Human Soil-transmitted Helminths and Lung Infections: A Guide Review for Respiratory Therapists

- DOI
- 10.2991/dsahmj.k.200916.002How to use a DOI?
- Keywords
- Parasites; soil-transmitted helminths; Ascaris lumbricoides; hookworm; Strongyloides stercoralis; lung; global health
- Abstract
Soil-transmitted helminths are among the neglected tropical diseases, although some 1.5 billion individuals are affected. Traveling to endemic regions, global immigration, and increase of immunocompromised cases, in addition to other factors, have an influence on global susceptibility to these parasites. These helminth parasites can affect the human respiratory system during their life cycles either directly or indirectly. A better understanding of all aspects related to these parasites will assist respiratory therapists in putting together a proper diagnosis and management. This review covers the morphology, life cycles, epidemiology, clinical aspects, glance about laboratory diagnosis, and control of soil-transmitted helminths.
- Copyright
- © 2020 Dr. Sulaiman Al Habib Medical Group. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Globally, the field of respiratory therapy is given more attention recently, and many universities established new bachelor programs among the Allied Medical Sciences Faculties. According to the World Health Organization (WHO), many parasitic infections are among the neglected tropical diseases. This review will be a start of a comprehensive series addressed to respiratory therapists to provide an overview of the important parasites involved as causative agents of lung infection. This paper discusses the soil-transmitted helminths and their impact on the lungs and other aspects.
The WHO estimated that globally more than 1.5 billion people are infected with soil-transmitted helminths. These helminths, which are roundworms related to the Phylum Nematoda, have among the highest infection rates in man, especially in tropical and subtropical areas [1]. Contaminated soil plays a vital role in these worms’ transmission and life cycles. The soil-transmitted helminths include Ascaris lumbricoides, hookworm (Ancylostoma duodenale and Necator americanus), Strongyloides stercoralis, and Trichuris trichiura. The first three parasites are commonly associated with lung infection and will be covered in this review (Figure 1). Very rare instances of pneumonitis caused by T. trichiura may occur when larvae penetrate the intestinal tissues and by way of the lymph and blood vessels reach the lungs [2].
