A haplotype at the MMP-9 locus is associated with high-blood pressure and arterial stiffness in patients with essential hypertension
- DOI
- 10.1016/j.artres.2009.01.002How to use a DOI?
- Keywords
- MMP-9; Polymorphism; Haplotype; Arterial stiffness; Aortic pulse wave velocity; Hypertension; Augmentation index
- Abstract
Background: Arterial stiffness is an independent predictor of cardiovascular events in hypertensive populations and is in part a heritable trait. Matrix metalloproteinase (MMPs) plays an important role in vascular remolding. MMP-9 levels predict cardiovascular risk and are associated with aortic stiffness. We investigated the influence of two MMP-9 polymorphisms (−1562C > T, 836G > A) on arterial stiffness and blood pressure in hypertensive subjects.
Methods: MMP-9 genotypes and plasma MMP-9 concentrations were determined in untreated patients (n = 217, mean age 46 ± 1 years). Supine blood pressure, carotid–femoral pulse wave velocity (PWV) and augmentation index (AIx) were assessed.
Results: Blood pressure and aortic PWV were higher in T allele carriers of the −1562C > T polymorphism and the A allele carriers of the 836G > A polymorphism. The two polymorphisms had a significant gene dose-dependent effect on PWV (p < 0.01). The −1562C/836A (AC) haplotype was the most frequent (58%). All haplotypes containing either −1562T or 836A alleles had significantly higher blood pressure and PWV compared with haplotypes that contained neither allele (p < 0.0001). These polymorphisms were also associated with higher aortic PWV after correction for confounding variables. In stepwise regression models, genetic variants emerged as independent determinants of PWV in addition to age; mean arterial pressure and heart rate (r2 = 0.45, p < 0.0001).
Conclusions: Aortic PWV and blood pressure were modulated by −1562C > T and −836G > A polymorphisms in the MMP-9 gene in this treatment naive hypertensive population. These genetic polymorphisms may help to identify hypertensive patients at increased risk of cardiovascular events.
- Copyright
- © 2009 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Increased arterial stiffness and augmented wave reflection have been shown to play an important role in the pathophysiology of hypertension, particularly systolic blood pressure (BP). Arterial stiffness, measured as carotid femoral pulse wave velocity (PWV), has been shown to be a marker of cardiovascular risk in the general population1 and an independent prognosticator of cardiovascular events in hypertension,2 stroke3 and end stage renal disease.4 Augmentation index (AIx), a measure of aortic wave reflection in the aorta and a global index of arterial stiffness, predicted all cause and cardiovascular mortality in patients with end stage renal disease5,6 and the extent of angiographic coronary artery disease (CAD) in men under the age of 60 years.7
Matrix metalloproteinase (MMP) are a family of zinc-containing enzymes with proteolytic activity against extracellular matrix components such as elastin, proteoglycans, and collagen in both physiologic and pathologic processes.8 MMPs play an essential role in vascular remodeling to allow blood vessels to change in size and structure for adaptation and repair. However, with excessive MMP expression and activity, inappropriate cardiovascular remodeling may occur resulting in arteriosclerosis, aneurysm formation and restenosis.9–11 MMP-9 (gelatinase B) is capable of degrading gelatin, fragments of collagen degraded by collagenase and type IV collagen, which forms part of the basement membrane. Circulating MMP-9 levels are increased in patients with isolated systolic hypertension,12 in type 2 diabetic patients with CAD13 and elevated MMP-9 levels are associated with premature coronary arteriosclerosis.14 Recently, plasma MMP-9 levels were identified as a predictor of cardiovascular mortality in patients with CAD15 and have been shown to be related to aortic stiffness.12
A number of single nucleotide polymorphisms (SNP) have been identified in regulatory and coding regions of the MMP-9 gene. Some of them have been reported to influence in vitro MMP-9 expression levels, enzymatic activity and susceptibility to various inflammatory and fibrotic conditions.16 A functional −1562C > T polymorphism in the promoter region of MMP-9 has been shown to relate to the presence and severity of coronary arteriosclerosis17 and associated with arterial stiffness in patients with CAD18 and healthy subjects.19 We have shown previously that the −1562C > T polymorphism is associated with arterial stiffness and BP.20 Another functional polymorphism 836G > A located in the exon 6 of the MMP-9 gene, leads to the substitution of a positively charged amino acid (arginine) by an uncharged amino acid (glutamine) possibly enhancing substrate binding.21–23
We tested the hypothesis that genetic variation of −1562C > T in the promoter and 836G > A in the coding regions of MMP-9 may play an independent role in modulating BP, arterial stiffness and plasma MMP-9 levels in a population of never-treated patients with essential hypertension.
