Shock index for outcome and risk stratification in acute pulmonary embolism✩
The study was conducted in St. Vincenz and Elisabeth Hospital Mainz (KKM).
Both authors were co-shared last authors.
The study was conducted in St. Vincenz and Elisabeth Hospital Mainz (KKM).
Both authors were co-shared last authors.
- DOI
- 10.1016/j.artres.2016.05.002How to use a DOI?
- Keywords
- Shock index; Tachycardia; Blood pressure; Thrombosis; Embolism; Right ventricular dysfunction
- Abstract
Introduction: Risk stratification of patients with acute pulmonary embolism (PE) is crucial in deciding appropriate therapy management. Shock index (SI) is rapidly available and a reliable parameter. We aimed to investigate SI for short term outcome in acute PE.
Materials and methods: Data of 182 patients with acute PE were analysed retrospectively. SI was defined as heart rate divided by systolic blood pressure. Logistic regression models were calculated to investigate associations between SI and in-hospital-death, myocardial necrosis and presence of right ventricular dysfunction (RVD) respectively. Moreover ROC curves and cut-off values for SI predicting in-hospital death, myocardial necrosis and RVD were computed.
Results: 182 patients (61.5% female, mean age 68.5 ± 15.3 years) with acute PE event were included in the study. 5 patients (2.7%) died an in-hospital death.
Logistic regression models revealed an association between SI and respectively in-hospital death (OR 5.854, 95% CI 1.876–18.274, P = 0.00234), myocardial necrosis (OR 5.043, 95% CI 1.362–18.674, P = 0.0154) and RVD (OR 53.539, 95% CI 6.810–420.914, P = 0.000155).
ROC analysis for SI predicting in-hospital death, myocardial necrosis and RVD revealed an AUC of 0.806, 0.636 and 0.713 respectively with respectively SI cut-off values of 0.89, 0.75 and 0.54.
Conclusions: SI is a significant predictor of in-hospital death, myocardial necrosis and RVD. The effectiveness of SI to predict in-hospital death is high with an optimal cut-off value of 0.89 for differentiation between PE patients with lower and higher risk to die in hospital after acute PE event.
- Copyright
- © 2016 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Current guidelines emphasise the exceptional importance of early risk stratification of patients with an acute pulmonary embolism (PE).1–3 Risk stratification should help to identify PE patients with higher risk of early death, who could benefit from more intensive surveillance and especially more aggressive therapy.1,4,5
Therefore, early risk stratification of patients with acute pulmonary embolism (PE) is crucial in deciding appropriate therapy management.
The Shock index (SI), defined as heart rate divided by systolic blood pressure, is a rapidly available and reliable parameter. Both parameters, heart rate and systolic blood pressure, are predictive for the outcome in PE on their own.3,6–11 Higher heart rate7,11–14 as well as lower systolic blood pressure6,8–11 were both predictive for poorer outcome in acute PE. Therefore heart rate and systolic blood pressure have been included in outcome scores such as pulmonary embolism severity index (PESI) as risk stratification parameters.9,11 Especially the systolic blood pressure is an important and powerful criterion in risk stratification and risk classification in patients with acute PE.3,9 Acute PE interferes with circulation, heart and lungs.2,15 If more than 30% of the pulmonary arterial bed is occluded by thrombus material, hemodynamic consequences of acute PE event occur.8,14 Severe obstruction of blood flow could result in right heart failure with insufficient maintenance of blood pressure and high risk of short term death.3,5,9,16 Hypotension with systolic blood pressure <90 mmHg was detected as one important prognostic factor in the International Cooperative Pulmonary Embolism Registry (ICOPER).6 In the PESI and the simplified PESI a systolic BP of <100 mmHg is one of the parameters to predict worse outcome.2,10,11 SI is a well-established and an independent predictor of short term mortality in acute PE (first 30 days after PE event),17,18 but limited data about optimal cut-off values for SI predicting short term outcome and especially in-hospital death in acute PE are available up to now.
The objective of this present study was to investigate SI as a risk stratification and outcome predicting parameter. We aimed to define optimal cut-off values for SI predicting in-hospital death, myocardial necrosis and right ventricular dysfunction (RVD) in patients with acute pulmonary embolism and to test the effectiveness of SI to predict these parameters.