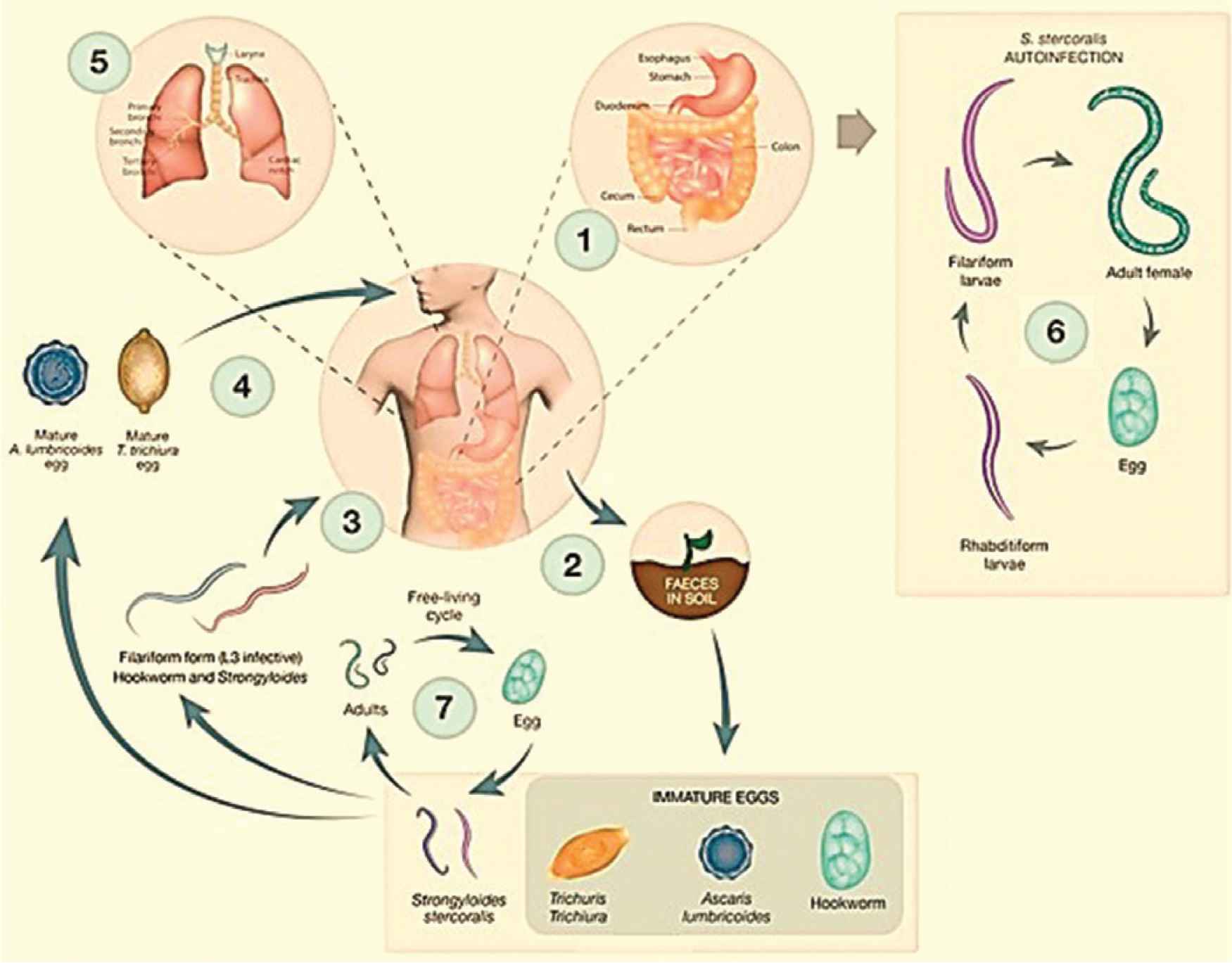
Life cycles of the human soil-transmitted helminths. Reproduced with modification from Gordon et al. [98]
2. ASCARIS LUMBRICOIDES
2.1. Introduction and Epidemiology
Ascaris lumbricoides belongs to the Family Ascarididae of the Suborder Ascaridina. It is the largest intestinal nematoda roundworm (male, 15–30 cm; female, 20–35 cm) and among the common helminths that infect humans. According to the WHO, ascariasis affects about 1 billion people worldwide in endemic countries, mainly children and adolescents [3,4]. Infection with A. lumbricoides is cosmopolitan, mainly in tropical areas with poor sanitation and personal hygiene. Those who live in areas where wastewater is used for irrigation, people defecate outdoors, or human feces are used as fertilizer, are at high risk of infection associated with the consumption of raw vegetables. Ascaris infection in the community is not age-dependent and can affect children, as well as young and elderly populations [5].
2.2. Life Cycle and Modes of Infection
Ascarislumbricoides transmission to humans is through direct fecal–oral transmission. It occurs mainly with soil contamination of hands or ingestion of contaminated foods or drinks containing mature second-stage larvated (embryonated) eggs. After hatching in the upper part of the small intestine, larvae penetrate the wall of intestinal mucosa, and transport to the vascular capillaries, then via the portal vein to the hepatic circulation. Some of the larvae can migrate to the liver directly through the intestinal wall. Larvae will molt to the third stage, then proceed to the right side of the heart, then to the pulmonary circulation [6]. They stay for several days, then make a trip across the alveolar wall and tracheobronchial tree to the larynx and esophagus to the small intestine. Here, larvae molt into the fourth stage and then finally become adults. After copulation, adult female worms begin to produce fertilized (fertile) eggs. Meanwhile, unfertilized (infertile) eggs can be seen in cases with absence of adult male worms. Fertilized eggs can survive and stay viable for several months or years depending on the surrounding warm and moist condition [3,5,6].