Methods
Patient population
We studied 217 never-treated Caucasian, apparently healthy hypertensive subjects with a diagnosis of essential hypertension based on three outpatient measures of BP greater than 140/90 mmHg and confirmed by ambulatory BP > 135/85 mmHg. The mean ± SEM age was 46 ± 1 years and 108 were male. Patients with a clinical history or electrocardiographic evidence of CAD, peripheral vascular disease, cerebrovascular disease, valvular heart disorders, cardiac rhythm disturbances and signs and symptoms of congestive heart failure, vasculitis, malignancies and other major systemic illnesses were excluded. The patients gave informed consent, the study had institutional ethics committee permission and the procedures followed were in accordance with institutional guidelines and the principles of the Declaration of Helsinki.
Study protocol
Height and weight were recorded and body mass index (BMI) calculated. The patients were studied in a quiet room having abstained from smoking, alcohol or caffeinated beverages in the 12 h prior to the study. The haemodynamic measurements were performed after a supine rest of 15 min.
Blood pressure measurement
Blood pressure was measured in the right arm using an automated oscillometric device (Omron Model HEM 705-CP, Omron Corporation, Tokyo, Japan). Three BP readings were taken at 1-min intervals and the mean used for data analysis. Peripheral pulse pressure was calculated as the difference between systolic and diastolic BP.
Assessment of arterial stiffness and wave reflection
Carotid–femoral PWV was measured on the right side in the supine position using an automatic device (Complior, Artech Medical, France) as described previously.20 Radial pressure waveforms were recorded with applanation tonometry and a corresponding aortic pressure waveform generated using a validated generalized transfer function (SphygmoCor, Version 8.0, AtCor Medical, Australia) as described previously.20 The aortic pressure waveform was used to calculate the augmentation index (AIx) as the difference in height between the first and second aortic systolic peaks expressed as a percentage of aortic pulse pressure.
Laboratory methods
Blood samples were drawn and the serum separated and stored at −80 °C. Total cholesterol, serum triglycerides and high-density lipoprotein (HDL) cholesterol and plasma glucose were measured using standard methods. Plasma MMP-9 concentrations were determined using commercially available ELISA assays (Quantikine, R&D Systems, Minneapolis, USA) according the manufacturer’s instructions. The detection limit of the MMP-9 assay was 0.156 ng/ml.
Genetic analysis
The MMP-9 −1562C > T polymorphism using polymerase chain reaction (PCR) – restriction fragment length polymorphism (RFLP). The MMP-9 promotor polymorphism −1562C > T was amplified from 100 ng of genomic DNA using the forward (5′-GCCTGGCACATAGTAGGCCC-3′) and reverse (5′-CTTCCTAGCCAG CCGGCATC-3′) oligonucleotide primers. PCR conditions included predenaturation at 95 °C for 15 min followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 65 °C for 1 min 30 s, extension at 72 °C for 1.5 min and a final extension at 72 °C for 10 min. The amplified PCR product (435 bp) was then digested with 10 U of the restriction enzyme, BbU overnight (Promega, USA) and the product run on a 2% agarose gel stained with ethidium bromide. CC homozygotes showed a single band at 435 bp, CT heterozygotes showed bands of 435, 247 and 188 bp, while TT homozygotes showed band sizes of 247 and 188 bp. Successful genotyping of the MMP-9 C/T polymorphism was achieved for all 261 cases.
The MMP-9 836G > A polymorphism was screened using restriction fragment length polymorphism (RFLP). An area surrounding the polymorphism was amplified by PCR using the oligonucleotide primers: forward (5′-CTCGCCCCAGGACTCTACA C-3′) and reverse (5′-GTGGAGGTACCTCGGGTCGGG-3′) PCR using thermo-start Taq (ABgene) and PCR conditions included enzyme activation at 95 °C for 15 min followed by 30 cycles of 95 °C for 30 s, 69 °C for 30 s, and 72 °C for 1 min, and a final extension at 72 °C for 10 min. The PCR product was digested with the restriction enzyme BsoBI (Biolabs, UK) and run on a 2% agarose gel stained with ethidium bromide. As one of the digest bands is only 15 bp, it cannot be shown on the agarose gel. GG homozygotes showed a single band of 194 bp, GA heterozygotes showed bands of 194, 179, while AA homozygotes showed band sizes of 179 only. Successful genotyping of the MMP-9 G > A polymorphism was achieved for 217 cases.