Methods and patients
A retrospective analysis of the patients with a confirmed diagnosis of acute PE, who were treated in the Internal Medicine department of the St. Vincenz and Elisabeth Hospital in Mainz (Germany) between May 2006 and June 2011, was performed. We identified the PE patients with a search in the hospital information system database for the diagnostic code of PE (ICD-Code: I26). In Germany, diagnoses are coded according to ICD-10-GM (International Classification of Diseases, 10th Revision with German Modification).
Data were recorded and analysed anonymously, and were retrospectively collected from patient files. Studies in Germany involving a retrospective analysis of diagnostic standard data do not require an ethics statement.
Enrolled subjects
Patients were eligible for this study.
- 1.
if diagnosis of acute PE was confirmed by identified filling defect in the pulmonary artery system in multidetector spiral computed tomography with computed tomography angiography (CT) of the chest or positive venous ultrasound/phlebography of an extremity consistent with DVT in patients with typical symptoms of PE (chest pain or dyspnoea) and a detected positive D-Dimer or scintigraphic ventilation-perfusion scan of the lungs read as high probability for PE;
- 2.
if the PE patients were treated in the Internal Medicine department of the hospital, and
- 3.
if the patients were at least 18 years old.
All CT and scintigraphic images were analysed by experienced radiologists. If diagnosis of PE was not confirmed by the criteria above, patients were not included in this study.
Shock index
The SI was determined at the patients’ admission to the hospital.
Definitions
Shock index
SI was defined as heart rate divided by systolic blood pressure.
PE severity stadium
PE stadium was defined according to the American Heart Association (AHA) scientific statement.3 Hemodynamically instable PE patients were classified as massive PE. Normotensive PE patients with RVD or elevated levels of cardiac biomarkers such as elevated cTnI, were categorized as submassive PE. Normotensive PE patients without RVD and normal biomarker levels were included in the low-risk PE group.3
Definition of right ventricular dysfunction
RVD was defined according to the AHA scientific statement from 20113 as enlarged right ventricle seen as right ventricular (RV) septal-lateral diameter in 4 chamber view divided by left ventricular septal-lateral diameter >0.9 in CT or transthoracic echocardiography, and/or RV hypokinesis and/or tricuspid regurgitation in transthoracic echocardiography.3
Definition of cardiac injury
Myocardial necrosis was defined as cardiac Troponin I (cTnI) elevation >0.4 ng/ml, according to the AHA scientific statement.3
Definition of in-hospital death
In-hospital death comprises all kinds of death during the hospital stay.
Study parameters
The retrospective analysis of the PE patients focused on SI, heart rate, blood pressure, echocardiographic signs and CT signs of RVD, cardiac biomarkers and in-hospital death.
Statistics
We performed uni-variable logistic regression models to investigate the association between SI and respectively in-hospital death, myocardial necrosis and RVD.
ROC analysis with area under the curve (AUC) for SI predicting respectively in-hospital death, myocardial necrosis and RVD were computed.
Commercially available software BIAS® (version 10.04) was used for the computerised analysis. P values of <0.05 were considered as statistically significant.
Results
Between May 2006 and June 2011, 182 patients (61.5% female, 38.5% male) with acute and confirmed PE met the inclusion criteria and were included in the study. PE patients’ mean age was 68.5 ± 15.3 years (female: 70.8 ± 15.1 years; male: 64.9 ± 15.0 years). PE diagnosis was made in 156 patients (85.7%) using CT. In 19 patients ventilation-perfusion scan read as high probability for PE (10.4%) lead to the PE diagnosis and in 7 patients (3.9%) diagnosis was made by positive venous ultrasound/phlebography of an extremity, which was consistent with DVT in patients with typical symptoms of PE (chest pain or dyspnoea) and positive D-dimer level.
5 of these 182 PE patients (2.7%) died in-hospital after the PE event. 7 PE patients (3.8%) presented with a hemodynamic instable PE (massive PE). Therefore, 175 PE patients (96.2%) were hemodynamically stable and were classified as non-massive PE patients.
