Cervical Cancer Care Continuum in South India: Evidence from a Community-based Screening Program
- DOI
- 10.2991/jegh.k.191111.001How to use a DOI?
- Keywords
- Care continuum; cancer screening; follow-up rate; sort it
- Abstract
In India, cervical cancer screening is conducted at various levels; however, after screening, the adherence to the cancer care continuum is barely understood. This study evaluated a community-based cancer screening program conducted in a rural setting (Tirunelveli and Tuticorin districts) in South India and reviewed the completion of care continuum. In this longitudinal descriptive study involving secondary data collection, data from the case records of 2192 women who were consecutively screened between March 2015 and May 2016 were included. All women underwent conventional cytology-based screening (Pap smear) and Visual Inspection with Acetic Acid (VIA). Those for whom either test was positive were referred for histopathological confirmation. Patients with confirmed precancerous conditions and unsatisfactory Pap smears were referred for further management. In total, 2192 women were screened [age range, 17–69 years; mean (standard deviation), 39.2 (8.5)]. Common symptom and sign were white discharge per vaginum (34.9%) and cervical erosion (34.4%), respectively. The VIA was positive for 24% (523/2178; 14 women did not cooperate for VIA) and 113 (5.1%) had epithelial cell abnormality in the Pap smear test. Per histopathology findings, one woman had non-keratinizing squamous cell carcinoma. Seven, three, and four had cervical intraepithelial neoplasia I, II and III, respectively. Of 2192, 807 were eligible for referral (597 had positive results on either Pap or VIA). Among the 807 women referred, only 74 (9.2%) women visited the referral center. The follow-up rate was very poor accounting to fragmentation of care continuum. The success of the screening program depends on the completion of the care continuum.
- Copyright
- © 2019 Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
India has the highest incidence of cervical cancer in Southeast Asia with approximately 132,000 new cases and 74,000 deaths annually [1,2]. More than 80% of cervical cancer patients are diagnosed at advanced stages resulting in high mortality [3]. Cervical cancer is preventable owing to easy accessibility of the primary site, availability of simple and cost-effective screening methods, and its long preinvasive period. Compared to no screening, even a single screening is effective in reducing the incidence and mortality of advanced cervical cancer [4]. Although proven strategies for cancer screening exist, the provision of accepted standard of care remains to be materialized, especially in rural India [5].
The recommended screening tests are human papillomavirus test, cytology (Papanicolaou—Pap test), and unaided Visual Inspection with Acetic Acid (VIA). These are used as single tests or sequentially.
In low- and middle-income countries, the resource-intensive demands of screening using cervical cytology and the inefficiencies along the continuum of care cause loss to follow-up. A considerable challenge to cancer screening in these countries is the poor systems in place interlinking screening, diagnosis, and treatment [6]. In India, opportunistic cervical cancer screening is conducted at the primary, secondary, and tertiary level health-care facilities; however, a systematic screening program covering the entire population is lacking [2,7]. Lack of awareness, financial constraints, cultural barriers, lack of social support, and stigma attached to the disease and denial of the screening outcome prevent the women from attending further evaluation, disrupting the care continuum [5]. Although projects such as the Tamil Nadu Health Systems Project (Tamil Nadu is a state/province in South India) have conducted long-standing breast and cervical cancer screening programs [8], the care continuum is not reported. This study assessed the completion of care continuum in a community-based cancer screening program conducted in rural settings in Tamil Nadu, India.
2. MATERIALS AND METHODS
2.1. Study Design and Population
This was a longitudinal descriptive study involving secondary data collection. Cervical cancer screening camps (n = 154) were conducted in 100 villages of Tirunelveli and Tuticorin districts (depicted in Figure 1), Tamil Nadu, India, between March 2015 and May 2016. All women (age ≥ 18 years) who underwent screening were included. The last day of follow-up before declaring that the referred patients did not visit Nellai Cancer Care Center (NCCC) was December 31, 2016.
Map depicting the districts selected for the study.
2.2. Study Setting
2.2.1. General setting
The study was conducted in two southern districts of Tamil Nadu: Tirunelveli and Tuticorin (only few villages adjacent to Tirunelveli). Tirunelveli has a population of about 3 million, with 50% of them living in rural areas. The sex ratio is 1023 females per 1000 males. Tirunelveli has a literacy rate of 82.5%, with male literacy 89.2% and female literacy 75.98% and per capita annual income of USD 771.27 [9]. The sex ratio of Tuticorin is 999 and the child sex ratio stood at 980. Tuticorin has an average literacy rate of 92.10% with male literacy being 94.84% and female literacy being 89.37% [10].
