Tick-borne Encephalitis—Need to know for Professionals outside Endemic Areas
- DOI
- 10.2991/dsahmj.k.190722.001How to use a DOI?
- Keywords
- Epidemiology; lyme; prevention; tick-borne encephalitis (TBE); travel
- Abstract
Tick-borne Encephalitis (TBE) is an arboviral infection widespread in Europe and northern parts of Asia. Ticks transmit the associated flavivirus usually between March and November to people with outdoor activities in the countryside, in gardens or, rarely, in urban parks. The clinical picture of TBE has two phases: after an uncharacteristic febrile illness and a brief interval in which neurological symptoms occur in a minority of the infected patients. In children, meningitis is the predominant syndrome, whereas the proportion of patients with more severe encephalitis and encephalomyelitis increases with growing age. Consequently, children may suffer from cognitive defects and adults from neurological defects after the acute phase. As there is no therapy for TBE, prevention is of paramount importance. The first line of defense is to avoid tick “bites” (actually stings). Various effective and well-tolerated vaccines are marketed in Europe, all parts of Russia, and in China.
- Copyright
- © 2019 Dr. Sulaiman Al Habib Medical Group. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Tick-borne Encephalitis (TBE) is an often severe infection ecologically associated with an arbovirus, taxonomically with a flavivirus [1,2]. Usually it is transmitted to humans as accidental hosts by a tick “bite”—more precisely, a sting. Transmission through unpasteurized milk occasionally has been described [3]. Not only thousands of residents within endemic areas are affected by TBE [4], but also a substantial number of foreign visitors who expose themselves to risk mainly outside the center of large cities become patients [5]. Thus, it is essential that health professionals in all parts of the world are aware of the essentials of this infection.
2. EPIDEMIOLOGY
Transmission of TBE usually occurs between March and November (temperature >8°C) at altitudes below 2100 m in the habitat of small rodents who are the main hosts and reservoirs for the virus [6,7]. Thus, the main risk areas are forested and agricultural lands, but also gardens of private homes and occasionally public parks in cities. Besides work in such areas, leisure activities—mainly hiking, camping, jogging, gardening, or collecting berries or mushrooms—may also lead to transmission [8]. Contaminated ticks do not fall from trees like paratroopers; rather, they climb on high grasses or branches up to an altitude of 1.5 m and they get on the clothing or skin of humans by direct contact. The risk of TBE is widespread in parts of Europe [4], in the Russian Federation [2], and in Northern Asia up to Japan [9,10] (Figures 1 and 2).
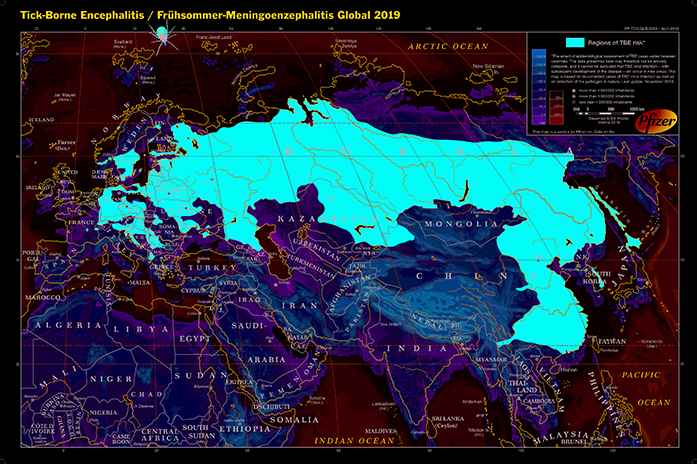
Tick-borne encephalitis (Frühsommer-Meningoenzephalitis), Global 2019.
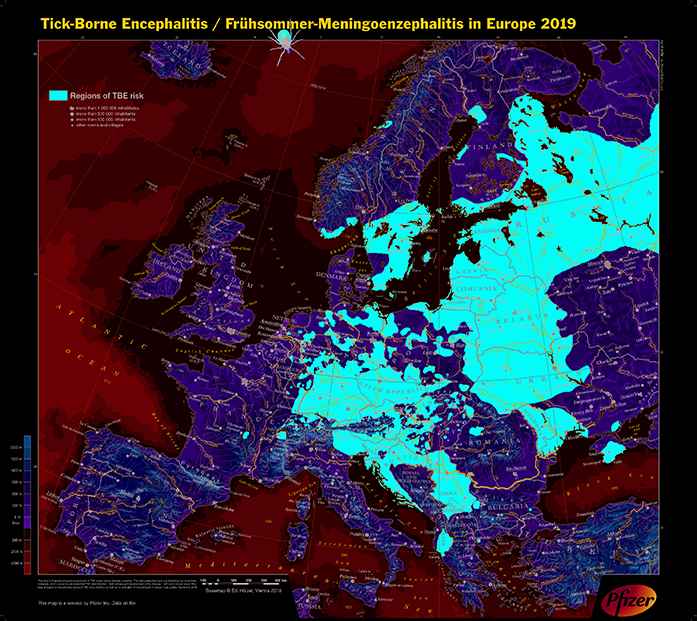
Tick-borne encephalitis (Frühsommer-Meningoenzephalitis) in Europe 2019.