Statistical analysis
Data were analyzed using JMP version 5.0 (SAS for Windows, Cary, NC, USA). Results are expressed as mean ± SEM for continuous variables and percentages for categorical data, p < 0.05 was considered significant. Differences between continuous variables were analyzed using one-way analysis of variance (ANOVA) and categorical variables with X2 testing. Stepwise multiple regression analysis was performed to analyze the relationship between the two MMP-9 polymorphisms, blood pressure and arterial stiffness adjusting for known or likely determinants of these variables in our patient population. Calculations of LD measurements (D′, r2) were performed using HAPLOVIEW24 and haplotype association tests were performed using HAPLOSCORE,25 which corrects for the ambiguity of phase reconstruction in association studies.
Results
The frequencies of the −1562C > T genotypes were CC: 68%, CT: 29% and TT: 3%. Allele frequencies were C: 82.6% and T: 17.4%. The frequencies of the 836G > A genotypes were GG: 14.1%, GA: 45.9% and AA: 40%. Allele frequencies were G: 37% and A: 63%. Neither genotype distributions differed significantly from that predicted by Hardy–Weinberg equilibrium.
Demographic and clinical characteristics of the patient population according to the MMP-9 −1562C > T and 836G > A genotype are given in Table 1. There were no significant differences in age, gender ratio, BMI, smoking status, lipids, glucose and creatinine levels according to the genotype.
| SNP 836 | SNP 1562 | |||||||
|---|---|---|---|---|---|---|---|---|
| GG (n = 31) | GA (n = 99) | AA (n = 87) | p | CC (n = 147) | CT (n = 64) | TT (n = 6) | p | |
| Age (years) | 45 ± 2 | 45 ± 1 | 47 ± 1 | 0.32 | 45 ± 1 | 47 ± 2 | 53 ± 3 | 0.33 |
| Gender, male (%) | 66% | 45% | 49% | 0.18 | 52% | 40% | 83% | 0.20 |
| BMI (kg/m2) | 30 ± 1 | 30 ± 0.5 | 29 ± 0.5 | 0.25 | 29 ± 0.4 | 30 ± 1 | 29 ± 2 | 0.46 |
| Waist (cm) | 98 ± 3 | 96 ± 1.5 | 95 ± 1.5 | 0.62 | 96 ± 1 | 96 ± 2 | 100 ± 5 | 0.59 |
| Hip (cm) | 107 ± 2 | 110 ± 1 | 104.5 ± 1 | 0.02 | 106 ± 1 | 109 ± 2 | 106 ± 3 | 0.13 |
| Smokers (%) | 20% | 29% | 27% | 0.59 | 28% | 32% | 33% | 0.77 |
| Total Cholesterol (mmol/l) | 5 ± 0.2 | 5.2 ± 0.1 | 5.1 ± 0.1 | 0.94 | 5 ± 0.1 | 5.2 ± 0.1 | 4.5 ± 0.4 | 0.18 |
| HDL cholesterol (mmol/l) | 1.3 ± 0.04 | 1.4 ± 0.04 | 1.3 ± 0.04 | 0.25 | 1.3 ± 0.03 | 1.4 ± 0.1 | 1.2 ± 0.1 | 0.49 |
| Triglycerides (mmol/l) | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.9 ± 0.2 | 0.60 | 1.6 ± 1 | 1.8 ± 0.1 | 1.5 ± 0.5 | 0.69 |
| Glucose (mmol/l) | 5.8 ± 0.3 | 5.5 ± 0.2 | 5.4 ± 0.1 | 0.71 | 5.3 ± 0.1 | 5.6 ± 0.2 | 6.5 ± 1.2 | 0.23 |
| Creatinne (μmol/l) | 86 ± 4 | 85 ± 1 | 84 ± 2 | 0.43 | 84 ± 1 | 85 ± 2 | 88 ± 5 | 0.80 |
| Brachial Systolic BP (mmHg) | 145 ± 3 | 153 ± 2 | 158 ± 2 | 0.004 | 151 ± 1 | 159.5 ± 2 | 167 ± 5 | 0.006 |
| Brachial Diastolic BP (mmHg) | 87 ± 2 | 90 ± 1 | 92.5 ± 1 | 0.02 | 89 ± 1 | 93 ± 1 | 97 ± 4 | 0.04 |
| Pulse Pressure (mmHg) | 58 ± 2 | 63 ± 1.5 | 65 ± 1.5 | 0.04 | 62 ± 1 | 66 ± 2 | 70 ± 4 | 0.06 |
| Mean Arterial Pressure (mmHg) | 106 ± 2 | 111.5 ± 1 | 114.5 ± 1 | 0.005 | 110 ± 1 | 115 ± 2 | 120 ± 4 | 0.009 |
