Patients with refractory angina have increased aortic wave reflection and wasted left ventricular pressure energy
- DOI
- 10.1016/j.artres.2014.01.003How to use a DOI?
- Keywords
- Left ventricular wasted energy; Wave reflections; Augmentation index; Refractory angina; Central aortic pressure; Arterial stiffness; SphygmoCor
- Abstract
Background: Early return of reflected blood pressure (BP) waves from the lower body augments central systolic BP and increases systolic pressure-time index (SPTI) and wasted left ventricular (LV) pressure energy, which increase afterload and myocardial oxygen (MVO2) demand. Accordingly, we sought to determine wave reflection characteristics and diastolic timing in a group of patients with chronic stable angina resistant to anti-anginal therapy.
Methods: Radial artery BP waveforms were recorded non-invasively by applanation tonometry in 36 patients with refractory angina (RA) and a reference control (CON) group of 36 successfully treated hypertensive patients without angina matched for age, gender, height, BMI, and mean BP. Pulse wave analysis (PWA) of the ascending aortic BP waveform was used to determine central hemodynamics, diastolic timing, wave reflection characteristics and wasted LV pressure energy (LVEw).
Results: Compared to the CON group, RA patients had higher central aortic augmented BP, augmentation index (Alx) and reflected pressure wave systolic duration (SDR). These modifications in wave reflection characteristics were associated with an increase in SPTI and LVEw and a decrease in pulse BP amplification and the diastolic pressure time fraction.
Conclusions: RA patients have changes in systolic wave reflections and diastolic timing that increase LV afterload, MVO2 demand and wasted LV energy and reduce coronary artery perfusion. These alterations in cardiovascular function contribute to an undesirable mismatch in the MVO2 supply/demand ratio that favors ischemia and angina pectoris and may precipitate future adverse cardiovascular events.
- Copyright
- © 2014 Published by Elsevier B.V. on behalf of Association for Research into Arterial Structure and Physiology.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Myocardial ischemia and angina pectoris occur when there is an imbalance between myocardial oxygen supply and demand, and the ischemia is usually entirely or predominantly subendocardial. Animal models have shown that relative subendocardial ischemia can be predicted from the ratio of two pressure-time areas: the area beneath the aortic pressure curve during left ventricular (LV) diastole (DPTI) and the area beneath the aortic pressure curve during LV systole (SPTI).1 Increased aortic stiffness increases aortic systolic blood pressure (SBP), decreases diastolic blood pressure (DBP) and widens pulse pressure (PP). These pressure changes negatively influence myocardial perfusion and coronary blood flow reserve and reduce the myocardial oxygen supply (DPTI)/demand (SPTI) ratio, which promotes subendocardial ischemia2–4; these adverse alterations are exaggerated in LV hypertrophy. Furthermore, numerous reports in humans suggest that aortic stiffness and wave reflections are associated with coronary artery atherosclerosis5–7 and predict adverse cardiovascular (CV) events and outcome.8–11 Recent studies have shown that central aortic PP, a surrogate for arterial stiffness, more strongly relates to adverse cardiovascular events and outcome than does brachial PP,12–14 therefore, measurement of central blood pressure and its components may improve our understanding of angina and aid in risk stratification.15
The central arterial BP wave is composed of a forward traveling wave generated by LV ejection and a later arriving reflected wave from the periphery.16 Chronic increase in stiffness of elastic arteries and the resulting increase in wave reflection are the primary cause of increased PP in subjects with degeneration and hyperplasia of the arterial wall. An increase in smooth muscle tone in peripheral arteries can also increase wall stiffness directly in these vessels and increase wave reflection in the central elastic aorta. As stiffness increases, transmission velocities of both forward and reflected waves increase, which causes the reflected wave to arrive earlier in the central aorta and augment pressure in late systole. Augmentation in central SBP increases LV mass, wasted pressure energy, SPTI and myocardial oxygen demand while a decrease in diastolic pressure time (DPT)17 and/or DPTI decreases myocardial perfusion causing a mismatch in ventricular/vascular coupling and an imbalance in the myocardial oxygen supply/demand ratio.18,19 Also, a minor decrease in DPT plus an increase in augmented pressure may have as much negative effect on coronary blood flow and reserve as a severe coronary artery stenosis.20 All of the above variables can be obtained from the central aortic pressure wave. Accordingly, the objective of the present study was to investigate indices of myocardial oxygen supply and demand non-invasively using central arterial pulse wave analysis (PWA)21 in a group of patients with refractory angina and compare these findings with a group of age, height, BMI, mean arterial BP and heart rate matched patients without angina and evidence for ischemic heart disease successfully treated for hypertension.
