Vitamin A Supplementation for Prevention and Treatment of Malaria during Pregnancy and Childhood: A Systematic Review and Meta-analysis
Present address: Department of Community Health Sciences, United Medical and Dental College, Karachi, Pakistan
- DOI
- 10.2991/j.jegh.2018.04.104How to use a DOI?
- Keywords
- Childhood; preventive; pregnancy; randomized controlled trials; supplementation; treatment; vitamin A
- Abstract
Animal studies have shown that vitamin A plays a role in immunity and protection against infectious diseases. Its role reducing incidence of diarrhea and measles, and childhood mortality is known, but its role in relation to malaria is unclear. Thus, a comprehensive, systematic literature search was conducted on PubMed and Cochrane Library to identify randomized controlled trials (RCTs) on the role of vitamin A during pregnancy and childhood for prevention and treatment of malaria. A total of 107 titles/abstracts were identified, of which 15 articles (11 studies) were selected for final inclusion. Based on the meta-analysis, vitamin A supplementation during pregnancy had no benefit for placental infection (relative risk = 1.09; 95% confidence interval (CI), 0.95–1.25; fixed effects, I2 = 0; 2 RCTs). Similarly, there was no effect on peripheral parasitemia or episodes of new clinical malaria. Preventive vitamin A supplementation in children younger than 5 years did not reduce the incidence of peripheral parasitemia or malaria mortality (latter rate ratio = 0.49; 95% CI, 0.07–3.26; random effects, I2 = 72%, 2 RCTs). Vitamin A as an adjunct treatment for cerebral or severe malaria in children did not have benefit on survival, fever resolution time, parasite clearance time, or incidence of neurological or other complications. Vitamin A has no benefit for malarial infection either as prevention or treatment in pregnancy or childhood based on RCT evidence.
- Copyright
- © 2018 Atlantis Press International B.V.
- Open Access
- This is an open access article under the CC BY-NC license (http://creativecommons.org/licences/by-nc/4.0/).
1. INTRODUCTION
According to the Global Burden of Disease and World Health Organization (WHO) 2016 estimates, although the incidence of malaria has decreased by 21% since 2010, it still accounts for 212 million cases and 429,000 deaths globally, with an attributable fraction of 1.32% of total deaths [1,2]. Malaria is a major public health burden with 90% of cases occurring in the African continent, followed by Southeast Asia (7%) and the Eastern Mediterranean region (2%) [2]. Malarial infection is common among the poor and underprivileged populations in developing countries, mostly affecting vulnerable adults, pregnant women, and children [3,4]. This necessitates making prevention and treatment efforts a priority in combatting this disease. Because there is a link between poverty and undernutrition, it is hypothesized that nutrition may play an important role in either primary prevention or treatment of malaria. Undernutrition is accompanied by deficiency of different micronutrients, and it is not clear if supplementation of single or multiple micronutrients can be of benefit or even increase the risk of infection in the setting of adequate nutrition.
Vitamin A is known to modulate innate and adaptive immune responses and is vital for host immunity against different infectious diseases. Vitamin A supplementation in randomized controlled trials (RCTs) is known to protect against incidence of diarrhea and measles in children younger than 5 years (by 15% and 50%, respectively—low to moderate quality evidence), and also reduce diarrhea-related and all-cause mortality [5]. Similarly, the effect of vitamin A supplementation on maternal clinical infections (mostly puerperal sepsis) is borderline significant with low-quality evidence [6]. It may, therefore, also play its part in malaria prevention and reduction in mortality due to malaria, the role of which is unclear. Various mechanisms have been proposed from animal studies for the protective role of vitamin A. Vitamin A may enhance phagocytosis of erythrocytes harboring the malarial parasite and attenuate the proinflammatory cytokine responses to infection [3]. Further animal model studies have also shown that vitamin A may play a major role in the functioning of the immune system, including lymphopoiesis, apoptosis, production of antibody, and the function of other immune cells such as neutrophils, natural killer cells, monocytes or macrophages, and lymphocytes [7].
The effect of vitamin A in preventing and treating malarial infections has not previously been studied in the form of a systematic review and meta-analysis based on evidence from RCTs. A meta-analysis would increase the power and allow for potentially statistically significant effects that have not been observed in individual studies. Moreover, this evidence has not been compiled in a comprehensive, organized, and simple manner to date. We therefore conducted this systematic review and meta-analysis to evaluate the effects of vitamin A supplementation during both pregnancy and childhood (younger than 5 years) for malaria prevention and treatment.