The uni-variable logistic regression model showed a significant association between in-hospital death and respectively SI (OR 5.854, 95% CI 1.876–18.274, P = 0.00234), SI ≥ 0.8 (OR 12.465, 95% CI 1.343–115.702, P = 0.0265), SI ≥ 0.9 (OR 11.775, 95% CI 1.835–75.556, P = 0.00932) and SI ≥ 1.0 (OR 7.762, 95% CI 1.186–50.791, P = 0.0325). The association between SI ≥ 0.7 and in-hospital death revealed a borderline significance (OR 6.727, 95% CI 0.728–62.182, P = 0.0930) (Table 1 ).
| Parameter | Odds ratio | P-value |
|---|---|---|
| Shock index | OR 5.854, 95% CI 1.876–18.274 | 0.00234 |
| Shock index ≥ 0.7 | OR 6.727, 95% CI 0.728–62.182 | 0.0930 |
| Shock index ≥ 0.8 | OR 12.465, 95% CI 1.343–115.702 | 0.0265 |
| Shock index ≥ 0.9 | OR 11.775, 95% CI 1.835–75.556 | 0.00932 |
| Shock index ≥ 1.0 | OR 7.762, 95% CI 1.186–50.791 | 0.0325 |
P values of <0.05 were considered as statistically significant.
Uni-variate logistic regression model to detect the association between shock index and in-hospital death.
Associations between myocardial necrosis on the one hand and SI (OR 5.043, 95% CI 1.362–18.674, P = 0.0154) as well as SI ≥ 0.7 (OR 2.798, 95% CI 1.431–5.470, P = 0.00262) and SI ≥ 0.8 (OR 4.048, 95% CI 1.900–8.621, P = 0.000290) on the other hand were respectively significant in uni-variate logistic regression model. The association between SI ≥ 0.9 and in-hospital death (OR 2.267, 95% CI 0.875–5.886, P = 0.0918) presented as a borderline significance. SI ≥ 1.0 and in-hospital death were not significantly associated (OR 1.755, 95% CI 0.580–5.311, P = 0.320) in uni-variate regression model (Table 2 ).
| Parameter | Odds ratio | P-value |
|---|---|---|
| Shock index | OR 5.043, 95% CI 1.362–18.674 | 0.0154 |
| Shock index ≥ 0.7 | OR 2.798, 95% CI 1.431–5.470 | 0.00262 |
| Shock index ≥ 0.8 | OR 4.048, 95% CI 1.900–8.621 | 0.000290 |
| Shock index ≥ 0.9 | OR 2.267, 95% CI 0.875–5.886 | 0.0918 |
| Shock index ≥ 1.0 | OR 1.755, 95% CI 0.580–5.311 | 0.320 |
P values of <0.05 were considered as statistically significant.
Uni-variate logistic regression model to detect the association between shock index and myocardial necrosis.
RVD on the one hand and SI (OR 53.539, 95% CI 6.810–420.914, P = 0.000155), SI ≥ 0.7 (OR 3.002, 95% CI 1.453–6.202, P = 0.00300), SI ≥ 0.8 (OR 4.112, 95% CI 1.657–10.207, P = 0.00230), SI ≥ 0.9 (OR 4.533, 95% CI 1.254–16.392, P = 0.0212) and SI ≥ 1.0 (OR 10.189, 95% CI 1.277–81.308, P = 0.0285) on the other hand were respectively significantly associated in uni-variate regression model (Table 3 ).
| Parameter | Odds ratio | P-value |
|---|---|---|
| Shock index | OR 53.539, 95% CI 6.810–420.914 | 0.000155 |
| Shock index ≥ 0.7 | OR 3.002, 95% CI 1.453–6.202 | 0.00300 |
| Shock index ≥ 0.8 | OR 4.112, 95% CI 1.657–10.207 | 0.00230 |
| Shock index ≥ 0.9 | OR 4.533, 95% CI 1.254–16.392 | 0.0212 |
| Shock index ≥ 1.0 | OR 10.189, 95% CI 1.277–81.308 | 0.0285 |
P values of <0.05 were considered as statistically significant.
Uni-variate logistic regression model to detect the association between shock index and right ventricular dysfunction.
The calculated ROC analysis for SI predicting in-hospital death revealed an AUC of 0.806 with a SI cut-off value of 0.89. The percentage of misclassification, the sensitivity, and the specificity were calculated as 16.2%, 81.4%, and 86.6% respectively (Fig. 1 ).