Methods
Institutional Review Board approved the study and written informed consent was obtained from each participant. Exclusion criteria included contraindications to provocative diagnostic testing, cardiomyopathy, New York Heart Association class III-IV congestive heart failure, recent myocardial infarction, and significant valvular or congenital heart disease. Demographic data, medical history, and symptoms were collected and are presented in Table 1. Thirty-six patients with refractory angina underwent non-invasive central aortic PWA studies and results were compared to a reference group of treated hypertensive patients without angina (N = 36) matched for, age, gender, height, BMI, mean arterial BP and heart rate. Non-invasive data were collected at least two hours after a meal and/or intake of coffee (or smoking) with the subject supine in a quiet, temperature-controlled room after a rest period of at least ten minutes.
| CON (N = 36) |
RA (N = 36) |
P | |
|---|---|---|---|
| Age (years) | 60 ± 12 | 65 ± 9.0 | 0.08 |
| Male/female | 27/9 | 27/9 | |
| Height (cm) | 174 ± 11 | 173 ± 8.5 | 0.73 |
| Weight (Kg) | 85 ± 18 | 90 ± 16 | 0.26 |
| BMI (Kg/m2) | 28 ± 4.7 | 30 ± 4.7 | 0.07 |
| HR (bpm) | 64 ± 8.4 | 63 ± 7.7 | 0.66 |
| Anginal episodes/week | 0.0 | 7.1 ± 6.0 | |
| Medications | Number of patients | ||
| Aspirin | 21 | 25 | |
| Plavix | 0 | 22 | |
| Lipid lowering agent | 22 | 32 | |
| Diuretic | 16 | 14 | |
| ACE inhibitor | 17 | 22 | |
| Angiotensin receptor blocker | 6 | 5 | |
| Calcium channel antagonist | 12 | 14 | |
| β-blocker | 17 | 25 | |
| Long acting nitrate | 1 | 29 | |
| Digoxin | 2 | 7 | |
CON = control group of treated hypertensive patients; RA = treated refractory angina patients; BMI = body mass index; HR = resting heart rate; ACE = angiotensin converting enzyme.
Patient characteristics.
Peripheral cuff blood pressure measurement
Brachial systolic, diastolic and pulse BP were measured in the left arm using a validated, automatic oscillometric BP monitor (Omron R3, Omron Healthcare, Kyoto, Japan) and an appropriate size BP cuff. Three measurements were taken at least two minutes apart and the latter two averaged and used in data analysis.
Central aortic pulse waveform analysis
Assessment of arterial wall properties, wave reflection characteristics, and event timing were performed non-invasively using the SphygmoCor system (AtCor Medical, Sydney, Australia). Radial artery pressure waveforms were recorded at the left wrist, using applanation tonometry with a high-fidelity micromanometer (Millar Instruments, Houston, Texas). After 20 sequential waveforms were acquired and ensemble averaged, a validated generalized mathematical transfer function was used to synthesize the central aortic pressure waveform.16,21,22 Indices of LV afterload, myocardial oxygen demand, and coronary artery perfusion