2. MATERIALS AND METHODS
A comprehensive, systematic literature search was conducted in July 2017 on PubMed and the Cochrane Library to identify RCTs on vitamin A supplementation in relation to malaria. The complete search term used was (“vitamin A”, suppl*, and malaria). Including synonym terms for vitamin A (such as retinol OR carotenoids) and malaria (such as plasmodium OR “remittent fever”) without suppl* yielded 275 titles/abstracts but no relevant additional RCTs. We also searched Google Scholar for gray literature with the original search strategy (first 100 hits) but found no additional studies.
We used the following inclusion/exclusion criteria. (1) RCTs where vitamin A supplementation should be the only difference between the intervention and placebo groups. If vitamin A was given with any other supplement such as zinc (2 × 2 factorial design), then those two groups were included that differed only in vitamin A (if no interaction with zinc) or vitamin A versus placebo groups (if an interaction). (2) The supplementation should be during pregnancy irrespective of the trimester or month of pregnancy. RCTs on post-partum supplementation were excluded. (3) Supplementation in children younger than 5 years. For those studies where age range exceeded 5 years, we included the overall results and did not report dissociated data for children younger than 5 years because such data were not found in any of the RCTs.
The primary/secondary outcomes are as follows. (1) The primary outcomes considered for prevention of vitamin A during pregnancy were incidence of clinical malaria and peripheral parasitemia. The secondary outcome considered was histopathological infection. (2) The primary outcomes considered for prevention in children were incidence of clinical malarial episodes and malaria-specific mortality. For treatment of malaria, the primary outcomes considered were survival and time to resolution of infection (fever). Secondary outcome was incidence of neurological and other complications.
The screening for studies and retrieval of full-text articles and review was done by a single author (M.Y.Y.). Standardized Excel sheets were prepared for data extraction. Data extraction was also done by the same author (M.Y.Y.) that was cross-checked through discussion by another author (M.Q.). A risk of bias table was prepared that included information on study authors, population, location, dose of intervention, randomization generation, allocation concealment, lost to follow-up (selection bias), and blinding.
If more than one study was found with an identical outcome, then meta-analysis was conducted. For dichotomous outcomes, the effect estimate was reported as relative risks/risk ratios (RRs) and for continuous outcomes as weighted mean differences (WMDs).The a priori model of choice was fixed effects, but if substantial heterogeneity was found (I2 > 50%), then the random-effects model was also reported. Fixed-effects models assume that all studies estimate a single true effect size and that the variance between effect sizes is attributable to sampling error only. Random effects models assume that the studies estimate their own true effect sizes, distributed around an average true effect, where variance is attributable to both sampling error and “real” between-study variance. Random effects, therefore, give more balanced weighting to smaller studies. If heterogeneity was substantial, we used the random effects model even though we did not attempt subgroup analyses or meta-regression because of the insufficient number of studies in each meta-analysis conducted.
A formal protocol of this review has not been published in any database. There was no funding source for this project.
3. RESULTS
A total of 107 titles/abstracts were screened, of which 57 articles were selected for detailed abstract/full-text review. One article was added from the references of the included articles. Of these 58, 15 articles or 11 distinct studies/trials [8–22] were finally selected for inclusion in this systematic review. The flow diagram of the literature search along with the reasons for 43 excluded studies is shown in Fig. 1. The risk of bias table for the 11 included studies is shown in Table 1.