2.3. Pathology and Clinical Aspects
Most intestinal ascariasis cases are asymptomatic or with limited symptoms. Symptomatic cases depend on the worm burden especially in children. The common nonspecific symptoms include abdominal pain, diarrhea, weight loss, weakness, and anorexia [7]. Complications related to ascariasis may include anemia caused by upper gastrointestinal bleeding, intestinal obstruction, intestinal peritonitis, pancreatitis, acute appendicitis, acute cholecystitis, gastric ascariasis, and hematemesis [8–10].
When larvae accumulate in the lung during the second week of infection, eosinophilic pneumonia, or Loeffler syndrome, may occur because of hypersensitivity reactions to larval stages. Light infection is usually asymptomatic. The main symptoms of heavy infections include chest pain, dry cough, dyspnea with abnormal breath sounds, bloody sputum (hemoptysis), and in rare cases, pleuritis or pleural effusion. In addition, respiratory failure was associated with ascariasis in immunodeficient patients. Some larvae can cause complications in other parts of the body. This could lead to granulomatous peritonitis, atopic dermatitis, and eye infection [11–17].
2.4. Diagnosis
The diagnosis of intestinal ascariasis depends on the macroscopic detection of adult worms in stool sample, in addition to microscopic identification of eggs (fertilized or/and unfertilized) using direct smears, thick smears, sedimentation and flotation techniques. Abdomen ultrasonography, Computed Tomography (CT), magnetic resonance imaging, and X-ray can show the adult worms of A. lumbricoides in the gut.
Immunoglobulin IgG4 antibodies against Ascaris hemoglobin could be used as a supportive marker for diagnosis [18,19].
Chest CT and radiographs may illustrate bilateral pleural effusion, consolidation, patchy alveolar infiltrates, and unilateral or bilateral transient migratory fleeting nonsegmental opacities of various sizes often peripherally situated [20–23]. Examination of sputum, bronchoalveolar specimens or gastric secretions may detect larvae, eosinophils, and Charcot–Leyden crystals. Elevated levels of total serum Immunoglobulin E (IgE) are associated with several parasitic infections including Ascaris larval infection [24,25].
3. HOOKWORM
3.1. Introduction and Epidemiology
Human hookworm includes two species: A. duodenale and N. americanus. They get their common name from the hook-like teeth in the mouth part of adult worms. Hookworms are classified under the Suborder Strongylina and Family Ancylostimatidea. Hookworms infect an estimated 450–740 million people worldwide [4,26]. The size of adult worms is about 1 cm long (male, 8–11 mm; female, 10–13 mm). A. duodenale is distributed more focally, although its common name is “Old World hookworm.” The sizes of male and female adult worms are 7–9 and 9–11 mm, respectively, being a little smaller than the preceding species. Although the common name of N. americanus is “New World hookworm,” this species widely found across developing regions of South Asia and sub-Saharan Africa. In several studies, both species coexisted in the same region with higher prevalence for N. americanus [27]. Infection with hookworms starts gradually from childhood and reaches the highest level during late teens and early adulthood. Intensity stabilization then begins during all age levels unless effective control measures are involved [28–31].
In general, the severity of iron deficiency anemia and protein malnutrition caused by hookworms depends on the amount of blood loss [32], which is associated with the worm burden, and depends on the nutritional and iron status of the host prior to the infection.
3.2. Life Cycle and Modes of Infection
Humans are the principal final hosts of hookworms, and infected persons pass thousands of eggs in feces daily. Eggs mature in soil or any favorable warm moist conditions outside the host, forming rhabditiform larvae (L1 and L2) and finally filariform larvae (L3). Humans become infected via skin penetration (transcutaneous) with L3 through bare feet (mainly N. americanus) or via the oral route in the case of A. duodenale. As in the case of A. lumbricoides, the larvae migrate through blood or lymph stream to the heart and lungs. Larvae reach the small intestine after penetrating the alveolar wall to the trachea, esophagus, and stomach. The larvae molt and become mature adult worms in the small intestine within 2 months, and are soon ready to start lay eggs [31].