As a consequence of global warming, and also of changing behavioral patterns of populations resulting in increased exposure and a variety of other reasons, an increase both with respect to endemic regions and incidence has been observed in the past decades [7,11]. In Europe, the main vector is Ixodes ricinus. Overall, 0.1–5% of these ticks harbor the TBE virus [12,13]. Thus, only a minority of individuals who experienced a tick “bite” will develop TBE. It has been extrapolated that for every 100,000 persons with tick “bites,” from six to 10 (in Asia 30) will develop TBE with neurological symptoms and from one to two (in Asia 20) will die.
3. CLINICAL CHARACTERISTICS AND OUTCOME
A tick “bite” often remains unnoticed; because of the anesthetizing effect of the tick’s saliva, only from one- to two-thirds of TBE patients will remember noticing a tick. Even after an infected tick bite, most individuals will remain asymptomatic, and 2–30% will develop an initial nonspecific flu-like illness lasting a few days, followed by an asymptomatic interval of 1–20 days. Only 10% among those who had the initial symptoms will develop neurological symptoms [14]. In children and adolescents, meningitis is the most frequent syndrome, but with growing age, the proportion of encephalitis and encephalomyelitis will continually increase [13]. Thus, neurological sequelae, which may result in incapacitation, are recorded as a consequence of TBE in adults and, particularly, in the elderly. The case fatality rate—which overall is from 1% to a few percent—increases with age. Although children only rarely have neurological sequelae, they often tend to have some cognitive impairment over a period of many years [15].
4. DIAGNOSTICS AND TREATMENT
The initial therapeutic step is to remove the tick as soon as possible. Even if it very rapidly may have deposited the TBE virus, this is beneficial to reduce the risk of transmission of borrelia, which are “vomited” much later. Removal should be done gently with tweezers, so as to ensure that the body of the tick is not compressed and the hypostome is also hopefully removed. There is no indication for surgical excision if “the head” has been separated and remains in the skin. Alternatively, a modified indented tick removal (credit card-sized) tool can be slipped under the tick to remove it (Figure 3).

“Tick card” to be used for removal of ticks from the skin (size of credit card).
For a confirmed case, the patient should meet clinical and laboratory criteria, that is, symptoms of inflammation of the central nervous system plus at least one of the following: TBE-specific Immunoglobulin M (IgM) and IgG antibodies in blood; TBE-specific IgM antibodies in Cerebrospinal Fluid (CSF); seroconversion or four-fold increase of TBE-specific antibodies in paired serum samples; detection of TBE viral nucleic acid in a clinical specimen; or isolation of TBE virus from clinical specimen.
Different definitions exist for probable and possible cases [16]. There is no specific treatment for TBE and or any need for isolation. The procedures are limited to supportive care, possibly in intensive care units. This includes maintenance of water and electrolyte balance, sufficient caloric intake, and medication to suppress pain, seizures, agitation, and fever. Physio- and ergotherapy are usually indicated [6]. Neurosurgical interventions are indicated on rare occasions.
5. PREVENTION
5.1. Options to Reduce Exposure
As there is no effective treatment for TBE, prevention is paramount. Theoretically, reduction of exposure is the first line of defense, but the options for tick avoidance at best have a limited effect [17]. Repellents offer no more than a moderate effectiveness [18]. Although battle-dress uniforms impregnated with permethrin reduced the tick-bite incidence by 98%, clothing covering so much of the body would be associated with minimal compliance at leisure [19,20]. Even if only 1% or less of all TBE cases are associated with the consumption of unpasteurized milk or dairy products, this must be avoided [3].
5.2. Vaccination
In Europe, two inactivated vaccines are marketed, both in an adult and in a half-dose pediatric formulation (additional vaccines in Russia [2,6,14]). The route of application is intramuscular injection (in exceptional cases, subcutaneously):
- •
Encepur (GlaxoSmithKline, Rixensart, Belgium, formerly Novartis, Chiron), based on K23 TBE viral strain 1.5 μg in 0.5 ml for adolescents ≥12 years and adults.