2.2.2. Specific setting
Nellai Cancer Care Center, an initiative of Udhavum Ullangal (meaning “helping hearts” in Tamil language), a non-governmental organization headquartered in Tirunelveli, is involved in cancer prevention activities in rural areas of Tirunelveli and Tuticorin [11]. The screening camps were led by a team of health professionals, including medical and paramedical staff, who were trained as per the World Health Organization (WHO) guidelines [12]. All the services were offered free of cost. After sensitization to cancer screening and prevention, villagers were encouraged to participate in the cancer screening program conducted in their locality. The camps were conducted in liaison with the community-based organizations working in the respective villages and volunteers from the villages. The breast, cervix, and oral cavity were the sites screened. Women who were healthy, married at the time of screening, not pregnant, had an intact uterus with no prolapse, had no history of cervical cancer, and living in the camp areas were included in the study.
The sociodemographic and clinical details were collected using a structured questionnaire before screening. All the women underwent Pap smear and VIA. First, the cervix was examined with the naked eye for any abnormalities. This was followed by a Pap smear for which the squamocolumnar junction was gently scraped using Ayre’s spatula and cytobrush and the smear was immediately fixed in 95% alcohol. Subsequently, VIA was performed to determine the presence of a well-defined opaque acetowhite lesion next or close to the squamocolumnar junction. The VIA results were classified as normal or positive (Table 1).
| Test definition | Characteristics |
|---|---|
| Normal | Normal-looking cervix, nabothian cysts |
| Positive (low threshold) | Cervicitis, erosion, polyp, wart, unhealthy cervix, reddish-looking cervix |
| Positive (high threshold) | Low-threshold features plus bleeding on touch, bleeding erosion, hypertrophied elongated cervix, growth, ulcer |
VIA—visual inspection of the cervix after acetic acid application is widely recommended as the method of choice in cervical cancer screening programs in resource-limited settings because of its simplicity and ability to link with immediate treatment.
The fixed slides of Pap staining were taken to NCCC. The results of Pap were recorded based on the Bethesda system [13] (Table 2).
| Test definitions | Characteristics |
|---|---|
| Negative | NILM |
| NILM without any infections or atrophy | |
| NILM with atrophy | |
| NILM with infections—bacterial vaginosis, Trichomonas vaginalis, Candida species, actinomycosis | |
| Precancerous | Epithelial cell abnormalities
|
| Cancerous |
|
| Advice for repeat test | Specimen adequacy—unsatisfactory staining/inadequate sampling and broken |
NILM, negative for intraepithelial lesion or malignancy; ASC-US, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells—cannot exclude HSIL; LSIL, low-grade squamous intraepithelial lesion; HPV, human papilloma virus; HSIL, high-grade squamous intraepithelial lesion; NKSCC, non-keratinizing squamous cell carcinoma; AGUS, atypical glandular cells of undetermined significance.
Classification of Pap results and its characteristics based on Bethesda system [13]
Referral was done to NCCC if (i) specimen were unsatisfactorily or insufficiently stained or broken for repeat cytology screening or (ii) VIA test and/or Pap test was positive. Women with reported abnormalities underwent colposcopic examination and endocervical curettage or biopsy if required.
Based on the diagnosis, further management was recommended with referral to the regional cancer centers or the government hospitals with cancer care facilities. Women with infections were treated with antibiotics and were advised to follow-up after 1 year. The care continuum followed is shown in Figure 2.