3.3. Pathology and Clinical Aspects
First, cutaneous dermatitis is caused during larval skin penetration. A red itchy papule is formed at the site of each penetration, the so-called “ground itch,” Scratching leads to secondary bacterial infection and formation of pustules. In rare cases, in heavy infections with extensive lesions the patients suffer from creeping eruption [33].
Filariform larvae that break through the pulmonary capillaries can cause bronchitis and bronchopneumonia. Larvae migration through pulmonary tissue may cause Loeffler syndrome, with fever, cough, wheezing, dyspnea, and hemoptysis [34]. Ingestion of a high number of larvae causes Wakana syndrome, which is characterized by eosinophilia and symptoms such as pneumonitis [26].
Adult worms inhabit the small intestine, mainly the upper part of the jejunum, biting the intestinal wall and sucking the mucosa with the cutting plates (N. americanus) or ventral teeth (A. duodenale). Symptoms are associated with continuous blood loss, intestinal inflammation, and mucosa necrosis. The patient may suffer from abdominal pain, diarrhea, fecal occult blood, and occasionally melena. In continuous blood loss in heavy infected cases, the patient suffers from severe iron deficiency, anemia with fatigue, short breath, headache, koilonychia, and rarely allotriophagy [35–40].
3.4. Diagnosis
Laboratory diagnosis during pulmonary infection depends on the detection and identification of L3 filariform larvae in sputum or bronchoalveolar lavage. Chest radiographs demonstrate transient pulmonary infiltrates, bronchitis, and transient nonsegmental consolidation [41].
Diagnosis of intestinal infection relies mainly on the detection of eggs in stool using direct iodine and concentration techniques [42,43]. In addition, rhabditiform and, in rare cases, filariform larvae can be observed in stool samples of constipation cases or delayed samples. Specimens from duodenum using Entero-capsule (duodenal string test) may detect the eggs and larvae [44].
4. STRONGYLOIDES STERCORALIS
4.1. Introduction and Epidemiology
This “threadworm’” is the smallest intestinal nematode. S. stercoralis are classified under the Suborder Rhabditina and Family Strongyloididae. The female adult worms occur in two different phases; the free living (1 mm × 65 µm) including the entire life cycle in the soil, and the parasitic (2 mm × 35 µm) generations in man, whereas all male adults (0.7 mm × 40 µm) are free living [45,46]. According to the WHO, strongyloidiasis affects up to 600 million people globally, and the risk of infection is associated with hygiene and walking barefoot, making children especially vulnerable to infection. Strongyloidiasis is among the most neglected tropical diseases, but was recently given more attention according to the WHO 2030 global target [1]. Infection with Strongyloides is not restricted to tropical and subtropical countries; it also seems to be endemic in temperate regions [47]. The infection could be serious in immunosuppression cases, certain malignancies, human T-cell lymphotropic virus type 1 infection, and alcoholism [48–53].
4.2. Life Cycle and Modes of Infection
The females of parasitic generations in infected final hosts inhabiting the mucosa of the duodenum or the jejunum lay eggs by parthenogenesis. These eggs grow promptly and hatch to become rhabditiform larvae (R-form) then pass in the stool. If the patient suffers from constipation or immunodeficiency, unpassed out R-form become infective filariform larvae (F-form), which invade the intestinal walls leading to internal autoinfection. Rarely in unclean persons, the larvae may penetrate the perianal skin leading to external autoinfection. Both types of autoinfection enable persistent infection for several years without any extrinsic exposure [48,54]. Meanwhile, under favorable conditions in soil, R-form passed in stool will continue the free living life cycle forming both male and female adult worms. Female worms then lay eggs, which mature and give rise exclusively to female R-form, then become infective F-form. These can infect humans both percutaneously (by penetration of the skin of the foot) and orally (by ingestion) [55,56], then enter the bloodstream and proceed to the heart and thereafter to the lung. The larvae migrate from the alveoli to the trachea, esophagus, stomach, then the mucosa of the small intestine, mainly in the duodenum, where they mature to female adult worms.