were derived from the central aortic pressure waveform using PWA.16,19 Morphology of the aortic pressure wave and its components are illustrated in Fig. 1. The merging point of forward and reflected waves (the inflection point, Pi) is identified on the pressure waveform. (Pi − Pd) is the amplitude of the forward wave (unaugmented pressure) created by LV ejection and (Ps − Pi) is the amplitude of the reflected wave from the lower body (augmented pressure). The aortic augmentation index (AIx) is defined as reflected wave amplitude, (Ps − Pi), divided by pulse pressure (Ps − Pd) and expressed as a percentage.23 The time, Tr, from the beginning upstroke of the synthesized aortic systolic pressure waveform to the upstroke of the reflected wave (inflection point, Pi) is the round-trip travel time of the pressure wave to and from the major reflecting site in the lower body.16,24 The time from the inflection point to the incisura (or dicrotic notch) is the systolic duration of the reflected wave (SDR).16 Increased pulse wave velocity (PWV) leads to a larger systolic duration of the reflected wave and a more rapid return of the reflected wave from the periphery to the heart. When the reflected wave returns during systole, as seen in Fig. 1, the aortic pressure is augmented and, therefore, the LV must generate enough energy to overcome this added boost in pressure and opposition to empting. This energy (LVEw), which takes into account both amplitude and systolic duration of the reflected wave, is wasted since it does not contribute to blood flow production and can be obtained as the area under the systolic portion of the reflected wave.24 SPTI = ΔSPTI + LVEw was estimated as the area under the systolic portion of the aortic pressure wave above zero and DPTI was estimated as the area under the diastolic portion of the pressure wave above zero. The ratio of these two area variables, DPTI/SPTI, represents the myocardial supply and demand ratio and is termed the myocardial viability ratio.18 The fraction (F) of time during the cardiac cycle the heart spends in systole (SPTF) and diastole (DPTF) was calculated as SPT/(cycle length) and DPT/(cycle length), respectively. Subendocardial perfusion is dependent upon the ratio DPTF/SPTF and is associated with aortic stiffness.25,26
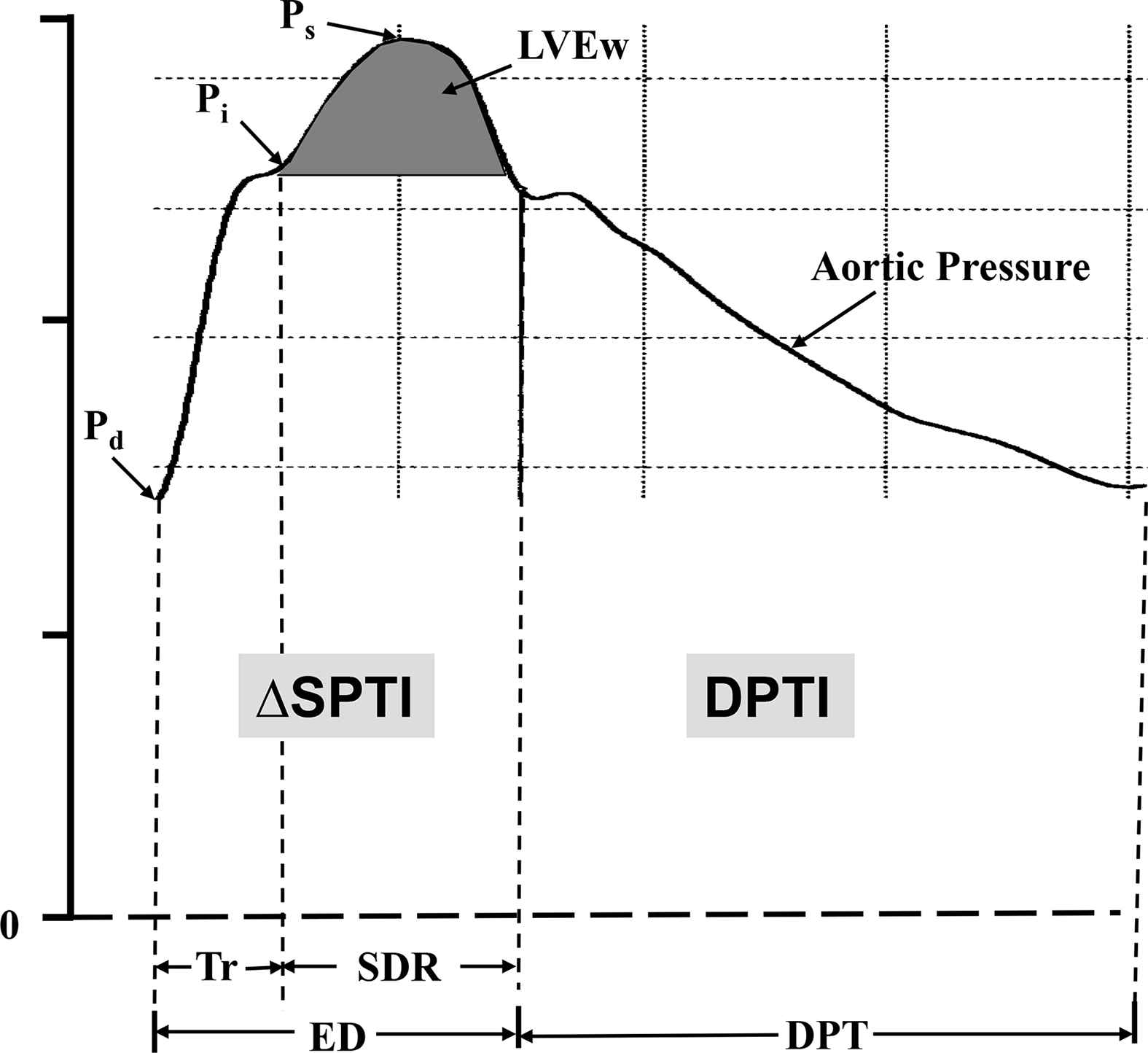
A synthesized central aortic pressure waveform. (Pi − Pd) indicates the first systolic pressure peak (or shoulder) and Pi indicates the merging (or inflection) point of the forward and reflected waves. The early part of the ascending aortic pressure wave of amplitude (Pi − Pd) is generated by LV ejection. The later part of the pressure wave with amplitude (Ps − Pi) is generated by the reflected wave from lower body reflection sites arriving during systole and adding to the forward pressure wave. Pulse pressure (PP) = (Pi − Pd) + (Ps − Pi) = (Ps − Pd) and augmentation index (AIx) = (Ps − Pi)/(Ps − Pd). Tr is the round-trip travel time of the pressure wave from the LV to the periphery and back and SDR is the systolic duration of the reflected wave; ED = ejection duration and DPT is diastolic pressure time. The area under the systolic portion of the reflected wave (dark shaded area) is defined as LV wasted energy (LVEw). SPTI = ΔSPTI + LVEw and is the area under the systolic portion of the pressure wave and DPTI is the area under the diastolic portion of the wave.
Only high-quality recordings, defined as an in-device quality index of >80% (derived from an algorithm including average pulse height, pulse height variation, diastolic variation, and the maximum rate of rise of the peripheral waveform) and acceptable curves on visual inspection, were included in the analysis.
Statistics
Data are presented as means and standard deviations for continuous variables. Comparisons between hemodynamic variable values obtained from the two matched groups were assessed using an unpaired two-tailed Student’s t test. A P < 0.05 was considered significant.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Subjects
Pertinent variables collected from the reference control patients and refractory angina patients are summarized in Tables 1 and 2. There were no significant differences in age (60 ± 12 vs. 65 ± 9.0 years, P = 0.08), gender (9 females in each group), height, BMI, heart rate or mean arterial BP between the two groups. Sixty-one percent of patients in the control group and 81% in the refractory angina group were overweight (BMI > 25 kg/m2). All patients in each group were on antihypertensive medication.
| CON (N = 36) |
RA (N = 36) |
P | |
|---|---|---|---|
| Brachial SP (mm Hg) | 123 ± 11 | 135 ± 19 | 0.001 |
| Brachial DP (mm Hg) | 72 ± 10 | 72 ± 9.7 | 0.96 |
| Brachial PP (mm Hg) | 51 ± 10 | 63 ± 17 | 0.001 |
| (Pi − Pd) (mm Hg) | 30 ± 5.7 | 36 ± 9.7 | 0.002 |
| Tr (ms) | 141 ± 16 | 140 ± 11 | 0.83 |
| Aortic Systolic Pressure (mm Hg) | 110 ± 9.5 | 123 ± 19 | 0.001 |
| Aortic Mean Pressure (mm Hg) | 88 ± 10 | 93 ± 13 | 0.06 |
| Aortic Pulse Pressure (mm Hg) | 38 ± 8.9 | 51 ± 15 | 0.001 |
| PP Amplification | 1.4 ± 0.17 | 1.3 ± 0.12 | 0.02 |
| Ejection Duration (ms) | 305 ± 27 | 328 ± 29 | 0.001 |
| (Ps − Pi) (mm Hg) | 8.1 ± 5.0 | 15 ± 7.5 | 0.001 |
| AIx (%) | 19 ± 10 | 27 ± 8.6 | 0.001 |
| SDR (ms) | 165 ± 29 | 189 ± 29 | 0.001 |
| Wasted LV energy (dyne-s-cm−2) | 1795 ± 1087 | 3053 ± 1878 | 0.001 |
| SPTI (mmHg-s/min) | 1924 ± 260 | 2231 ± 470 | 0.001 |
| DPTI/SPTI | 1.8 ± 0.22 | 1.6 ± 0.30 | 0.002 |
| Cardiac cycle length (ms) | 960 ± 150 | 967 ± 114 | 0.82 |
| SPTF | 0.32 ± 0.03 | 0.34 ± 0.04 | 0.01 |
| DPTF | 0.68 ± 0.03 | 0.66 ± 0.04 | 0.01 |
| DPTF/SPTF | 2.1 ± 0.31 | 1.9 ± 0.0.33 | 0.02 |
SP, systolic pressure.