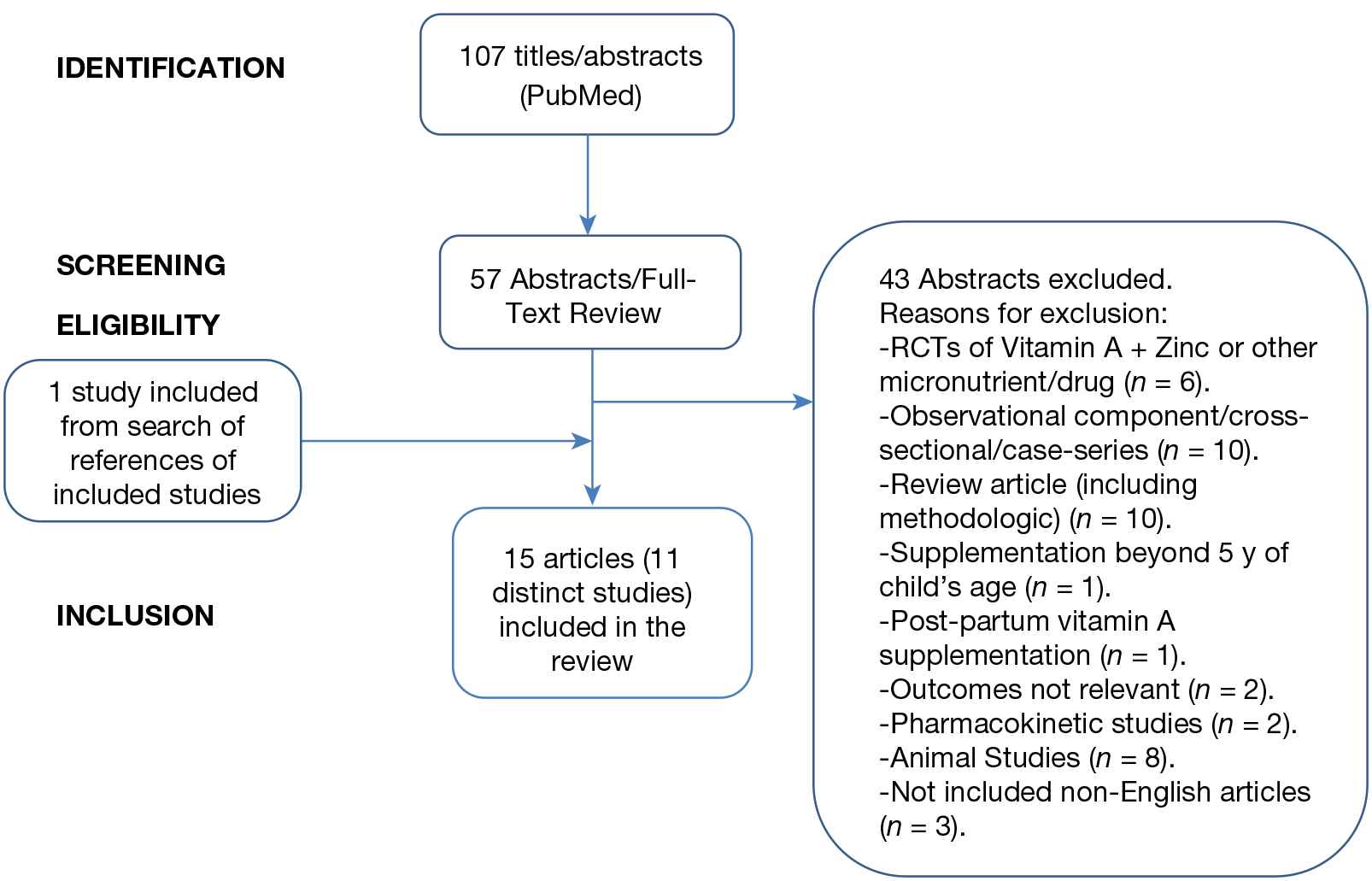
Flow diagram showing the steps in the literature search. RCTs, randomized controlled trials
| Study authors | Study population | Country | Dose of vitamin A | Randomization | Allocation concealment | Lost to follow-up | Blinding | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevention of malaria during pregnancy | ||||||||||||
| Cox et al. [13] | 98 Pregnant women (<24 week gestation) | Central Ghana | 10,000 IU of vitamin A weekly or placebo. | Balanced block randomization. | The coding was unknown to the investigators, until after completion of the trial and the capsules were identical in appearance. | Two were excluded due to early miscarriage or false pregnancy. One lost to follow-up prior to delivery. 10 women missed the late pregnancy visit. | Double-blind. | |||||
| Darling et al. [8] | 2500 Screened pregnant women during early gestation | Dar es Salaam, Tanzania | Vitamin A 2500 IU, zinc 25 mg, vitamin A 2500 IU plus zinc 25 mg, and placebo. The treatment was given orally each day until delivery. | Individual randomization in equal numbers. Computer-generated randomization sequence using blocks of size 20 by a scientist not involved in the data collection. | Each study clinic was issued regimen bottles that were prelabeled according to randomization sequence by study pharmacists who had no contact with participants. At enrollment, each participant was assigned to the next numbered bottle at their site. | Birth outcomes were obtained for 2434 of the 2500 randomized participants because participants later left Dar es Salaam for cultural reasons (e.g., at the request of their mothers or in-laws) or delivered at a nonstudy facility. | Double-blind. Neither the participants nor the study personnel had access to the masking information. The tablets were indistinguishable from one another in appearance and taste. | |||||
| Olofin et al. [9], Villamor et al. [11] | 1078 HIV-infected pregnant women | Dar es Salaam, Tanzania | A factorial design to receive a daily oral dose of 1 of 4 regimens: placebo, vitamin A alone (30 mg beta-carotene with 5000 IU preformed vitamin A), multivitamins without vitamin A, or multivitamins with vitamin A. At delivery, women received an additional 200,000 IU oral vitamin A in the vitamin A groups. | Eligible women were randomly assigned in blocks of 20. | The regimen was provided in bottles labeled with the participants’ names and identification numbers, and made active tablets and placebo indistinguishable. | 28 Women were excluded from analyses—27 were lost to follow-up immediately after enrollment and 1 had protracted malaria parasitemia starting at baseline. | Double-blind study. Active tablets and placebo were identical in size and color. All participants and study staff were blinded. | |||||
| van den Broek et al. [12] | 700 Women with singleton pregnancies at 12–24 weeks and with Hb < 11.0 g/dL | Rural community in southern Malawi, Central Africa | One of three daily oral interventions: (a) 5000 IU vitamin A, (b) 10,000 IU vitamin A, or (c) a placebo. | Randomization was done with a random-generation procedure to assign treatments to each individual, using consecutive numbers. | Neither the statisticians nor the midwives involved revealed the randomization schedule to anyone involved in the conduct of the trial and the schedules were placed in sealed envelopes. | 93 Women were lost to follow-up for different reasons given in the article. | The supplements in vitamin A and placebo treatments allocated were prepared in identical capsules and were packaged in bottles containing supplies for 14 days, according to the randomization schedule by midwives who were not involved in the trial conduct. | |||||
| Treatment of malaria during pregnancy No studies found. | ||||||||||||
| Prevention of malaria in children | ||||||||||||
