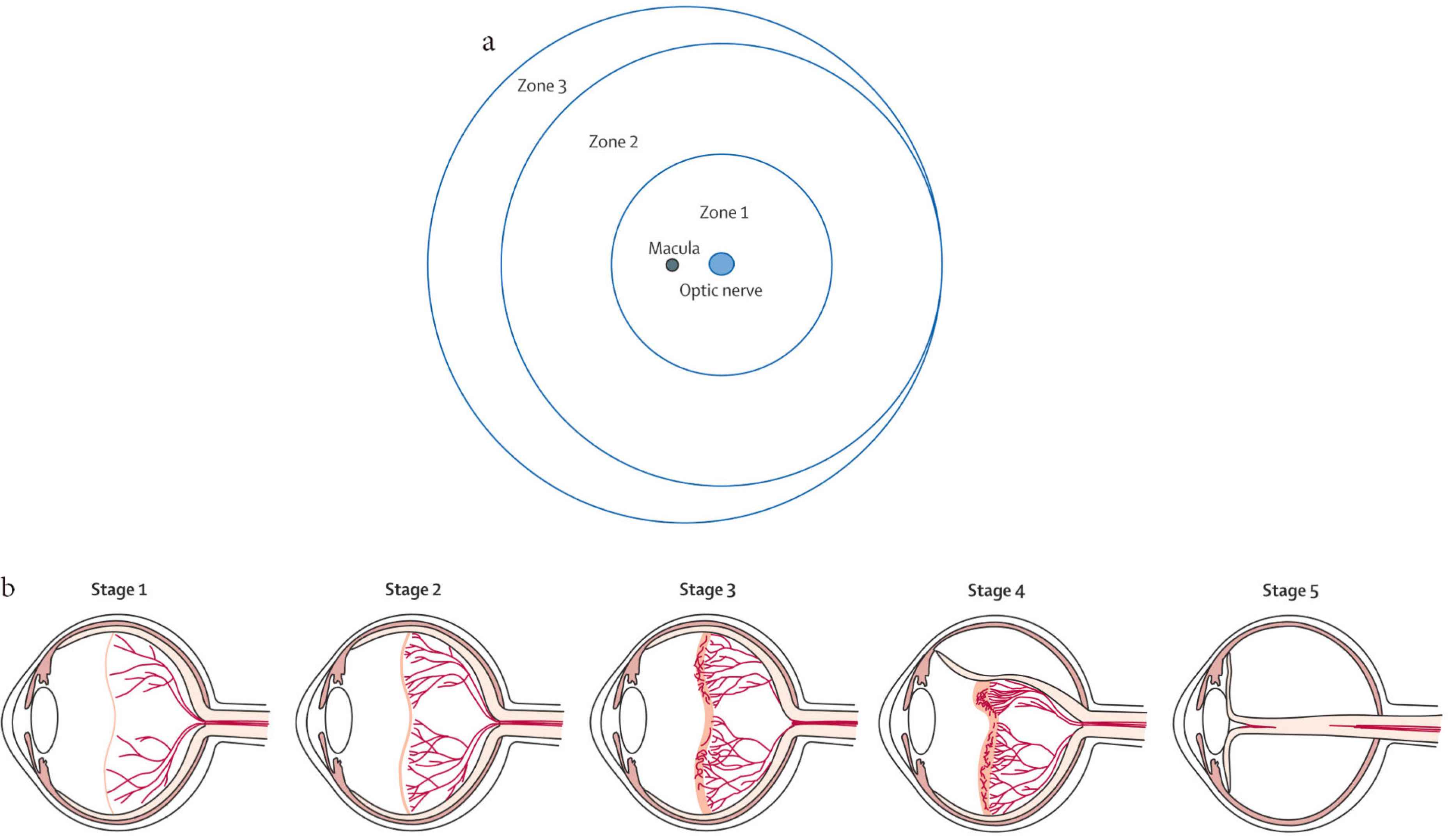
Zones and stages of retinopathy of prematurity. (a) Diagram shows the retina in the right eye is divided into three zones. (b) Classification of retinopathy of prematurity: Stage 1, a thin demarcation line between vascularized and nonvascularized retina; Stage 2, a ridge; Stage 3, an extraretinal fibrovascular proliferation; Stage 4, partial retinal detachment; and Stage 5, total retinal detachment.