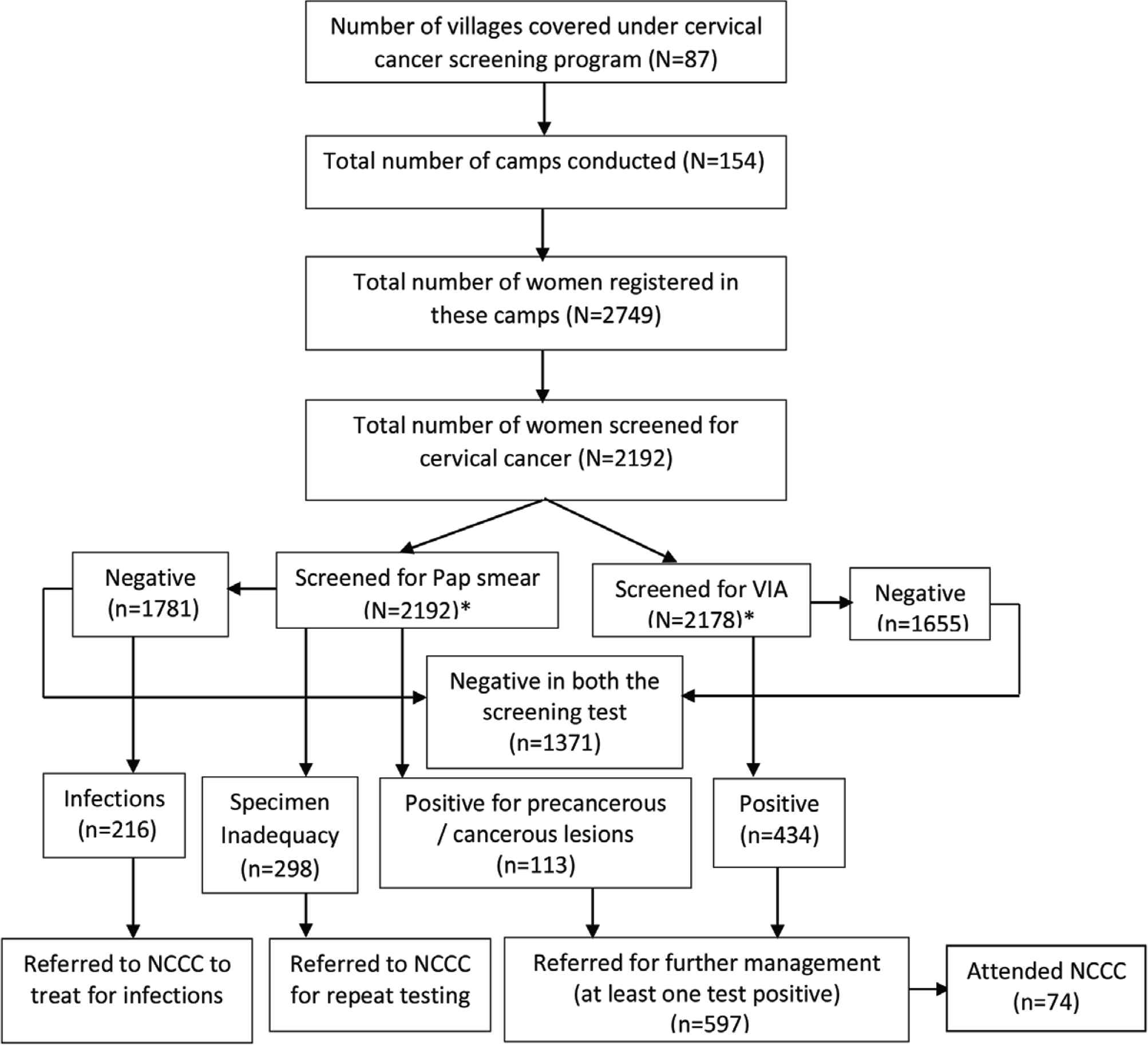
Flowchart showing the cancer care continuum among women screened in screening camps. *The number of women screened for Pap smear and visual inspection with acetic acid is not mutually exclusive. NCCC, Nellai Cancer Care Center.
2.3. Data Variables and Source of Data
The sociodemographic (current age, age at marriage, education, occupation, marital status, and tobacco use) and clinical (chief complaints, age at menarche, parity, age at first and last child births, and history of previous screening) variables were extracted from the paper-based case records. VIA, cytology, and histopathology findings were also drawn from the records. The care continuum details were collected. The cancer care continuum describes the phases through which a person moves to prevent and control cancer. It includes timely referral and follow-up investigations, referral to treatment if the screening test is positive, and processes that ensure follow-up and return for retesting if the test is negative.
2.4. Analyses and Statistics
The data were single entered and analyzed with EpiData (version 3.1 for entry and 2.2.2.183 for analysis; EpiData Association, Odense, Denmark). The percentage distribution of socioeconomic characteristics and clinical signs and symptoms are presented. We reported the proportion of the patients with cervical abnormalities (VIA) and a positive Pap smear. Cross tabulation was done to associate Pap smear and VIA findings of screened women. The cancer care continuum and follow-up status were assessed by (i) the proportion of women screened who were eligible for referral for further confirmation and disease management and (ii) the proportion of women, those who visited NCCC referred to tertiary care facilities.