| Fawzi et al. [19], Villamor et al. [14,21,22], | 687 Children were enrolled in the trial who were admitted to the hospital with pneumonia | Dar es Salaam, Tanzania | A dose of 200,000 IU/mL vitamin A or placebo on the day of admission, another dose on the following day and a third and fourth dose at 4 and 8 months after discharge from hospital. Infants received half the dose. | Study children were randomized in blocks of 20. | Vitamin A and placebo were dispensed out of a dropper from identical 25-mL opaque bottles that were labeled with one of four batch numbers. The batch number code was retained by the manufacturer until the end of the study. | After 4 months, 89% of children were followed up (611/687). Of the remaining 76 children, 18 left the hospital and could not be traced, 25 moved out of the study area during the follow-up period and the remaining moved within the city and their new address was not known. | Double-blind trial. Vitamin A and placebo were dispensed out of a dropper from identical 25-mL opaque bottles. | |||||
| Binka et al. [17] | Child Survival Study (mortality). 21,906 children aged 6–90 months. | Kassena-Nankana District in upper east region of Northern Ghana | Children were given vitamin A (200,000 IU retinol equivalent; 100,000 IU for infants) or placebo every 4 months for 2 years (September 1989– December 1991). | The Survival Study was divided into 185 geographical areas (clusters) and cluster was used as unit of randomization in blocks. | Randomization was done in London by an independent statistician who held the randomization code. Vitamin A or placebo were supplied in opaque bottles. Each bottle had a unique number and was labeled with a cluster code prior to dispatch to Ghana. | 892 (4.1%) died, 102 (0.5%) developed xerophthalmia, 5 (<0.1%) withdrawn by parents, 60 (0.3%) changed treatment groups, 1847 (8.4%) children were lost to follow-up. | Double-blind trial. Vitamin A and placebo were similar in taste and color. | |||||
| Ghana VAST Study Team [18] | Child Health Study (morbidity). 1455 children aged 6 months to 5 years | Kassena-Nankana District in upper east region of Northern Ghana | Children were given vitamin A (200,000 IU retinol equivalent; 100,000 IU for infants) or placebo every 4 months for average of 9.8 months (June 1990–August 1991). | Individual children were randomly assigned in blocks. | Randomization was done in London by an independent statistician who held the randomization code. Vitamin A and placebo were supplied in gelatin capsules, individually packaged in opaque envelopes with one child’s unique identification number. | 26 (1.8%) died, 14 (1.0%) developed xerophthalmia, 5 (0.3%) developed measles, 4 (0.3%) were withdrawn by parents, and 119 (8.2%) children were lost to follow-up | Double-blind trial. Vitamin A and placebo were similar in taste and color. | |||||
| Shankar et al. [16] | 480 Children between 6 and 60 months of age | North Wosera District of East Sepik Province, in northwest Papua New Guinea | Quarterly high-dose vitamin A (200,000 IU or half for infants) supplements vs. placebo. | Children were stratified by age and geographical regions and within these strata were individually randomized by computer-generated randomly permutated codes. | The code was kept offsite by personnel who were not involved in the study. | 201/239 completed follow-up in vitamin A group and 209/241 in placebo at 13 months. | Double-blind trial. Vitamin A and placebo capsules were identical. | |||||
| Treatment of malaria in children | ||||||||||||
| Mwanga-Amumpaire et al. [10] | 142 Children aged 6–59 months admitted through emergency unit with a confirmed diagnosis of cerebral malaria | Mulago Hospital, Uganda | 200,000 IU of vitamin A for children older than 1 year and half for infants (n = 74) vs. placebo (n = 68). | Randomization was done in blocks of variable sizes (4–10) using a set of computer generated random numbers. | The randomization sequence was generated by an independent statistician not involved in the study design, data processing, or analysis. Instructions for treatment were produced and placed in opaque sealed envelopes with unique identification numbers by an independent pharmacist not involved in the study. | Two children in total (one in each group) were lost to follow-up. | Double-blind. The vitamin A capsules and placebo were exactly the same in color, shape, and size. | |||||