DP, diastolic pressure.
PP, pulse pressure.
(Pi − Pd), unaugmented pressure (or amplitude of forward wave).
Tr, travel time of wave to and from periphery.
MP, mean pressure.
(Ps − Pi), augmented pressure (or amplitude of reflected wave).
AIx, aortic augmentation index.
SDR, systolic duration of reflected wave.
SPTI, systolic pressure time index.
DPTI, diastolic pressure time index.
DPTI/SPTI, myocardial viability ratio.
SPTF, systolic pressure time fraction.
DPTF, diastolic pressure time fraction.
Pertinent variables of the reference controls and refractory angina patients.
Components of peripheral and central blood pressure
All subjects had acceptable radial artery pressure waveforms on visual inspection and all aortic pressure waves obtained from the control patients and refractory angina patients exhibited an in-device quality index of >80% (80–93%). Composite data from the two groups indicate that brachial SBP (123 ± 11 vs. 135 ± 19 mmHg, P < 0.001) and brachial PP (51 ± 10 vs. 63 ± 17 mmHg, P < 0.001) were higher in refractory angina patients; while DBP (72 ± 10 vs. 72 ± 9.7 mmHg, P = 0.96) and mean BP (88 ± 10 vs. 93 ± 13 mmHg, P = 0.06) were similar in the two groups of patients. In addition, central aortic SBP (110 ± 9.5 vs. 123 ± 19 mmHg, P < 0.001) and central PP (38 ± 8.9 vs. 51 ± 15 mmHg, P < 0.001) were greater in the refractory angina patients compared to the reference control patients (Table 2).
Wave reflection indices
A deleterious effect on wave reflection characteristics was associated with the elevated central SBP and PP in the refractory angina patients. The amplitude (8.1 ± 5.0 vs. 15 ± 7.5 mmHg, P < 0.001) and duration (165 ± 29 vs. 189 ± 29 ms, P < 0.001) of the reflected wave were both greater in refractory angina patients compared to control patients (Table 2). These differences in wave reflection characteristics produced a higher AIx (19 ± 10 vs. 27 ± 8.6%, P < 0.001) and caused a reduction in PP amplification (1.4 ± 0.12 vs. 1.3 ± 0.12, P < 0.02).
Indices of myocardial oxygen demand
The changes in arterial wall properties and wave reflection characteristics produce a significant elevation in pulsatile LV afterload. These afterload changes were associated with an increase in indices of myocardial oxygen demand. The increase in wave reflection amplitude and duration in the refractory angina patients caused the LV to generate more wasted energy (1795 ± 1087 vs. 3053 ± 1897 dyne s cm−2, P < 0.001) compared to the control patients. Also, the elevated systolic pressure and prolongation of ejection duration (305 ± 27 vs. 328 ± 29 ms, P < 0.001) in refractory angina patients caused an increase in estimated SPTI (1924 ± 260 vs. 2231 ± 470 mmHg s/min, P < 0.001) compared to control patients.
Determinants of myocardial oxygen supply (coronary artery perfusion)
Heart rate (64 ± 8.4 vs. 63 ± 7.7 beats/min, P = 0.66) (Table 1) and cardiac cycle length (960 ± 150 vs. 967 ± 114 ms, P = 0.82) (Table 2) were likely influenced by β-blockade and were similar in the two groups of patients. However, coronary artery perfusion time fraction (DPTF) was abbreviated in refractory angina patients (0.68 ± 0.03 vs. 0.66 ± 0.04, P < 0.01) compared to control patients which, along with the increase in SPTF (0.32 ± 0.03 vs. 0.34 ± 0.04, P < 0.01), was associated with a reduced myocardial supply/demand (2.1 ± 0.31 vs. 1.9 ± 0.33, P < 0.02), and viability ratios (1.8 ± 0.22 vs. 1.6 ± 0.30, P < 0.002).