2.5. Ethics Approval
Ethics approval was obtained from Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease (The Union), Paris, France (EAG Number 97/17, dated 16/11/2017). Administrative approval was obtained from NCCC. Because the study involved review of secondary, waiver for informed consent was sought and approved by the ethics committee.
3. RESULTS
3.1. Sociodemographic and Clinical Characteristics
The participation in screening programs was very minimal, ranging from 5 to 48 women in each camp, and more than 50% of the camps registered <20 women. Of the 2749 women registered, 2192 were screened for cervical cancer and were included in this study. The remaining were not screened (n = 557) because of a history of hysterectomy, menstruation at the time of screening, old age, or unwillingness to participate. The sociodemographic and clinical characteristics of 2192 women are summarized in Table 3.
| Characteristics | n(%) |
|---|---|
| Total | 2192 (100) |
| Age (years) | |
| <30 | 342 (15.6) |
| 31–40 | 967 (44.1) |
| 41–50 | 655 (29.9) |
| 51–60 | 203 (9.3) |
| >60 | 24 (1.1) |
| Missing | 1 (–) |
| Marital status | |
| Married | 2135 (97.4) |
| Never married* | 5 (0.2) |
| Divorced/separated/widow | 27 (1.2) |
| Missing | 25 (1.2) |
| Education | |
| No formal education | 408 (18.6) |
| Primary schooling | 573 (26.1) |
| Secondary schooling | 802 (36.6) |
| Higher secondary | 165 (7.5) |
| Degree or above | 220 (10.1) |
| Missing | 24 (1.1) |
| Occupation | |
| Professionals | 122 (5.57) |
| Government employed | 30 (1.37) |
| Private job | 116 (5.29) |
| Self-employed | 90 (4.11) |
| Daily wages | 560 (25.55) |
| Bidi workers | 292 (13.32) |
| Homemaker | 966 (44.07) |
| Retired | 1 (0.05) |
| Missing | 15 (0.68) |
| Religion | |
| Hindu | 1645 (75.0) |
| Muslim | 211 (9.6) |
| Christian | 324 (14.8) |
| Missing | 12 (0.5) |
| Age at menarche (years) | |
| <10 | 11 (0.5) |
| 11 | 37 (1.7) |
| 12 | 164 (7.5) |
| 13 | 504 (23) |
| 14 | 592 (27) |
| >15** | 860 (39.2) |
| Missing | 24 (1.1) |
| Age at marriage (years) | |
| <18 | 544 (24.8) |
| 19–21 | 857 (39.1) |
| 22–25 | 583 (26.6) |
| >25 | 182 (8.3) |
| Missing | 26 (1.2) |
| Parity | |
| Nulliparous | 15 (0.7) |
| Parous | 2177 (99.3) |
| Number of children (n = 2177) | |
| 1 | 195 (9.0) |
| 2 | 837 (38.4) |
| 3 | 594 (27.3) |
| >4 | 551 (25.3) |
| Age at first child birth (years) | |
| <18 | 217 (10.0) |
| 19–25 | 1456 (66.9) |
| 25–30 | 386 (17.7) |
| 31–35 | 40 (1.8) |
| >35 | 5 (0.2) |
| Missing | 73 (3.4) |
These women were aged above 50 years, and considering their age as a risk factor, they were included in the study even though they were not married.
Mean age at menarche in India is estimated as 13.76 years; however, it may not be a true estimate due to recall bias and there is a possibility of overestimation because the age at menarche is always collected retrospectively [14]. Similar assumption can be made in this context also.