| Uscategui et al. [20] | 93 Children with malaria, aged 4–10 years. | Municipalities of El Bagre and Turboin Columbia | Random allocation to one of the following groups: (1) treatment with antimalarial and retinol supplement (Group MA); (2) treatment with antimalarial-retinol supplement and antiparasitic drug (Group MAP); (3) treatment with antimalarial and antiparasitic drug (Group MP), and (4) treatment only with antimalarials (Group M). | Assignment was randomized. | – | 8/93 Children were excluded. | Nonblinded study. | |||||
| Varandas et al. [15] | Children between 6 and 72 months with a diagnosis of severe malaria | Central Hospital of Maputo, Mozambique | 100,000 IU of vitamin A + 20 IU of vitamin E (n = 333) vs. 20 IU of vitamin E (placebo) (n = 316). | Randomization was done through a predetermined simple random list. | Capsules of vitamin A or placebo were put into an envelope labeled with a code number and the number was available only to a team member not involved in recruitment or treatment of patients. | 213/280 in vitamin A group and 140/290 in placebo completed follow-up at 6 weeks. | Double-blind. Identical looking placebo capsules. | |||||
HB, hemoglobin; HIV, human immunodeficiency virus; RCT, randomized controlled trial.
Methodological characteristics of included RCT studies including the risk of bias
3.1. Vitamin A Supplementation for Prevention of Malaria during Pregnancy
A total of four RCTs were identified [8,9,11–13]. Three RCTs reported on peripheral parasitemia [9,11–13], two RCTs on placental histopathological infection [8,13], one on placental infection through polymerase chain reaction (PCR) [8], and one on clinical malaria [9,11]. Meta-analysis could not be carried out on the outcome of peripheral parasitemia because only one study [9,11] reported on incident parasitemia, i.e., new episodes after baseline assessment. In the other two studies [12,13], we could not ascertain the new cases of parasitemia at the end of pregnancy/post-partum/follow-up, relative to baseline.
The meta-analysis based on two trials of histopathologically diagnosed placental malaria did not show evidence of benefit of vitamin A [RR = 1.09, 95% confidence interval (CI), 0.95–1.25; fixed effects, I2 = 0] (Fig. 2). The details of these two trials are presented as follows. A randomized, double-blind placebo-controlled trial [8] was conducted in Dar es Salaam, Tanzania, where 2500 screened pregnant women during early gestation were assigned to four groups: vitamin A 2500 IU, zinc 25 mg, vitamin A 2500 IU plus zinc 25 mg, and placebo. The treatment was given orally each day until delivery. Placentas were obtained after delivery for histopathological examination and PCR testing. In marginal structural models, vitamin A administration had no impact on histopathology-positive infection (57/679, 8% vs. 58/682, 9%; RR = 1.03; 95% CI, 0.82–1.29) nor on PCR-positive placental malaria (88/579, 15% vs. 83/579, 14%; RR = 1.00; 95% CI, 0.70–1.45). These indicated any infection—acute, chronic, or past. All infections detected by these two methods were Plasmodium falciparum-positive. In another randomized, double-blind placebo-controlled trial [13], 98 Ghanaian women were randomized to receive 10,000 IU of vitamin A weekly or placebo. Placentas were available from 76 women and malarial infection was determined by placental blood smear and histology. All grades of placental malarial infection were 35/38 (92%) in vitamin A group vs. 31/38 (81%) in the placebo group. Of this, active infection was 2/38 (5%) vs. 4/38 (11%), active chronic infection 12/38 (36%) vs. 15/38 (39%), and past-chronic infection 21/38 (55%) vs. 12/38 (32%).