- •
Encepur N children/Kinder 0.25 ml indicated from the first until the 12th birthday.
- •
Primary vaccination course, preferably to be started during winter:
- ○
Conventional: Day 0/1–3 months/9–12 months after second dose (three doses);
- ○
Accelerated: Day 0/Day 14/9–12 months after second dose (three doses);
- ○
Rapid: Days 0/7/21 (then possible seroconversion) plus fourth dose = first booster at 12–18 months.
- ○
- •
FSME-Immun (Pfizer Corporation, Vienna, Austria, formerly Baxter, Immuno AG), based on Neudörfl TBE viral strain 2.4 μg in 0.5 ml, in some countries marketed as TicoVac, for use from 16th birthday.
- •
FSME-Immun Junior 0.25 ml (=TicoVac Junior in the UK) indicated from the first until the 16th birthday.
- •
Application: intramuscularly;
- •
Primary vaccination course, preferably to be started during winter:
- ○
Conventional: Day 0/1–3 months (seroconversion 3–5 weeks later 96%)/5–12 months after the second dose (three doses);
- ○
Rapid: Days 0/4/third dose at 5–12 months.
- ○
Some studies initiated by the respective vaccine producers suggested that their product was superior to that of their competitor. However, as neither reviewers, nor the World Health Organization position paper, nor the authors in the TBE chapter the latest edition of the Plotkin textbook expressed a preference for either of the two vaccines, there is apparently general agreement that both are very similarly effective and well tolerated [6,14]. There is limited clinical and more research evidence that both vaccines induce protective immunity not only against the homologous European TBE virus subtype, but also against the Siberian and Far Eastern strains [14,21]. It is possible to switch from one vaccine to the other during primary immunization and for boosters [22], which is relevant, for example, when the originally used vaccine is temporarily out of stock.
No controlled efficacy trials have been performed with either of the two vaccines, but the impact of vaccination programs is well documented particularly basing on Austrian data. It is the only country with official universal TBE vaccination recommendation. The immunization coverage of 85% (2011) and 82% (2013), respectively, has resulted in an overall reduction of TBE to 16% as compared to the pre-vaccination era [6,23,24]. In children aged 0–14 years, field effectiveness rates for the vaccine of 94.0% (95% confidence interval, 88.0–97.0) and 88.6% (95% confidence interval, 63.3–96.5), respectively, have been recorded.
Duration of protection as per package insert is initially limited to 3 years after basic immunization. In children and adolescents, subsequent boosters are recommended every 5 years in most countries. It is only in Switzerland that booster injections are recommended every 10 years for all age groups. In adults younger than 50 years, seropersistence of TBE virus antibodies was 88.6% after 10 years subsequent to a three-dose primary series plus a first booster with FSME-Immun [25].
Similar to other immunizations, TBE vaccine protection does not reach the full 100% rate. A Swedish and a Slovenian series with clinical and serological evidence of TBE despite adequate immunization have been presented [26,27]. There is evidence that such breakthroughs occur mainly in adults, particularly in older age.
Overall, “the currently available vaccines are considered to be safe with no serious adverse events reported” [6]. As with other inactivated vaccines systemic reactions can occasionally occur, mainly after the first dose, particularly headache, fatigue, malaise, muscle, and joint pain.
6. CONCLUSION
Tick-borne encephalitis is a potentially devastating neurological infection endemic in large parts of Europe and in Northern Asia. In view of the fact that there is no treatment option that guarantees a favorable outcome without sequelae and incapacitation or death, prevention is the strategy of choice. Although reduction of risk exposure is plausible, it has only a limited effectiveness. Thus, immunization must be recommended to those at potential risk. Because TBE vaccines often are not available outside countries with endemicity, it is important that persons at risk be exposed as soon as possible upon arrival in such regions. Awareness about this health risk must be improved not only among all who will become expatriates there, but also among tourists or other temporary visitors during the period of transmission. People visiting only in winter (e.g., for skiing) and those who will remain only in urban centers (e.g., attending meetings or cultural indoor events) are not at risk.
CONFLICTS OF INTEREST
The author has received honoraria and reimbursement for travel and lodging in connection to lectures by both producers of TBE vaccines mentioned above.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Robert Steffen PY - 2019 DA - 2019/08/02 TI - Tick-borne Encephalitis—Need to know for Professionals outside Endemic Areas JO - Dr. Sulaiman Al Habib Medical Journal SP - 65 EP - 69 VL - 1 IS - 3-4 SN - 2590-3349 UR - https://doi.org/10.2991/dsahmj.k.190722.001 DO - 10.2991/dsahmj.k.190722.001 ID - Steffen2019 ER -