Sociodemographic characteristics of women who underwent cervical cancer screening in Tirunelveli and Tuticorin districts, Tamil Nadu, India, between March 2015 and May 2016
The patients’ age ranged from 18 to 69 years, with a mean [standard deviation (SD)] age of 39.2 (8.4) years. Nearly three-fourths of the women were in the reproductive age-group (<45 years). Among those employed, 24.1% (292/1210) were bidi workers. Tobacco use (smokeless) was found among 1.4% (n = 31) of the women. Of the women screened, 1.8% (n = 40) reported to have undergone screening for cervical cancer in the past. Clinical signs and symptoms among 2192 women are listed in Table 4.
| Symptoms and signs | n(%) |
|---|---|
| Total | 2192 (100) |
| Symptoms | |
| No complaints | 1238 (56.5) |
| White discharge per vaginum | 764 (34.9) |
| Postcoital bleeding | 8 (0.4) |
| Postmenopausal bleeding | 5 (0.2) |
| Intermenstrual bleeding | 6 (0.3) |
| Urinary symptoms | 13 (0.6) |
| Missing | 151 (6.9) |
| Signs | |
| Healthy cervix | 1013 (46.2) |
| Polyp | 56 (2.6) |
| Cervical erosion | 753 (34.4) |
| Cervicitis | 25 (1.1) |
| Nabothian follicles | 66 (3.0) |
| Cervical leukoplakia | 7 (0.3) |
| Condyloma acuminatum | 6 (0.3) |
| Bleeds on touch | 28 (1.3) |
| Suspicion of vaginal vault lesion | 1 (0.0) |
| Atrophy, urinary tract infections | 76 (3.5) |
| Not recorded | 267 (12.2) |
Clinical signs and symptoms of screened women in screening camps conducted in Tirunelveli and Tuticorin districts, Tamil Nadu, India, between March 2015 and May 2016
The most common symptom reported was white discharge per vaginum (n = 764, 34.9%), and the most common sign was cervical erosion (n = 753, 34.4%). Two in five women had an unhealthy cervix on inspection.
3.2. Pap Smear Findings
Of 2192 women, 1781 (81.3%) were negative for intraepithelial lesion or malignancy (NILM), 216 (9.9%) had NILM with infections, 112 (5.1%) had epithelial cell abnormalities, 298 (13.5%) had unsatisfactory staining or inadequate sampling or broken slides, and 1 was suggestive of squamous cell carcinoma (SCC) (Table 5).
| Pap smear findings* | n(%) |
|---|---|
| Total | 2192 (100.0) |
| NILM (n = 1781, 81.3%) | |
| NILM without any infections or atrophy | 1486 (67.8) |
| NILM atrophy | 79 (3.6) |
| NILM with infection (n = 216, 9.9%) | |
| NILM—bacterial vaginosis | 162 (7.4) |
| NILM—Trichomonas vaginalis | 24 (1.1) |
| NILM—Candida species | 28 (1.3) |
| NILM—actinomycosis | 2 (0.1) |
| Precancerous | |
| Epithelial cell abnormalities (n = 112, 5.1%) | |
| Squamous cell | |
| ASC-US | 71 (3.2) |
| ASC-H | 2 (0.1) |
| LSIL | 14 (0.6) |
| LSIL-HPV | 1 (0.0) |
| HSIL | 14 (0.6) |
| Glandular cell | |
| AGUS | 5 (0.2) |
| AGUS with ASC-US | 5 (0.2) |
| Cancerous | |
| SCC | 1 (0.0) |
| Specimen adequacy | |
| Unsatisfactory staining/inadequate sampling and broken** | 298 (13.5) |
Based on the Bethesda System.
NILM, negative for intraepithelial lesion or malignancy; ASC-US, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells—cannot exclude HSIL; LSIL, low-grade squamous intraepithelial lesion; HPV, human papilloma virus; HSIL, high-grade squamous intraepithelial lesion; SCC, squamous cell carcinoma; AGUS, atypical glandular cells of undetermined significance.
Unsatisfactory staining: n = 5; inadequate sampling: n = 268; broken: n = 25.
Pap smear findings of screened women in screening camps conducted in Tirunelveli and Tuticorin districts, Tamil Nadu, India, between March 2015 and May 2016
It is observed that women who reported to have epithelial changes reported some signs or symptoms during initial screening.
3.3. VIA Results
The VIA results were positive among 24% (523/2178; 14 women did not cooperate for VIA). Of 2192, 1880 women had both Pap smear and VIA results (14 did not undergo VIA and 298 had unsatisfactory Pap smears). The VIA results have been compared with Pap results in Table 6.
| Pap findings** | VIA findings* | |
|---|---|---|
| Negative (n = 1446) | Positive (n = 434) | |
| n(%) | n(%) | |
| Negative (NILM: n = 1768) | 1371 (77.5) | 397 (22.5) |
| Positive (precancerous: n = 111; cancerous: n = 1) | 75 (66.9) | 37 (33.0) |
Excluded:
14 women did not undergo VIA testing;
298 had unsatisfactory Pap smears. Percentage calculated based on Pap findings.