Figure 1b shows that the stages of ROP started from incomplete vascularization (no stage) to descriptions of the retinal appearance at the junction between the vascularized and avascular retina. Plus disease is defined by dilation and tortuosity of posterior retina blood vessels, and this sign indicates the disease progression Figure 2.
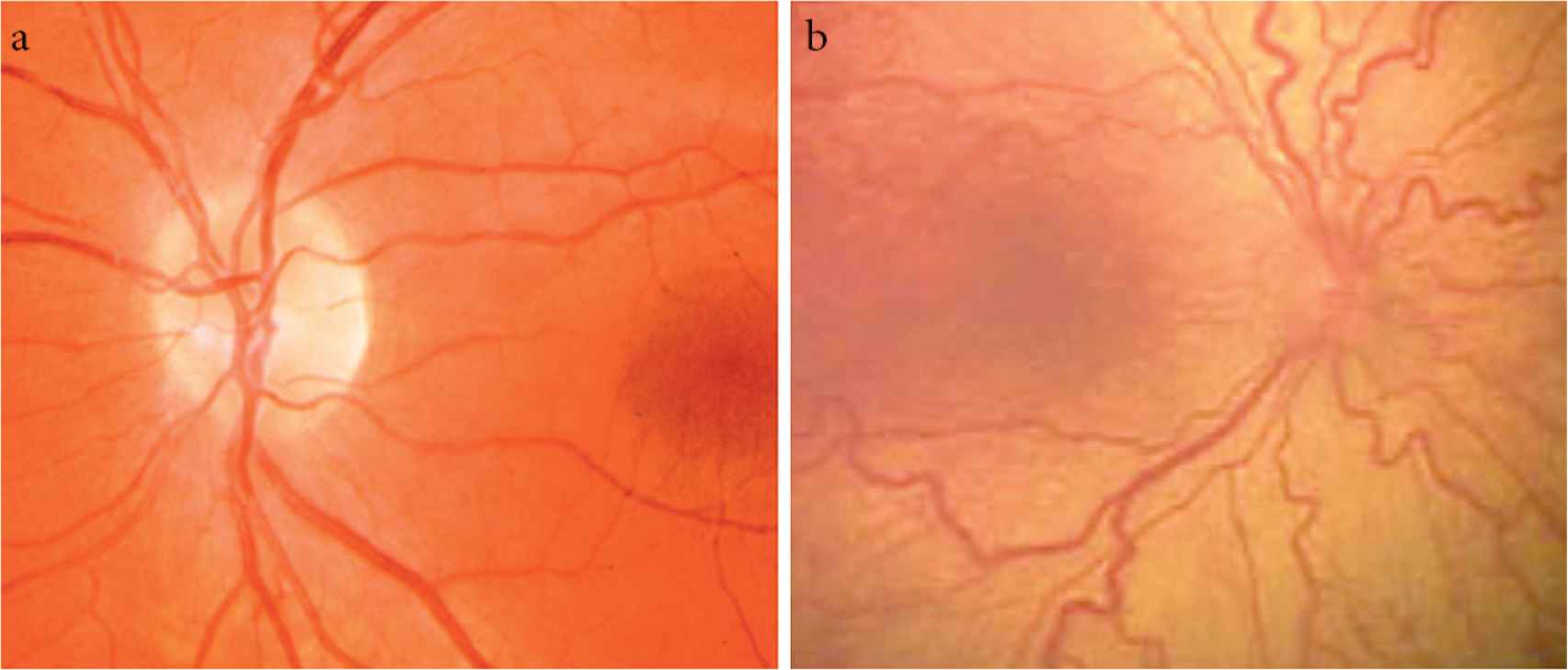
Stages of retinopathy of prematurity. (a) Normal retina vessels. (b) Plus disease showing venous dilation and increased arterial tortuosity of posterior vessels.