| PWV (m/s) | 9 ± 0.4 | 10 ± 0.2 | 10.4 ± 0.2 | 0.003 | 9.8 ± 0.1 | 10.6 ± 0.3 | 11 ± 0.9 | 0.004 |
| AIx (%) | 23 ± 2.5 | 29 ± 1 | 31 ± 1 | 0.03 | 29 ± 1 | 29 ± 1 | 27 ± 2 | 0.94 |
| Heart rate (min−1) | 70 ± 2 | 69 ± 1 | 70.5 ± 1 | 0.38 | 71 ± 1 | 70 ± 2 | 69.5 ± 3 | 0.95 |
| MMP-9 level (ng/mL) | 75 ± 10 | 90 ± 10 | 125 ± 19 | 0.13 | 81 ± 9 | 97 ± 11 | 213 ± 53 | 0.006 |
Clinical characteristics of the hypertensive patients according to MMP-9 genotype at positions 836 and 1562 (n = 217, mean ± SEM).
Association between MMP-9 −1562C > T and 836G > A polymorphisms and blood pressure
For the −1562C > T polymorphism, systolic and diastolic BP were significantly higher in TT homozygotes compared with CT heterozygotes and CC homozygotes as reported previously (Table 1).20 Similarly, for the 836G > A polymorphism, both systolic and diastolic BP were significantly higher in AA homozygotes compared with GA heterozygotes and GG homozygotes (Table 1). Both polymorphisms exhibited a gene–dose effect on systolic (p < 0.01), diastolic (p < 0.05) and mean arterial pressure (p < 0.01) (Table 1). As shown previously for the −1562C > T polymorphism,20 −836G > A polymorphism emerged as independent determinant of both systolic and diastolic BP (Tables 2 and 3). When both polymorphisms were included in the regression model, each emerged as an independent predictor of BP (Table 4) with greater contribution from the 836G > A polymorphism.
| −1562C > T | r2 = 47%, p < 0.0001 | |||
|---|---|---|---|---|
| Parameters | β | Standard Error | r2 Change (%) | Significance |
| Age (y) | 0.07 | 0.008 | 33 | <0.0001 |
| Mean arterial pressure (mmHg) | 0.05 | 0.006 | 12 | <0.0001 |
| Heart Rate (min−1) | 0.02 | 0.008 | 2 | <0.01 |
| −1562C > T | 0.21 | 0.10 | 1 | <0.01 |
| −836G > A | r2 = 45%, p < 0.0001 | |||
|---|---|---|---|---|
| Parameters | β | Standard Error | r2 Change (%) | Significance |
| Age (y) | 0.07 | 0.008 | 28 | <0.0001 |
| Mean arterial pressure (mmHg) | 0.05 | 0.008 | 13 | <0.0001 |
| Heart Rate (min−1) | 0.02 | 0.009 | 2 | <0.01 |
| −836G > A | 0.28 | 0.14 | 3 | <0.01 |
Smoking status, total cholesterol, fasting glucose and creatinine levels did not enter the model.
Results of the stepwise multiple regression analysis with aortic PWV-dependent variable (n = 217).
| A. Systolic Blood Pressure r2 = 26%, p < 0.0001 | ||||
|---|---|---|---|---|
| Parameters | β | Standard Error | r2 Change (%) | Significance |
| Age (y) | 0.55 | 0.09 | 13 | <0.0001 |
| 836G > A | −6.3 | 1.7 | 4 | <0.001 |
| Heart rate (min−1) | 2.8 | 1.1 | 3 | <0.001 |
| Gender (female) | −3.4 | 1.6 | 3 | <0.01 |
| Body mass index (kg/m2) | −0.48 | 0.22 | 1 | <0.05 |
| B. Diastolic Blood Pressure r2 = 15%, p < 0.0001 | ||||
|---|---|---|---|---|
| Parameters | β | Standard Error | r2 Change (%) | Significance |
| Gender (female) | −3.3 | 1.6 | 6 | <0.0001 |
| 836G > A | −3.0 | 1.0 | 4 | <0.01 |
| Heart rate (min−1) | 0.18 | 0.06 | 3 | <0.001 |
Smoking status, total cholesterol, fasting glucose and creatinine levels did not enter the model.