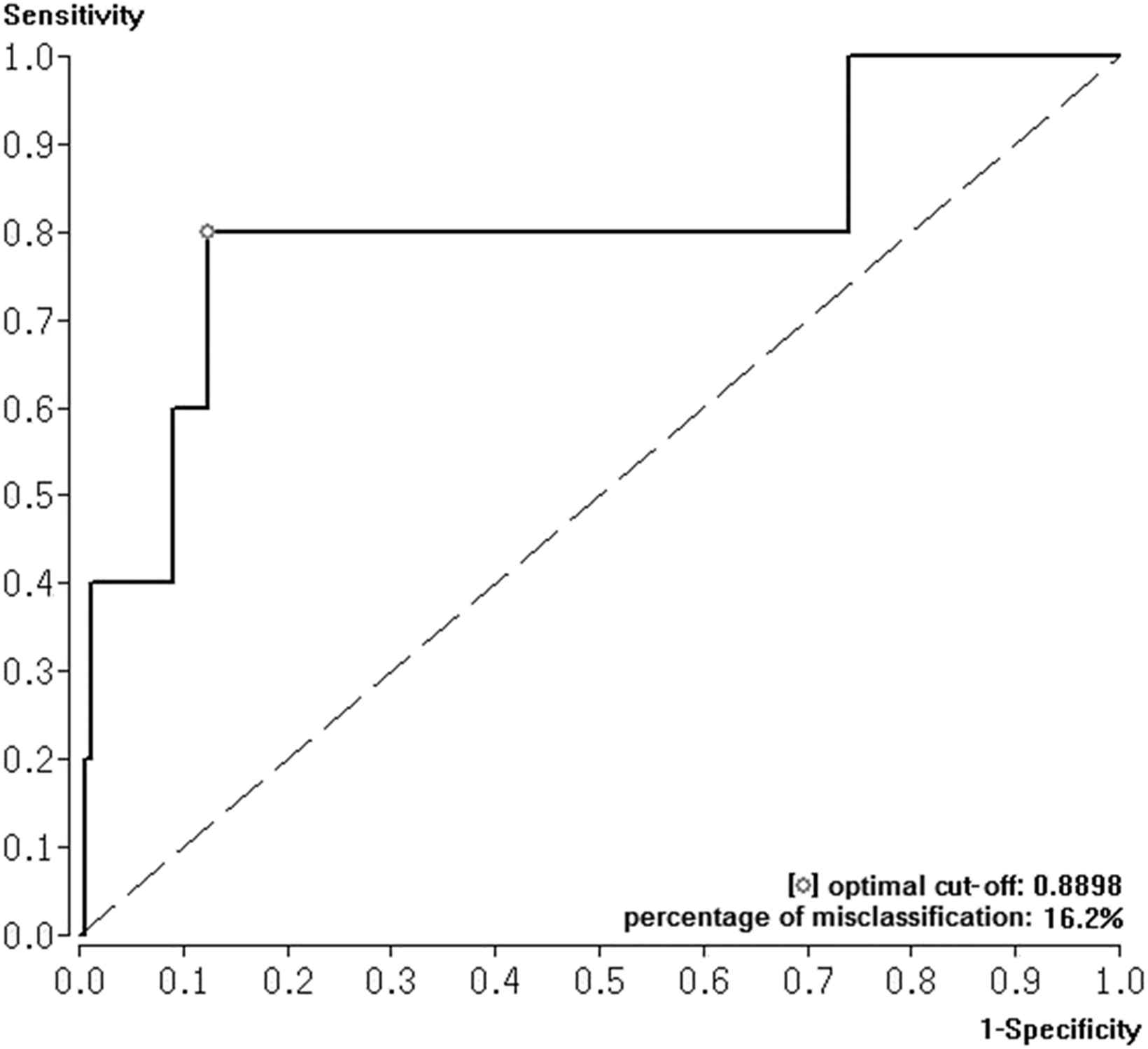
Receiver Operating Characteristic (ROC) curve with area under the curve (AUC) and Youden Index were calculated to test the effectiveness of shock index to predict in-hospital death in acute PE.
ROC analysis for SI predicting myocardial necrosis (cTnI > 0.4 ng/ml) showed an AUC of 0.636 with a SI cut-off value of 0.75. The percentage of misclassification, the sensitivity, and the specificity were calculated as 34.2%, 62.3%, and 72.1% respectively (Fig. 2 ).