Discussion
This study investigated arterial properties, wave reflection characteristics and time intervals (systolic and diastolic) in a group of CAD patients with stable angina resistant to anti-anginal therapy (refractory angina). Data from this study group (age range 48–85 years) were compared to those collected from a reference group of patients without chest pain and successfully treated for hypertension matched for age, height, BMI, mean arterial pressure, and heart rate in addition to vasoactive drugs that may alter arterial pathology (Table 1); no patient in either group was a current smoker or had diabetes. Our findings confirm and extend previous reports suggesting an association between increased arterial stiffness and chest pain27–30 while other studies have suggested that arterial stiffness is increased in patients with signs and symptoms of myocardial ischemia in the absence of CAD and higher values of stiffness are predictive of coronary artery atherosclerosis.5–9 These studies consistently show that elastic artery stiffness increases in relation to the severity of CAD.5–7 Also, other studies have shown an inverse relation between aortic stiffness and coronary endothelial function31 which has been proposed as the mechanism for angina in patients with normal coronary angiograms and referred to as “microvascular angina”.32 Another possible mechanism, which appears to have been overlooked in the past, is the contribution of pressure and time during diastole when the LV is being perfused.33 This is reduced in older women with stiffened arteries, particularly when the LV is hypertrophied.34 Indeed, a significant decrease in diastolic duration can have the same effect as an increase in coronary stenosis.17 Perhaps the “continuing dilemma” is created by the assumption that reduced coronary flow is always due to arterial narrowing.
To our knowledge this is the first report to examine wave reflection characteristics and diastolic timing in patients with refractory angina. We found in this study, using PWA of the non-invasively obtained central aortic pressure waveform, that refractory angina patients have adverse alterations in large conduit arterial properties, wave reflection characteristics and indices of myocardial oxygen supply and demand.
Pulse BP (peripheral and central) and to a lesser degree SBP are dependent upon heart rate, ejection duration, peak flow and arterial stiffness while mean pressure is dependent upon arteriolar caliber and peripheral resistance. Central and peripheral BP are not the same and cardiovascular risk factors can exert differential effects on them.16 Both SBP and PP increase markedly while diastolic and mean BP decrease slightly as the pressure wave travels from the heart toward the periphery. This difference between peripheral and central SBP and PP is greater in young subjects with compliant elastic arteries and less in older subjects with stiff elastic arteries. The “amplification” (brachial PP/central PP) of the pressure pulse is due to greater stiffness of peripheral arteries (compared to central arteries) and enhanced wave reflection amplitude, which depends upon the difference between elastic moduli of the respective arteries and distance to major reflecting sites.16 As a consequence, these two pulsatile BP components are greater in arteries of the extremities than in the central aorta. This difference is important since the major organs (e.g. brain, heart and kidney) ‘see’ central arterial BP and not brachial BP. Therefore, brachial SBP and PP measured with a sphygmomanometer in the arm are not always reliable measures of central aortic SBP and PP.