Results of the stepwise multiple regression analysis with systolic (A) and diastolic (B) blood pressure as the dependent variables (n = 217).
| A. Systolic Blood Pressure r2 = 30%, p < 0.0001 | ||||
|---|---|---|---|---|
| Parameters | β | Standard Error | r2 Change (%) | Significance |
| Age (y) | 0.51 | 0.09 | 13 | <0.0001 |
| 836G > A | −6.1 | 1.6 | 4 | <0.001 |
| Heart rate (min−1) | 0.32 | 0.9 | 4 | <0.001 |
| Gender (female) | −3.2 | 1.1 | 4 | <0.01 |
| −1562C > T | −0.40 | 1.2 | 2 | <0.01 |
| Body mass index (kg/m2) | −0.50 | 0.21 | 2 | <0.05 |
| B. Diastolic Blood Pressure r2 = 16%, p < 0.0001 | ||||
|---|---|---|---|---|
| Parameters | β | Standard Error | r2 Change (%) | Significance |
| Gender (female) | −2.8 | 0.69 | 6 | <0.0001 |
| 836G > A | −3.0 | 0.76 | 7 | <0.001 |
| −1562C > T | −2.1 | 0.77 | 3 | <0.01 |
Smoking status, total cholesterol, fasting glucose and creatinine levels did not enter the model.
Results of the stepwise multiple regression analysis with systolic (A) and diastolic (B) blood pressure as the dependent variables (n = 217).
MMP-9 −1562C > T and 836G > A polymorphisms and arterial stiffness
Aortic PWV was significantly higher in −1562TT homozygotes and 836AA homozygotes (p < 0.01) (Table 1). There was a significant gene–dose effect of both the −1562C > T (p = 0.02) and 836G > A (p = 0.003) on aortic PWV. In a stepwise multiple regression model, with PWV as the dependent variable and the known or likely confounders of arterial stiffness as independent variables, age and mean arterial pressure emerged as independent determinants of aortic PWV with a significant contribution from both the −1562C > T and 836G > A polymorphisms (Table 3). When the two polymorphisms were included in the regression model, both emerged as independent determinants of aortic PWV, further confirming that the observation was not due to chance alone.
The AIx was significantly higher in the presence of the A allele of the −836G > A polymorphism compared with GG homozygotes (Table 1). However, when AIx was adjusted for major confounders such as age, gender, mean arterial pressure, heart rate, height and smoking status, the −836G > A did not emerge as an independent determinant of AIx.
MMP-9 −1562C > T polymorphism and plasma MMP-9 levels
Mean plasma MMP-9 levels were higher in subjects carrying the −1562T allele (TT & CT) compared with CC homozygotes (112 ± 13 vs. 78 ± 9, p = 0.03) and the difference was more significant after adjusting for age, gender and MAP (136 ± 5 vs. 93 ± 3, p < 0.001). Also, there was a dose-gene effect of the −1562C > T polymorphism on the MMP-9 levels (Table 1). There was no significant relationship between the 836G > A polymorphism and serum MMP-9 levels.
MMP-9 haplotypes
The effects of the A allele of the MMP-9 836 and the T allele of the MMP-9 −1562 polymorphisms on BP and arterial stiffness were of a similar magnitude (13 and 14 mmHg differences between homozygotes in systolic BP and 1.4 and 1.3 m/s differences in PWV, respectively). The two SNPs were in modest linkage disequilibrium (D′ = 0.32, r2 = 0.03, p = 0.04). The haplotype distribution was AC: 58%, GC: 24%, GT: 13% and AT: 5%. The effects of the two genotypes were additive, with a direct linear relationship observed between the number of −1562T and 836A alleles carried and systolic BP (p < 0.001) (Fig. 1), diastolic BP (p < 0.001), PWV (p = 0.001) (Fig. 2) and MMP-9 levels (p < 0.01) (Fig. 3). Only 3 subjects (1.3%) were homozygous for both the −1562T and the 836A alleles. Twenty-three (11%) of the study population had neither at-risk allele, −836GG nor −1562CC genotype which is unambiguous for gametic phase (only GC haplotype is possible). These subjects with this protective GC haplotype had significantly lower PWV and systolic BP (both p < 0.05) than any of the other genotypes containing one or more of the at-risk alleles (Figs. 1 and 2). Even though the global haplotype score is not significant for PWV (global-stat = 6.89723, df = 3, p = 0.07525) adjusted for age, gender and MAP, one of the haplotypes AT, does seem to be associated (p = 0.033).