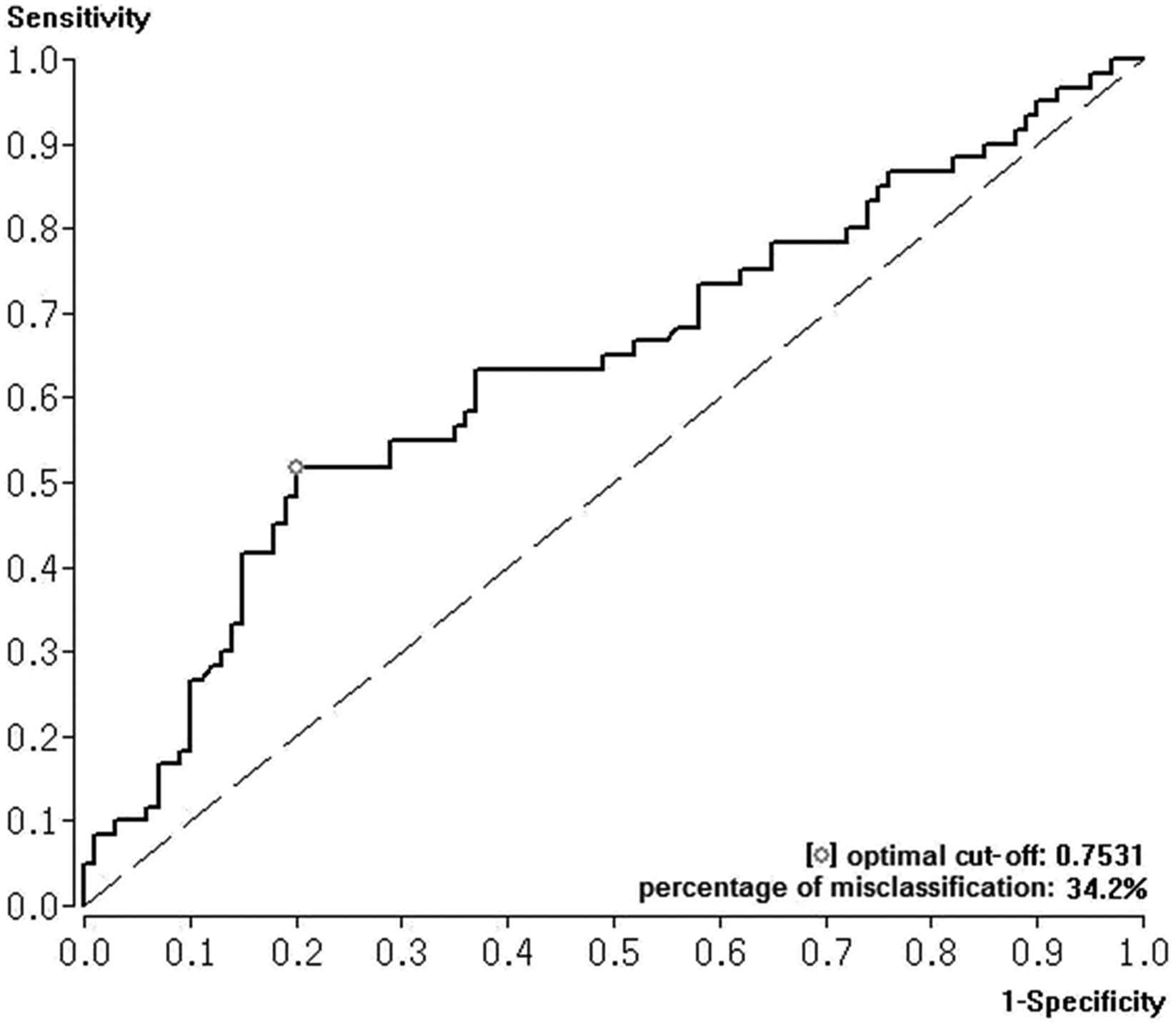
Receiver Operating Characteristic (ROC) curve with area under the curve (AUC) and Youden Index were calculated to test the effectiveness of shock index to predict myocardial necrosis in acute PE.
ROC analysis for SI predicting RVD revealed an AUC of 0.713 and a SI cut-off value for predicting RVD of 0.54. The percentage of misclassification, the sensitivity, and the specificity were calculated as 34.4%, 72.2%, and 62.1% respectively (Fig. 3 ).