VIA, visual inspection with acetic acid; NILM, negative for intraepithelial lesion or malignancy.
Association of Pap smear and VIA findings of screened women in Tirunelveli and Tuticorin districts, Tamil Nadu, India, between March 2015 and May 2016 (N = 1880)
Among those who were Pap negative, 22.5% (397/1768) were VIA positive. About 66.9% (75/112) were Pap positive but VIA negative; 33% (37/112) showed both Pap and VIA positive. One woman who had SCC in Pap was VIA negative. More than 50% (n = 8/14) of women with high-grade squamous intraepithelial lesion findings were also positive on VIA. Of 298 unsatisfactory Pap smears, 89 women (29.9%) showed VIA positivity.
3.4. Referral for Further Management
Of the 2192 women screened, 807 were eligible for referral (597 had positive results on either Pap or VIA). Among 298 with unsatisfactory Pap smear, 209 women were VIA negative. Among the 807 women referred, only 74 (9.2%) women visited NCCC. Of the 74 women, 56 women had positive results on either Pap or VIA, 14 women for repeat test, and four women for treatment of infections. About 9.4% (56/597) of women who were tested positive on either Pap or VIA attended NCCC.
4. DISCUSSION
In this study from South India, retention in cervical cancer care continuum remained poor. There was a large gap between those referred for further care and those reaching the referral clinic. The camps were conducted in rural areas without access to cervical cancer screening and the women were mobilized with the support of community-based organizations in the same locality to enhance trustworthiness. Despite this, the participation in the camps was limited.
Even in developed countries like the United States, a significant health disparity was reported among rural and urban residents, with lack of health-care providers and awareness about screening procedures among the rural residents identified as the deterrent factors [15,16]. In developing countries like India, where the health disparity is much higher, cervical cancer screening and management of precancerous conditions are yet to become standard of care.
The women in the screening locations were at high risk due to early marriage, low socioeconomic status, early pregnancy and child birth, and poor access to health-care facilities. Therefore, screening camps conducted by NCCC did not restrict any age-group and included young girls aged 17 years if they were married and also unmarried women aged more than 50 years to provide screening for all women. Srinivasan et al. posited that poverty is a risk factor for developing cervical cancer and withholding poor women from undergoing screening, despite the availability of effective lifesaving screening procedures, further increases the risk of mortality due to invasive cervical cancer. In the several trials conducted by Shastri et al. [17] and Sankaranarayanan et al. [18], a total of 548 women were recorded to have died during trials, with 254 of them belonging to the no-screening control arms. Therefore, denying screening because of age for trials was highly debated and criticized as ethics dumping [19]. Thus, it is imperative to allow all women to undergo screening utilizing every possible opportunity to address health inequality.
Vaginal discharge was reported by 34.9% of the women and cervical erosion by 34.4% in this study, whereas in the study at Punjab, India, by Bal et al., it was comparatively higher at 59%. The prevalence of positive cytology (5.1%) in our study is in line with the study by Bal et al. [20] (5%).
Approximately one in five women had positive VIA results and one in 20 women had abnormality in Pap findings. All women who were reported to have epithelial changes reported some signs or symptoms during initial screening. Lack of awareness of risk factors and symptoms for cancer may lead to late diagnosis and poor prognosis [21]. Simon et al. [22] opined that raising awareness of cancer symptoms and signs might increase people’s ability to detect early symptoms and signs of cancer. It is also known that early help seeking through awareness and knowledge on cancer warning symptoms is beneficial in cancer prevention [23].
Inflammatory smear (NILM with infections) was reported in 9.9% of women (n = 216) in our study. But mixed results were reported by Kulkarni et al. [24] with a higher rate of 73.7% and Lawley et al. [25] observed a lower rate of 14.3% inflammatory smears.
This study had higher unsatisfactory Pap smears (13.5%) compared to 3.8% in the study by Sankaranarayanan et al. [26]. The reasons for unsatisfactory smears need to be explored including the training needs.