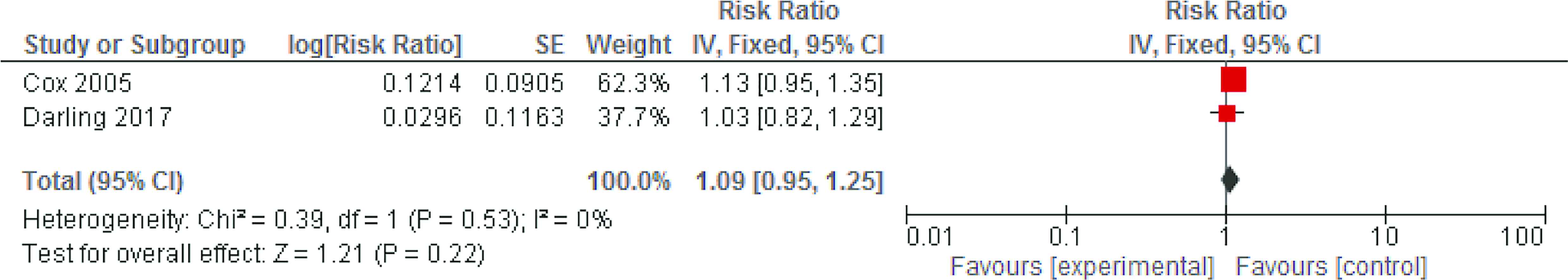
Meta-analysis based on two trials of the preventive effect of vitamin A supplementation on histopathologically diagnosed placental malaria infection. CI, confidence interval; SE, standard error
The remaining two trials were not included in any meta-analysis. In an RCT [9,11] from Dar es Salaam with a factorial design, 1078 HIV-infected pregnant women were allocated into four groups to receive vitamin A alone (30 mg β-carotene with 5000 IU preformed vitamin A), multivitamins without vitamin A, multivitamins with vitamin A, and placebo. All women received 5 mg folic acid and 120 mg ferrous iron along with weekly 500 mg chloroquine prophylaxis. The vitamin A arm was prematurely stopped because of increased risk of mother-to-child transmission of HIV. However, based on person-time contributed by this arm until second pregnancy, lost to follow-up, death, or end of study, vitamin A did not impact on the risk of developing maternal clinical malaria (rate ratio = 0.97; 95% CI, 0.82–1.14; p = 0.70) nor of malarial parasitemia (RR = 1.02; 95% CI, 0.84–1.24; p = 0.84). In a single-center RCT conducted in southern Malawi, central Africa, 700 women with singleton pregnancies with anemia were randomized to receive until delivery oral daily supplementation of 5000 or 10,000 IU vitamin A or placebo [12]. All women received iron tablets (60 mg) and 0.25 mg folic acid and antimalarial prophylaxis (500 mg sulfadoxine with 25 mg pyrimethamine) at 20 and 34 weeks of gestation. There was no difference in the proportion of peripheral malaria parasitemia in the three groups at final assessment of 36–38 weeks of gestation: 13.0%, 17.5%, and 15.0% (p = 0.56); similarly for parasite density >1000 (p = 0.72).
3.2. Vitamin A Supplementation for Treatment of Malaria during Pregnancy
No trials were identified for vitamin A supplementation for treatment of malaria during pregnancy.
3.3. Vitamin A Supplementation for Prevention of Malaria in Children
A total of four RCTs were identified [14,16–19,21,22]. The details of these RCTs are mentioned below. In a double-blind randomized trial [14,19,21,22], a total of 687 children between 6 and 60 months with pneumonia in Dar es Salaam, Tanzania, were randomized to receive 200,000 IU of vitamin A or placebo on the day of admission, a second dose the next day, and then at 4 and 8 months after discharge. Children younger than 12 months received 100,000 IU. Vitamin A supplementation showed a trend in reduction of malaria-specific mortality (1/326, 0.3% vs. 7/322, 2.3%; hazard ratio = 0.14; 95% CI, 0.00–1.07; p = 0.06). In Ghana, two companion high-dose vitamin A supplementation trials were conducted [17,18]. In the mortality Child Survival Study, 21,906 children aged 6–90 months were visited every 4 months for more than 2 years, and in the morbidity Child Health Study, 1455 children aged from 6 months to 5 years were visited weekly for 1 year. There was no difference between vitamin A supplemented versus placebo groups in malaria mortality rates (rate ratio = 1.03; 95% CI, 0.74–1.43) or fever incidence (nonspecific fever: rate ratio = 1.01; 95% CI, 0.96–1.07; or fever accompanied by malaise, headache, diarrhea, or vomiting: rate ratio = 1.04; 95% CI, 0.92–1.18). In both the trials, there were also no effects of vitamin A on malaria parasitemia rates, parasite densities in children with a positive blood smear, and rates of probable malaria illness. Based on a meta-analysis of two RCTs [14,17], there was no benefit in malaria-specific mortality (rate ratio = 0.98; 95% CI, 0.71–1.36; fixed effects). Significant heterogeneity was found in this analysis (I2 = 72%), but the random effects was also nonsignificant (RR = 0.49; 95% CI, 0.07–3.26) even though the point estimate suggested 51% benefit (Fig. 3).