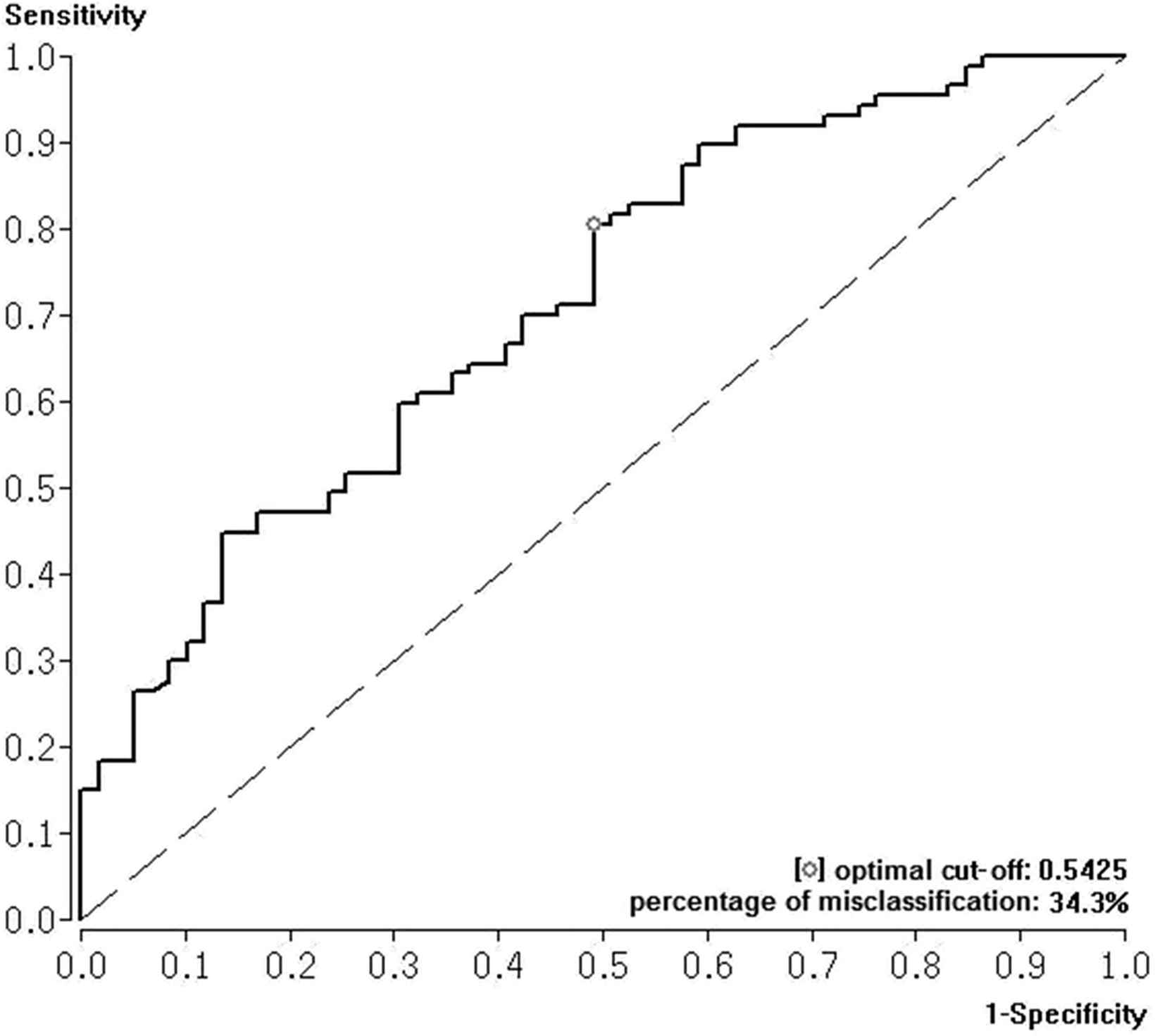
Receiver Operating Characteristic (ROC) curve with area under the curve (AUC) and Youden Index were calculated to test the effectiveness of shock index to predict right ventricular dysfunction in acute PE.
Discussion
Risk stratification of patients with acute PE is crucial in deciding appropriate therapy management. The central task of risk stratification in acute PE is to identify PE patients with higher risk of early in-hospital death and adverse outcome, who could benefit from more intensive surveillance and especially more aggressive therapy.1,4,5 The SI is a rapidly available and reliable parameter and a tool for risk stratification in many emergencies as trauma or myocardial infarction.1,2,19–27 There is a lack of data about the optimal cut-off values for SI predicting adverse short term outcome and especially in-hospital death in acute PE. To our knowledge, no cut-off values for SI predicting in-hospital death were published as of writing. Therefore, we aimed to investigate SI for short term outcome in acute PE.
Our study results confirmed a significant association between SI and in-hospital death. We calculated an optimal cut-off value of 0.89 for the differentiation between PE patients with lower and higher risk to die in hospital after acute PE event.