The investigators of the Early Treatment for Retinopathy of Prematurity (ET-ROP) study [28] also reclassified ROP type 1 (Figure 3) (requires treatment) and type 2 (Figure 4) (to be followed up). Type 1 now includes a more virulent form of retinopathy in extremely low-birth-weight babies [aggressive posterior ROP (APROP)], which involves very central neovascularization with plus disease (Figure 5).
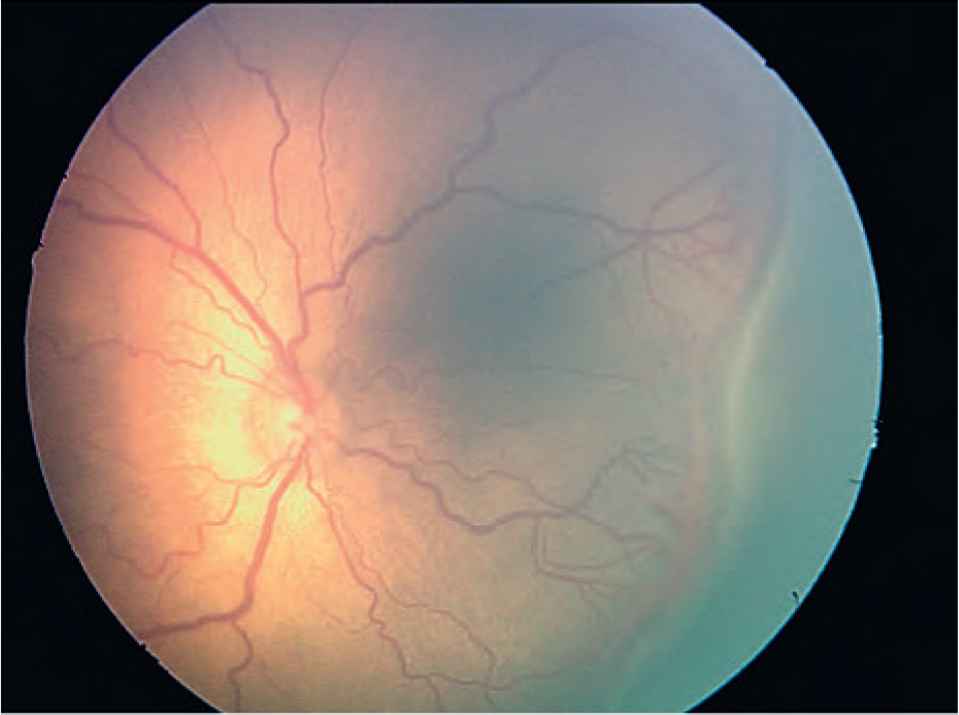
Type 1 retinopathy of prematurity (plus disease and posterior Stage 3).
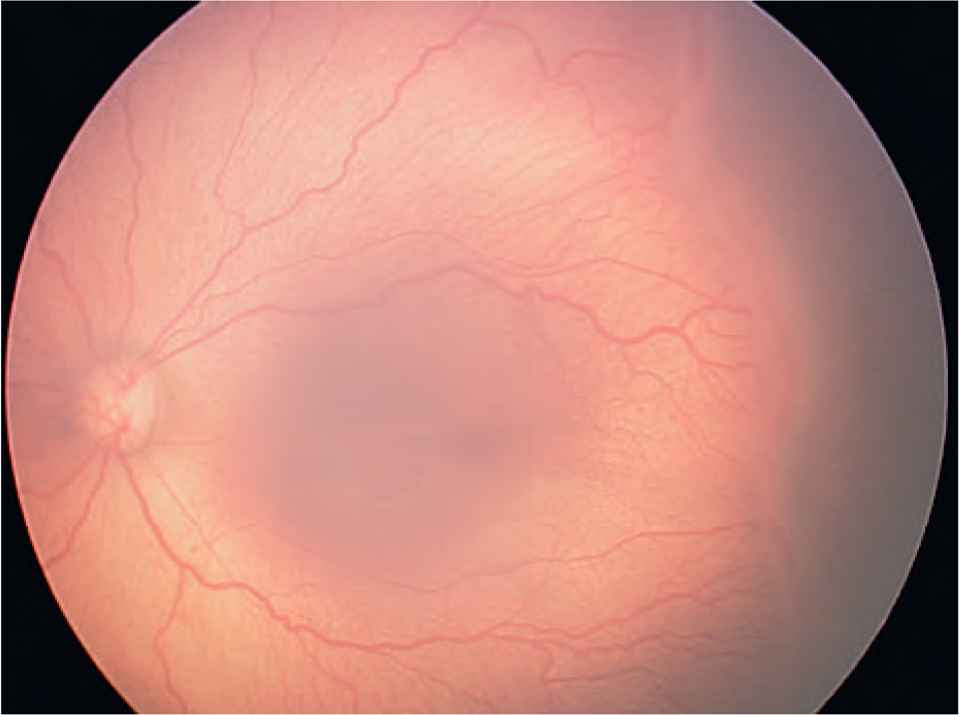
Type 2 retinopathy of prematurity (Stage 3 disease in Zone II no plus disease).