4.3. Pathology and Clinical Aspects
Percutaneous invasion by F-form larvae may give rise to dermatitis at the site of penetration. Like hookworm, itching and erythema are noticed, but in some cases, larvae can move over intracutaneously leading to “creeping eruption” [57–59].
Strongyloides stercoralis larvae gathering in the pulmonary tissue can cause cough and appear in the sputum. In addition, patients may present Loeffler syndrome with eosinophilia, hypoalbunemia, and elevated IgE. This can lead to dyspnea, bronchopneumonia, and massive bleeding caused by alveolar hemorrhage [60]. Acute Respiratory Distress Syndrome (ARDS) and septicemia related to intestinal transmural migration of bacteria can occur as a result of pulmonary hyperinfection or disseminated strongyloidiasis [61–63]. In addition, acute anemia, acute renal failure, and systemic inflammatory response syndrome are also reported in hyperinfection [64–66].
Symptoms of intestinal infection include diarrhea, abdominal discomfort, upper abdominal pain, and fever. In heavy chronic autoinfection cases, nausea, mucous with bloody stool, and anemia are seen. These symptoms are related to the destruction, damage, and mucosal inflammation caused by the adult and larval stages of the parasite, particularly in the duodenum and jejunum, but may take place in the bile and pancreatic ducts. In immunosuppressed patients, autoinfection progresses further to hyperinfection (in the lung and intestine) and disseminates into multiple organs, mainly the liver, brain, heart, and urinary tract [67–71]. Hyperinfection can become severe and even fatal or occur decades after the initial infection [72–75]. This is related to a deficient T helper 2 (Th2) immune response, so it is difficult to control the multiplication of huge number of larvae. Additional ulcers/lesion caused by F-form larvae, with possible destruction of muscular layers, which may lead to perforation, are also seen [76,77].
4.4. Diagnosis
During pulmonary involvement, chest X-ray, and CT findings include patchy, migratory airspace consolidation, with areas of cavitation with irregular walls. A miliary pattern has also been reported [78–81]. Sputum, bronchoalveolar lavage can be examined microscopically for larvae detection [82–85].
Stool is the best sample for laboratory diagnosis of intestinal infection. The main applicable techniques for detection of R-form larvae (the common diagnostic stage) are wet mount preparation and sedimentation techniques. Rarely, in certain cases, F-form larvae, adult worms, and eggs can be detected in stool samples. Moreover, several fecal cultures are available, but infrequently used. Duodenal specimens using Entero-Test are useful for detection of eggs, larvae, and/or parasitic female adult worms [44,86].
Hyperinfection cases can be diagnosed by identifying female adult worms, R-form larvae, F-form larvae, or eggs in biopsies from the affected organs. Enzyme-linked immunosorbent assay and polymerase chain reaction of blood and cerebrospinal fluid have shown reasonable results [87].
5. IMMUNE RESPONSE
Helminths antigens or helminth-derived Excretory/Secretory (ES) activate dendritic cells, which act as antigen-presenting cells, presenting the antigen to T cells to commence an immune response mediated by Th2 cells [88–91]. There are multiple mechanisms in which helminths trigger a type 2 immune response through the lymphatic/circulatory system. Prior to adult stages formation and establishment, the infective larvae invade the lungs or intestinal mucosa. This damage triggers tuft cells and other epithelial cells to secrete a group of alarmins, including thymic stromal lymphopoietin, Interleukin-33 (IL-33), and IL-25. These alarmins then promote the activation and differentiation of type 2 innate lymphoid cells and CD4 Th2 cells, leading to the release of many cytokines. IL-5 promotes eosinophilia, and in integration with IL-4, IL-9, and IL-13, in addition to IgE, which bind with high-affinity with Fc epsilon receptor, leading to activation of mast cells and basophils and release of inflammatory mediators (e.g., histamine and heparin). Furthermore, IL-4 and IL-13 lead to an increased contractility of smooth muscle cells, goblet cells, hypersecretion of mucus, and enhance intestinal permeability to help expulsion of adult parasites in the lumen. In addition, IL-4 and IL-13 activate the alternative activated macrophages, which inactivate the production of Th1, Th2, and Th17. These cytokines also promote isotype class switching in B cells to produce IgG.