Even SI and myocardial necrosis as well as SI and RVD were significantly associated. The optimal cut-off values of 0.75 and 0.54 respectively for SI predicting myocardial necrosis and RVD were lower than the cut-off value to predict in-hospital death.
Effectiveness of SI to predict in-hospital death was with an AUC of 0.806 good to excellent. The effectiveness of SI for prediction of RVD was accurate with an AUC of 0.713. In contrast to these results, SI was less effective to predict myocardial necrosis (AUC 0.636).
PE patients with a SI of >0.89 had a 11.8-fold higher risk for in-hospital death than in those patients below this cut-off. Although the AUC of the ROC analysis revealed a good to excellent effectiveness for SI to predict in-hospital death, the 95% confidence interval of the association between SI ≥ 0.9 and in-hospital death showed a wide range resulting in a weakening of this calculated effectiveness.
However, in consensus with our results, Otero et al.17 reported that SI is an independent predictor of short term mortality in acute PE (first 30 days after PE event).17,18 A SI value ≥ 1 was correlated to RVD, higher systolic pulmonary arterial pressure and short term mortality in the first 30 days after acute PE event.17,23,28
In most of the studies, that investigate SI for predicting outcome in acute PE, a fix cut-off point of SI ≥ 1 for worse outcome was set in the study protocols.1,17,18,23,29 To our knowledge, this study is the first one to investigate the optimal cut-off value of SI to differentiate between patients with lower and higher risk of in-hospital death after acute PE event. In contrast to the chosen SI value of 1.0 in several studies,1,17,18,23,29 our study reveals a lower optimal SI cut-off point of 0.89.
In healthy adults SI values of 0.5–0.7 represent normal results.22,24 Higher SI values indicate for cardiovascular effect of diseases or emergencies. In the AHA statement a persistent SI of >1 indicates for circulatory failure in acute PE.3 The results of our study emphasise that the so far recommended SI cut-off values indicating a higher mortality risk in acute PE should be revised.
Furthermore Sam et al.1 described that the simplified pulmonary embolism severity index (PESI) was better to quantify the 30-day prognosis than the SI.1,2 But as mentioned before, the chosen cut-off value was set in an arbitrary manner to a SI of 1.0.1 Therefore, maybe the results would have been different, if a SI of 0.9 would have been chosen instead of 1.0.
However, haemodynamically unstable PE patients with shock or hypotension should immediately be identified as patients with high-risk PE.2 Our study results emphasise that SI is a good and useful tool for rapid risk stratification in acute PE. In our study SI reveals a better effectiveness to predict in-hospital death than cardiac Troponin I (AUC 0.719).30 In contrast to our results, Janata et al.31 reported an higher effectiveness (AUC 0.92) for cardiac Troponin T predicting in hospital death.31 In the study of Kucher et al.32 the AUC for predicting adverse outcome was 0.89 for cardiac Troponin I alone and AUC 0.90 for the combination of cardiac Troponin I in combination with RVD in echocardiography.32
Our study results suggest a useful role for SI in risk stratification process of acute PE, which should be confirmed by large studies in the future.
Limitations
The most important study limitations are the small number of included PE patients and the retrospective study character. Therefore, follow-up examinations are missing. Beside the outcome marker in-hospital death, several study results have already shown the connection between myocardial necrosis as well as RVD and elevated mortality in the follow up. Therefore the surrogate parameters of myocardial necrosis and RVD are established and widely used risk stratification markers for the outcome of PE.
Further limitations are the variability of our study data and the fact that conclusions are drawn upon regression analyses.
Conclusions
SI is a significant predictor of in-hospital death, myocardial necrosis and RVD. The effectiveness of SI to predict in-hospital death is high with an optimal cut-off value of 0.89 for differentiation between PE patients with lower and higher risk to die in hospital after acute PE event.
Funding
None.
Disclosures/Conflicts of interest
None.
References
Cite this article
TY - JOUR AU - Karsten Keller AU - Meike Coldewey AU - Martin Geyer AU - Johannes Beule AU - Jörn Oliver Balzer AU - Wolfgang Dippold PY - 2016 DA - 2016/06/07 TI - Shock index for outcome and risk stratification in acute pulmonary embolism✩ JO - Artery Research SP - 30 EP - 35 VL - 15 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2016.05.002 DO - 10.1016/j.artres.2016.05.002 ID - Keller2016 ER -