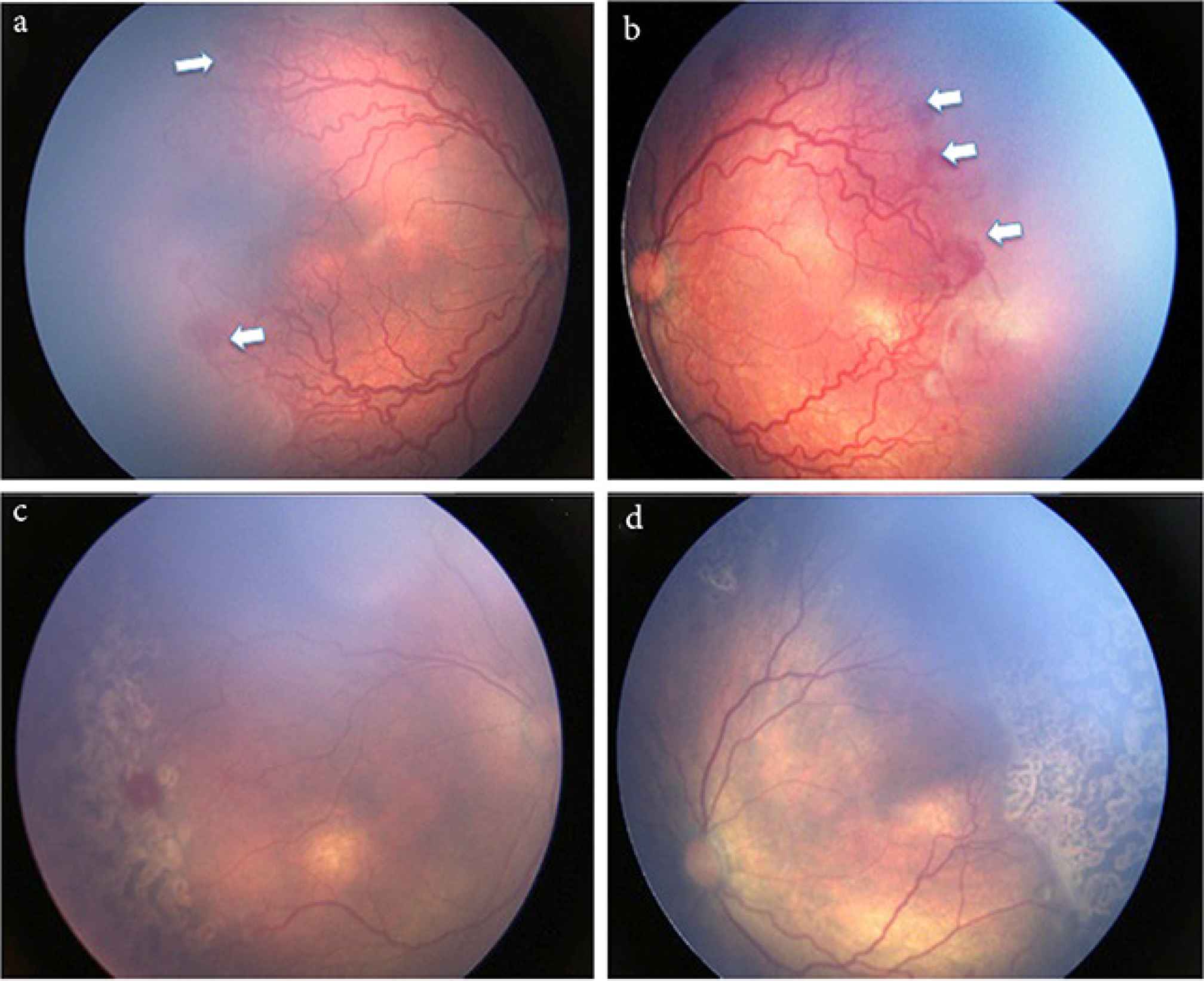
(a and b) Both eyes demonstrating Aggressive Posterior Retinopathy of Prematurity (APROP) that show neovascularization at the junction of vascular and avascular retina in Zone I (white arrow) and with presence of plus disease. (c and d) After laser photocoagulation showing regressed ROP and laser scars.
5.1. Screening
The purpose of the ROP screening guidelines is to (1) detect preterm infants who are at risk of developing ROP and thus closely monitor their retinal development after birth, and (2) identify preterm infants with severe disease that requires prompt treatment. Globally the recommended dilated fundus examination screening time ranges from 30 to 35 weeks’ gestational age at birth and from birth weights of 1500–2000 g. This was dependent on the extent and quality of neonatal intensive care available. Eye examination can be very painful for preterm infants, even when done by a skilled ophthalmologist [29]. It was reported that only about 5–10% of screened infants in high-quality neonatal intensive care mandate treatment; therefore, safely decreasing the number of stressful and costly screening examinations would be beneficial.
Recently, the Saudi National Eye Health Program (NEHP) and Neonatology Services Improvement Program at Ministry of Health (MOH) formed the ROP Screening program [30] and published screening guidelines and treatment recommendation for ROP mostly based on (ET-ROP) study recommendation [28] (discussed in the following subsections).
5.2. Inclusion Criteria