6. CONTROL OF SOIL-TRANSMITTED HELMINTHS
The main key in adapting control programs for any parasite is to understand the life cycle and modes of infection. In simple terms, the goal of any control program is to cut or interrupt the life cycle of the parasite.
The first line of soil-transmitted helminthiasis control is treatment of infected people. The WHO supports and recommends regular and mass treatment using drugs such as albendazole, mebendazole, and ivermectin [1,6,92,93]. The WHO’s global target for morbidity control is to eliminate soil-transmitted helminths as a public health problem by the end of 2020 [94].
Second, use of educational materials to provide information about the modes of infections is very effective in reducing disease burden and prevention of infection. Awareness about proper disposal of fecal matter/use of latrines/avoid soil contamination, use of footwear, washing hands, proper food preparation, consumption of washed food, and eliminating the use of untreated human feces as fertilizer, are examples related to these fecal-borne parasites [95,96]. Improvement in water, sanitation, and hygiene has important short- and long-term impacts on infection rates. As these parasites can be transmitted to humans directly or indirectly, the community should therefore be supplied with information, particularly on appropriate sanitation and hygiene standards and cleanliness practices [95–97].
In addition, we believe that the role of large-scale screening by accurate clinical/diagnostic techniques and involving well-trained physicians/respiratory therapists/laboratory technologists have a significant impact on the management and control programs.
Table 1 outlines the symptoms, signs, investigation, and treatment of human soil-transmitted helminths involved in lung diseases.
| Parasite | Mode of infection | Clinical presentation | Radiological features | Laboratory tests | Treatment |
|---|---|---|---|---|---|
| Ascaris lumbricoides | Ingestion of larvated eggs in contaminated food or drink (fecal–oral) | Eosinophilic pneumonia, cough, wheeze, dyspnea, hemoptysis | Transient nodular or diffuse pulmonary infiltrates, basal opacities, spontaneous pneumothorax | Stool: adult worms and eggs | Mebendazole and albendazole |
| Pulmonary: larvae | |||||
| Blood: eosinophilia | |||||
| Hookworm | L3 larval skin penetration (and oral route in case of A. duodenale) | Eosinophilic pneumonia, cough, wheeze, dyspnea | Bronchitis, bronchopneumonia, transient pulmonary infiltrates, transient nonsegmental areas of consolidation | Stool: eggs and larvae | Mebendazole and albendazole |
| Pulmonary: larvae | |||||
| Blood: eosinophilia | |||||
| Strongyloides stercoralis | L3 larval skin penetration | Eosinophilic pneumonia, chest pain, fever, cough, wheeze, dyspnea, hyperinfection syndrome, ARDS, intra-alveolar hemorrhage | Bronchopneumonia, pulmonary infiltrates, miliary nodules, airspace opacities ARDS in severe disease, rarely granulomatous changes | Stool or duodenal specimens: larvae | Ivermectin and albendazole |
| Pulmonary: larvae | |||||
| Blood: eosinophilia |
Overview of soil-transmitted helminths and human lung infections
CONFLICTS OF INTEREST
The author declares no conflicts of interest.
REFERENCES
Cite this article
TY - JOUR AU - Majed H.M. Wakid PY - 2020 DA - 2020/09/21 TI - Human Soil-transmitted Helminths and Lung Infections: A Guide Review for Respiratory Therapists JO - Dr. Sulaiman Al Habib Medical Journal SP - 144 EP - 150 VL - 2 IS - 4 SN - 2590-3349 UR - https://doi.org/10.2991/dsahmj.k.200916.002 DO - 10.2991/dsahmj.k.200916.002 ID - Wakid2020 ER -