Meta-analysis based on two trials of the preventive effect of vitamin A supplementation on malaria-specific mortality in children younger than 5 years. CI, confidence interval; SE, standard error
In a randomized, double-blind placebo-controlled trial, 480 children between 6 and 60 months of age were randomized to receive high dose vitamin A (200,000 IU; 100,000 IU for <12 months of age) every 3 months for 13 months (n = 239) or placebo (n = 241) in Papua New Guinea [16]. There was no difference seen between groups in prevalence of parasitemia (P. falciparum, Plasmodium vivax, or Plasmodium malariae) at the end of study. There was, however, a trend toward a 36% decrease in P. falciparum density (95% CI, −8 to 62; p = 0.093) and an 11% (−1 to 22; p = 0.075) decrease in spleen enlargement compared with children given placebo.
3.4. Vitamin A Supplementation for Treatment of Malaria in Children
Three RCTs were identified [10,15,20]. One RCT had participants with cerebral malaria [10], one with severe malaria [15], and one with malaria [20]. Two RCTs reported on survival, fever and parasite clearance times, and incidence of neurological complications [10,15]. One RCT [20] with malaria patients as participants reported only on reduction in inflammation and other clinical outcomes not relevant to this study.
In a randomized, double-blind placebo-controlled trial [10], 142 children aged 6–59 months admitted with cerebral malaria in Kampala, Uganda were randomized to receive either vitamin A (n = 74) or placebo (n = 68) as adjunct treatment therapy. Vitamin A was administered as a single dose of 100,000 IU for those younger than 1 year and 200,000 IU for those older than 1 year. There was no difference in fever or parasite clearance times (p = 0.92 and 0.12, respectively) or in survival (p = 0.14), or occurrence of neurological complications (p = 0.44 for coma resolution time and p = 0.37 for resolution of convulsions) after 7 days of follow-up. In Mozambique, children aged between 6 and 72 months admitted with diagnosis of severe malaria were randomized to a single oral dose of 200,000 IU (100,000 IU if <12 months old) vitamin A (n = 280) or placebo (n = 290) and followed up to 6 weeks after discharge [15]. Seven of 277 (2.5%) and 13 of 288 (4.5%) children died in the experimental and control groups (RR of death = 0.56; 95% CI, 0.23–1.38; p = 0.201). This difference was clinically rather than statistically important. The risk of death, however, was significantly higher in vitamin A group compared with placebo 5 hours after admission but not during the first 5 hours. Overall, 4/82 (4.9%) vs. 2/78 (2.6%) among patients with cerebral malaria developed neurological sequelae (RR = 1.90; 95% CI, 0.36–10.09; p = 0.682). The incidence of frequency of complications (RR = 0.92; 95% CI, 0.66–1.29; p = 0.623) and blood transfusion (RR = 0.94; 95% CI, 0.87–1.02; p = 0.161) was not different between vitamin A supplemented and placebo groups. The time to resolution of fever (p = 0.151), resolution of coma (96/102 vs. 88/99; p = 0.746), and clearance of parasitemia were not different (73/123 vs. 88/164; p = 0.833).
A nonblinded, randomized experimental study [20] was conducted in 93 children with malaria aged 4–10 years. The children were allocated into four groups: (1) treatment with antimalarial and retinol supplement, (2) treatment with antimalarial-retinol supplement and antiparasitic drug, (3) treatment with antimalarial and antiparasitic drug, and (4) treatment only with antimalarials. The groups were observed for 30 days. Inflammation was measured in terms of C-reactive protein >8 mg/L. Although in total, inflammation was reduced from 96% to 18%, there was no difference in frequency of inflammation either at baseline or end of study among the groups (p = 0.512 and 0.454, respectively). This indicates that inflammation was reduced in all groups, which could be attributable to antimalarials, and vitamin A did not have any specific effect in reducing inflammation related to malarial infection.