The recommended inclusion criteria were as follows. (1) Neonates with birth weight of ≤1500 g and/or gestational age of ≤32 weeks (as defined by the attending neonatologist). (2) Preterm neonates (<36 weeks) receiving supplemental oxygen for ≥50 days. (3) Special attention should be paid to larger preterm neonates at risk of ROP who receive frequent red blood cell transfusions or exchange transfusions to treat anemia of prematurity or rhesus hemolytic disease of the newborn.
5.3. Timing of First Examination
(1) Any preterm neonate of gestational age of ≤27 weeks should have the first fundus examination at postmenstrual age of 31 weeks. (2) Any preterm neonate of gestational age of ≥28 weeks should have the first fundus examination at 4–6 weeks chronological (postnatal) age. (3) Any eligible stable preterm neonate planned for discharge prior to the scheduled fundus examination should have the first fundus examination at the time of discharge.
5.4. Follow-up Examinations
(1) 1 week or less follow-up if Stage 1 or 2 ROP in Zone I (without plus disease) or Stage 3 ROP in Zone II (without plus disease). (2) 1–2 weeks of follow-up in case of immature vascularization in Zone I with no ROP, Stage 2 ROP in Zone II, or regressing ROP in Zone I. (3) 2 weeks of follow-up in case of Stage 1 ROP in Zone II/regressing ROP in Zone II; or in case of immature vascularization in Zone II with no ROP, Stage 1 or 2 ROP in Zone III or regressing ROP in Zone III. (4) Three weeks or more follow-up in the following findings: Zone III retinal vascularization attained without previous Zone I or II ROP, regression of ROP.