Despite extensive awareness programs, repeated house visits, phone calls, and free services, only a few women (15.7%) attended the referral center for further evaluation and management. Distance could have been a major deterrent as 50% of the camps were 40 km away from the health facilities. In a study conducted in a Nigerian community, the reasons reported for nonparticipation in the screening program were fear of cancer diagnosis, disagreement of the husband, perception of low risk, negative attitude toward cervical cancer screening, and fear of being perceived as promiscuous [27]. Although, the reasons are yet to be understood in Indian context, this evidence underlines the significance of educating the rural population, both men and women, about the advantages of cervical cancer screening [28,29]. In this study, of the 807 women referred, only 74 (9.2%) women visited NCCC. As most did not visit the NCCC for follow-up, risk factor analysis (of who were at higher risk to be lost to follow-up) would not serve the purpose. If the follow-up rate was 80%, we would want to increase the follow-up to 100%, and it would be of interest to look for the women who are at higher risk of not following up. In addition, we have followed it up with a qualitative study to explore provider and patient perspectives for reasons for not following up. The poor participation and follow-up status in this study proves the underutilization of the screening facilities and it is understood that there is a need for systematic qualitative enquiry on the reasons for nonparticipation.
Cancer care continuum is important for patients who are screened positive but some patients discontinue their care and are lost to follow-up at the cancer center where they were screened and referred for treatment. In a study at a community cancer center, 50% of patients who were lost to follow-up were lost within 1 year of treatment. Low socioeconomic status is associated with less adherence to treatment and follow-up [30], and it is also reported that being married and having good social support relate with better compliance [31].
In this study, among the patients who attended NCCC, 36 women (48.7%) were reported of precancerous Pap results. It is understood that 284 women did not attend for investigation as advised during the screening, may be at high risk for cancer. This becomes unethical because the women whom we know are at risk have not given the same level of protection and consideration, whereby the care continuum is fragmented.
Mapping the continuum of care has, for communicable diseases, led to successful system-level interventions to retain individuals in care and improve outcomes [32]. For example, in India, various tracking mechanisms and incentivization for health workers and patients were devised to complete the care continuum of patients with tuberculosis to decrease the tuberculosis-related morbidity and mortality. Although, in Tamil Nadu, documentation was ensured through technology for cancer screening as part of Tamil Nadu Health Systems Project supported by World Bank, there is no information about the completion of care continuum for patients screened for cancer.
The global burden of disease study [33] reported the success in executing continuum of care whereby increasing life expectancy through prevention and treatment of communicable diseases. Similarly, non-communicable diseases require a mix of services that are integrated and coordinated across a continuum of care, which needs to be sustained over time [34].
4.1. Limitations
The data were drawn from a single cancer prevention center. As the screening camps were conducted with the help of community-based organizations, coverage bias could have existed.
5. CONCLUSION
The follow-up rate was very poor accounting to fragmentation of care continuum. The success of the screening program depends on the completion of the care continuum. Therefore, reducing cancer care fragmentation is important.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
EV principal investigator and corresponding author, conception/design of the protocol, acquisition of data, data analysis/interpretation, drafting/critically reviewing the paper, giving approval for the final version to be published. KN data analysis/interpretation, critically reviewing the paper, giving approval for the final version to be published. HS and SM role of mentor/senior investigator during conception, design, acquisition of data, critically reviewing the paper, giving approval for the final version to be published.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
ACKNOWLEDGMENTS
We thank the staff of Nellai Cancer Care Center, Bavithra, A., Seeniammal, Dhanalakshmi K., Jenifer, G., Vijayalakshmi, N., Sangeetha Keerthana, G., Chermakani, RamaGanesal, H., and Parvathy, A., for their contribution in the extraction of data from case records, data entry, and verifying the clinical details. We thank eNoah iSolution India Private Limited, Chennai, for developing a software for efficient data collection in NCCC. We acknowledge Dr. Sinthia Saroja for verifying the clinical details and the terms used in the records. We thank Dr. Priyadarshini for helping in collation of information for drafting. We thank the screening team and the participants of the screening program.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - E. Vidhubala AU - K. Niraimathi AU - Hemant Deepak Shewade AU - Sankar Mahadevan PY - 2019 DA - 2019/11/25 TI - Cervical Cancer Care Continuum in South India: Evidence from a Community-based Screening Program JO - Journal of Epidemiology and Global Health SP - 28 EP - 35 VL - 10 IS - 1 SN - 2210-6014 UR - https://doi.org/10.2991/jegh.k.191111.001 DO - 10.2991/jegh.k.191111.001 ID - Vidhubala2019 ER -