4. DISCUSSION
In this review, we found that vitamin A supplementation during pregnancy had no benefit in reducing placental malarial infection nor in peripheral parasitemia. Similarly, vitamin A supplementation in children had no impact on malaria-specific mortality. Although prior evidence has shown that vitamin A reduces diarrhea and measles incidence, this review shows that it has no such protective effect either for malaria incidence or for malaria-specific mortality. Regarding treatment effects of vitamin A as an adjunct therapy, based on RCTs of children with cerebral or severe malaria, there was no difference between the groups in survival, fever and parasite clearance times, or in incidence of neurological sequelae or other complications. Similarly, no benefit was found for prevention of malaria. According to the best of our knowledge, this is the first systematic review and meta-analysis studying vitamin A supplementation for both prevention and treatment of malaria.
Different animal model and in vitro studies have been conducted to study the role of vitamin A supplementation in decreasing malarial parasitemia or infection intensity with conflicting results. An animal study [23] on mice showed that vitamin A supplementation alone had no effect on parasitemia development and even increased the risk when administered with diphtheria–tetanus–pertussis (DTP) vaccine. The authors had earlier concluded [24] that it depended on the pathogenesis of disease because vitamin A plus DTP tended to increase parasitemia and significantly suppressed cytokine responses in mice with cerebral malaria, but this was not the case in mice dying of anemia. Similarly, another murine model study [25] showed that retinol, when administered prior to Plasmodium berghei inoculation, reduced end-point parasitemia by 24% (p = 0.001 vs. controls) but was not effective when given both prior to and after infection (11% reduction, p = 0.47). This effect of retinol was mediated through increase in tissue and liver retinol stores rather than to changes in oxidant status. In an in vitro study of P. falciparum [26], all stages of parasite development including merozoite invasion were sensitive to retinol levels. There was evidence of membrane rupture of parasites and increased vacuolization of parasite food particles with retinol treatment. This indicated a potential antimalarial role of vitamin A as these effects were similar to those seen with quinoline and artemisinin compounds. In another animal study in rats with P. berghei infection [27], parasitemia developed at a faster pace in vitamin A, protein energy-undernourished rats compared with those supplemented with vitamin A. A higher number of red blood cells infected with the parasite was noticeable 6–7 days after the infection, at which point most of the animals in the former group died.
In contrast to mixed findings from animal studies, human cross-sectional and observational studies have more consistently indicated an inverse association between vitamin A supplementation and malarial parasitemia. In a cross-sectional study based on 39 individuals, there was an inverse correlation of retinol levels with parasitemia (r = −0.338, p = 0.035) [28]. In a recent observational national survey from sub-Saharan Africa [29], in more than 8000 children aged 6–59 months over an 18-month period, vitamin A supplements reduced parasitemia by 54% (RR = 0.46; 95% CI, 0.39–0.54) and P. falciparum-related antigenemia by 77% (RR = 0.23; 95% CI, 0.17–0.29). In a secondary analysis of a cluster-randomized trial of peer counseling for exclusive breastfeeding [30], among 483 infants aged between 3 and 12 months who did not receive vitamin A, there was a higher risk of prevalent parasitemia compared with those who received vitamin A after controlling for use of bed nets, age, and residence (prevalence ratio = 6.1; 95% CI, 2.1–17.6). Because of the study design and limited adjustment for confounding, the cause–effect relationship in these studies, however, remains unclear. Furthermore, our meta-analysis based on RCT evidence does not show a benefit or support the findings of these studies.
The main strengths of our review include a comprehensive, systematic search to identify RCTs on the topic, compiling evidence on both preventive and treatment roles of vitamin A in pregnancy and childhood, and conducting meta-analyses where more than one outcome was found—for placental malarial infection (for supplementation in pregnancy) and malaria-specific mortality (for supplementation in childhood). The main limitation was inability to distinguish prevalent versus incident peripheral parasitemia from published articles that precluded meta-analysis of this outcome.
5. CONCLUSION
We did not find any evidence of benefit of vitamin A supplementation during pregnancy on peripheral parasitemia or placental malarial infection. Similarly, the effects of supplementation in children also showed no benefit either in parasitemia or mortality rates due to malaria. Potential reasons should be explored as to why cross-sectional and observational studies have shown a benefit, whereas RCT evidence shows otherwise; moreover, differences in findings should be reconciled through more studies if needed.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
Cite this article
TY - JOUR AU - Mohammad Yawar Yakoob AU - Murad Qadir AU - Omm-e-Hany PY - 2018 DA - 2018/12/31 TI - Vitamin A Supplementation for Prevention and Treatment of Malaria during Pregnancy and Childhood: A Systematic Review and Meta-analysis JO - Journal of Epidemiology and Global Health SP - 20 EP - 28 VL - 8 IS - 1-2 SN - 2210-6014 UR - https://doi.org/10.2991/j.jegh.2018.04.104 DO - 10.2991/j.jegh.2018.04.104 ID - Yakoob2018 ER -