6. MANAGEMENT
6.1. Cryotherapy
The multicenter trial of Cryotherapy for ROP (Cryo-ROP) [31] earlier proved the usefulness of peripheral retinal cryotherapy in reducing unfavorable outcomes. Treatment at threshold resulted in nearly 50% reduction in rates of retinal detachment (21.8% in eyes treated with cryotherapy compared to 43% in the untreated eyes). The follow-up report of the 10-year study [32] confirmed that unfavorable structural outcomes were reduced from 48% to 27%, and unfavorable visual outcomes (i.e., best corrected visual acuity worse than 20/200) were reduced from 62% to 44%. However, cryotherapy is a destructive time-consuming procedure that requires a trained retina specialist. Furthermore, the outcomes are still not optimum.
6.2. Retinal Laser Photocoagulation
The standard of care for ROP treatment is laser ablation to the peripheral avascular retina and showed good success rates in treatment of threshold ROP as reported in earlier studies [33–37]. The procedure can be performed under general anesthesia with endotracheal intubation or with only sedation in the operating room or in the neonatal nursery. Laser spots should be of moderate intensity and approximately half to one spot size apart, extending anteriorly from the ridge to the ora serrata. Later on, the ET-ROP randomized trial showed a reduction in unfavorable structural outcomes from 15.6% to 9.1% at 9 months with laser treatment done at high-risk pre-threshold stage compared to treatment at threshold [28]. The study confirmed the effectiveness of treatment for severe ROP and redefined the indications for treatment as follows: (1) Zone I ROP—any stage with plus disease; (2) Zone I ROP—Stage 3 with no plus disease; (3) Zone II ROP—Stage 2 or 3 with plus disease. Treatment should be initiated between 48 and 72 h of diagnosis with special attention for APROP to minimize the risk of retinal detachment.
It was found that the presence of posterior Zone I disease, gestational age <29.5 weeks, presence of pre-retinal hemorrhages prior to laser photocoagulation, and or fibro vascular proliferation after laser photocoagulation can increase the risk for progression to retinal detachment significantly even in prompt laser treatment [38]. Despite the favorable outcome of laser photocoagulation for ROP management, it has limitations in managing certain cases that had poor fundus view in which laser delivery is difficult—for instance, eyes with tonic small pupil with or without posterior synechia or eyes with fresh vitreous hemorrhage. Moreover, APROP cases still showed unfavorable outcomes even after prompt laser photocoagulation.
6.3. Antivascular Endothelial Growth Factor Therapy
The use of Antivascular Endothelial Growth Factor (anti-VEGF) therapy was investigated in a few reports. The Bevacizumab Eliminates the Angiogenic Threat of ROP (BEAT-ROP) study [39] was the largest study that showed the efficacy of intravitreal bevacizumab (IVB) monotherapy (0.625 mg in 0.025 mL) or conventional laser, and it showed that the ROP recurrence in the bevacizumab group was significantly less (4%) than that in the laser-treated group (22%; p = 0.002). A significant treatment effect was found for Zone I (p = 0.003) but not for Zone II disease (p = 0.27). Moreover, the IVB group showed the growing up of peripheral retinal blood vessels, thus avoiding the peripheral retinal destruction that was seen in the laser photocoagulation treatment group. In addition, the study did not show any bevacizumab-related side effects. However, a concern about anti-VEGF therapy risks was raised by the investigators as it might interfere with the normal VEGF secretions that are needed for normal pulmonary, brain, and kidney development in the premature infant [40]. Therefore, anti-VEGEF therapy can be used with caution as rescue therapy in cases where laser delivery failed.
6.4. Surgical Treatment of ROP
Surgical intervention is indicated for Stages 4A, 4B, and 5 ROP. The choice of surgery depends on the ROP stage and surgeon preference. For instance, scleral buckling or Lens-Sparing Vitrectomy (LSV) has been advocated for Stages 4A and 4B, whereas combined lensectomy and vitrectomy was suggested for Stage 5 ROP. The reported anatomic success rates of LSV for Stage 4A ROP range from 84% to 100%, and those for Stage 5 ROP range from 14.3% to 45.5% [41,42].