We found in this group of elderly patients with refractory angina that central systolic augmented pressure resulting from increased reflected wave amplitude was elevated and amplification of the pulse was reduced. Reflected wave amplitude and PP amplification are directly related to arterial stiffness and inversely related to major reflecting site distance.16 Therefore, it was assumed from these observations that arterial stiffness was abnormally elevated in the refractory angina cohort. This contention is supported by other studies that found an increase in arterial stiffness in patients with chest pain.27–29
Increased arterial stiffness causes an increase in transmission velocity of both forward and reflected waves which in-turn causes the reflected wave to arrive earlier with greater amplitude and duration in the central aorta.16 Our results support the fact that arterial stiffness and the resulting alteration in wave reflection amplitude can alter the aortic pressure wave and pulsatile LV afterload independent of changes in brachial cuff BP. This mechanism probably explains why central aortic BP is a better predictor of cardiovascular events and a better guide for hypertension management than peripheral BP.13,14,35–37
The later part of the aortic pressure wave with amplitude (Ps − Pi) is generated by the reflected wave arriving during systole and adding to the forward pressure wave by the superposition principle. This augmentation of the aortic pressure wave can be estimated as an augmentation index (AIx) and is dependent upon the elastic properties of the entire arterial tree, the velocity of the reflected wave and distance to the major reflecting site.16 These mechanisms are the major cause of increased central systolic and pulse BP with advancing age and in patients with hypertension and other cardiovascular risks27 such as those present in the refractory angina patients. These modifications in wave reflection characteristics causes a decrease in PP amplification and indicates that refractory angina patients have stiffer arteries than control patients which may be related to chronic inflammation,27 superoxide generation in the arterial wall36 and/or peripheral and coronary artery endothelial dysfunction.28,29 Previous studies in humans and animals have shown that exogenous inhibition of nitrite oxide (NO) and oxidative stress cause an increase in elastic artery pulse wave velocity (PWV).38,39 In support of this, recent studies showed that AIx was positively associated with plasma levels of asymmetric dimethyl-arginine, an endogenous inhibitor of endothelial NO synthase39 and inversely associated with global endothelial function.40 The augmentation in late central SBP and PP increases LV afterload, wasted LV energy, SPTI and myocardial oxygen demand and likely decreases systolic coronary blood flow. According to classic studies LV oxygen requirements are closely related to SPTI, rather than to LV blood output.41–43 Wasted LV energy is that component of SPTI which is attributable to wave reflection. Furthermore, wasted LV energy is also deleterious to the circulation since it causes a reduction in ejected blood volume during blood flow deceleration. Previous studies have shown that long term exposure of the LV to elevated arterial stiffness and energy expenditure causes left ventricular hypertrophy and eventually leads to cardiac failure.24,44
At a given arterial pressure, subendocardial perfusion is dependent upon the ratio between the time the heart is in diastole and the duration of a complete cardiac cycle. This ratio is defined as the diastolic pressure time fraction (DPTF).25,26 DPTF indicates the duration of absence of compression of intramural vessels during a heartbeat and has been used as input into theoretical models on coronary artery perfusion.45 The patients in the refractory angina group had a longer ejection duration than patients in the control group. This prolonged ejection duration decreased diastolic time and DPTF indicating reduced blood supply to the subendocardium. This adverse change in timing of cardiac cycle components and presumed decrease in coronary artery perfusion during both systole and diastole coupled with the increase in LV afterload causes a mismatch in ventricular/vascular coupling and an imbalance in the supply/demand ratio. This contention is supported by reduction in both DPTF/SPTF and the myocardial viability ratio in the refractory angina patients. This scenario is exaggerated during exercise and thus can cause angina at a lower workload even in individuals with normal coronary arteries.16,17
In summary, this study showed for the first time that compared to a reference control group, patients with refractory angina have higher LV afterload and abbreviated diastolic pressure time. These hemodynamic alterations probably cause a mismatch in ventricular/vascular coupling and an imbalance between myocardial oxygen supply and demand resulting in myocardial ischemia and angina.
Limitations
This study in patients with refractory angina has some limitations. No direct measure of arterial stiffness was attempted. Only surrogates of arterial stiffness and indices of wave reflection and time intervals were measured. Although the results strongly imply an increase in arterial stiffness, we do not know, with certainty, which arterial segment is altered, central elastic arteries or peripheral muscular arteries or both. We do know, however, that LV afterload and systolic time fraction are increased and diastolic time fraction is decreased. The cause of these changes in arterial stiffness and wave reflection characteristics could be a change in arterial wall properties of the elastic arteries or a change in smooth muscle tone of the muscular arteries or both.
Disclosure
Wilmer W. Nichols is a consultant for Millar Instruments. Alvaro N. Gurovich, Randy W. Braith and C. Richard Conti have no conflict of interest.
References
Cite this article
TY - JOUR AU - Alvaro N. Gurovich AU - Wilmer W. Nichols AU - Randy W. Braith AU - C. Richard Conti PY - 2014 DA - 2014/01/31 TI - Patients with refractory angina have increased aortic wave reflection and wasted left ventricular pressure energy JO - Artery Research SP - 9 EP - 15 VL - 8 IS - 1 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2014.01.003 DO - 10.1016/j.artres.2014.01.003 ID - Gurovich2014 ER -