7. LONG-TERM OUTCOMES
It is well known that definite retinal detachment and blindness can result from untreated ROP. However, the risk of complication can occur even in babies that have undergone treatment. These risks can persist throughout the patient’s lifetime. The most common complication of ROP is myopia, which can worsen throughout childhood. Dragging of the macula with pseudo strabismus that can cause decreased vision is another frequent complication. Other complications include glaucoma, cataract, irregularities of corneal curvature, band keratopathy, acute hydrops, amblyopia, strabismus, and retinal detachment. Therefore, close monitoring throughout childhood is mandatory to detect any late complications [27,28,43,44].
8. AREAS OF CONTROVERSY
8.1. Inositol Supplements
Inositol is a naturally occurring six-carbon sugar derivative found in most foods including breast milk. It is an important component of surfactant [45]. Inositol supplementation increases the amount of saturated phosphatidylcholine in surfactant in infants. Several studies have investigated the use of inositol to prevent the incidence/severity of ROP and preterm comorbidities with uncertain results regarding benefit and safety. Recently, a meta-analysis study [46] investigated the efficacy and safety of inositol supplementation in preterm infants for preventing ROP. The investigators found that inositol has no effect on the incidence of severe ROP and in all ROP stages; in addition, the study found that there was a trend toward an increase on mortality in preterm infants <32 weeks GA. Therefore, the authors did not recommend the use of routine inositol supplementation in preterm infants.
8.2. Hypothermia
Experimental evidence suggests that hypothermia reduces brain damage as well as retinal gliosis associated with perinatal hypoxia [47]. One recent study from the Canadian Neonatal Network determined that the lowest rate of the composite outcome of severe neurological injury, severe ROP, necrotizing enterocolitis, bronchopulmonary dysplasia, and nosocomial infection was an admission temperature of 36.8°C (range, 36.5–37.2°C) [48]. It is noteworthy that neonatal hypothermia is associated with higher mortality and morbidity [49].
8.3. Other Factors
Other studied factors that can influence the rate of ROP that have not showed beneficial effects include the effects of light reduction on ROP clinical trial [50], which investigated the effect of increased light to premature infants and severity of ROP; and the use of antioxidants supplements such as vitamin E, lutein/zeaxanthin [51,52]. However, these studies did not show an effect on rate and severity of ROP.
Currently, the future research direction is on genetic and epigenetic factors to assess the genetic variants in ROP [23], and in stem cell or progenitor cells to repair damaged retinal tissues [53]. A number of recent reviews have been published on ROP and are in the October 2019 issue of Clinics in Perinatology [54].
9. CONCLUSION
Retinopathy of prematurity is considered a potentially childhood blindness disorder. As the survival rate of preterm births is increasing worldwide, including in Saudi Arabia, this reflects the increase in the frequency of ROP cases. Close monitoring of the risk factors such as body weight, way of oxygen delivery, infection prevention, restrictive blood transfusion, together with adherence to the protocol of ROP screening guidelines, are the gold standard of care to prevent ROP blindness. Parents should be informed about the importance of regular eye examination of their infants to prevent ROP blindness. Current treatments include laser peripheral retinal photocoagulation for patients with prethreshold type 1 ROP. The recent development of anti-VEGF inhibitors such as bevacizumab has shown promising results for treatment of aggressive Zone I ROP; however, there is a need for further research into the systemic and long-term effects of these medications in premature infants. Surgical intervention is indicated for Sages 4A, 4B, and 5 ROP with guarded anatomical and visual outcomes, especially for Stage 5 ROP. Therefore, timely screening and laser treatment requiring coordinated teamwork between neonatologists and trained ophthalmologists in neonatal intensive care unit are mandatory to prevent ROP complications.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Saba Al Rashaed PY - 2019 DA - 2019/12/24 TI - Retinopathy of Prematurity—A Brief Review JO - Dr. Sulaiman Al Habib Medical Journal SP - 58 EP - 64 VL - 1 IS - 3-4 SN - 2590-3349 UR - https://doi.org/10.2991/dsahmj.k.191214.001 DO - 10.2991/dsahmj.k.191214.001 ID - AlRashaed2019 ER -