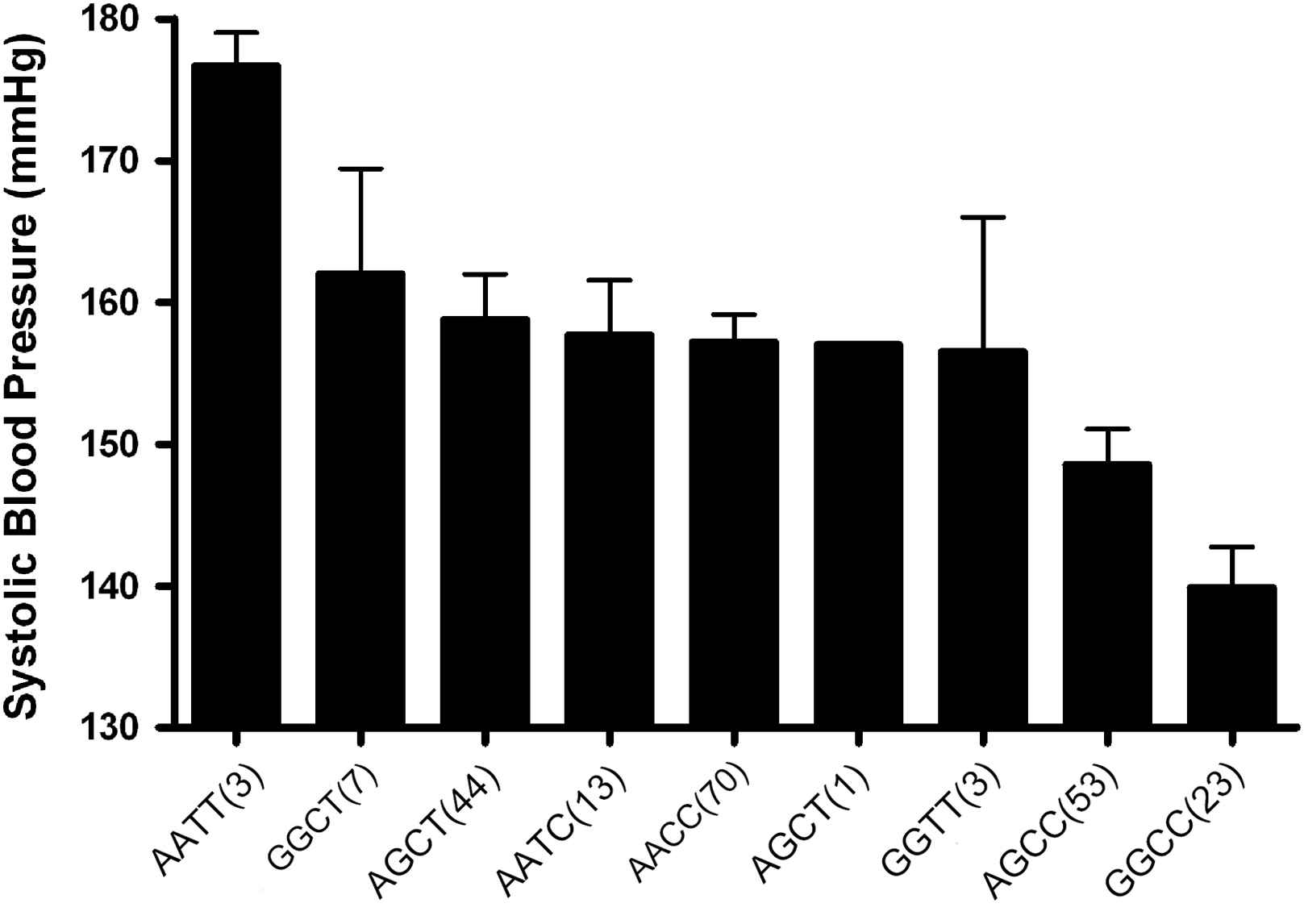
MMP-9 whole genotypes influence on systolic blood pressure in a hypertensive population. The MMP-9 genotypes have a significant dose-dependent effect on systolic blood pressure (p < 0.001), independent of confounding factors. Numbers in parenthesis indicate number of subjects in each genotype (mean ± SEM).
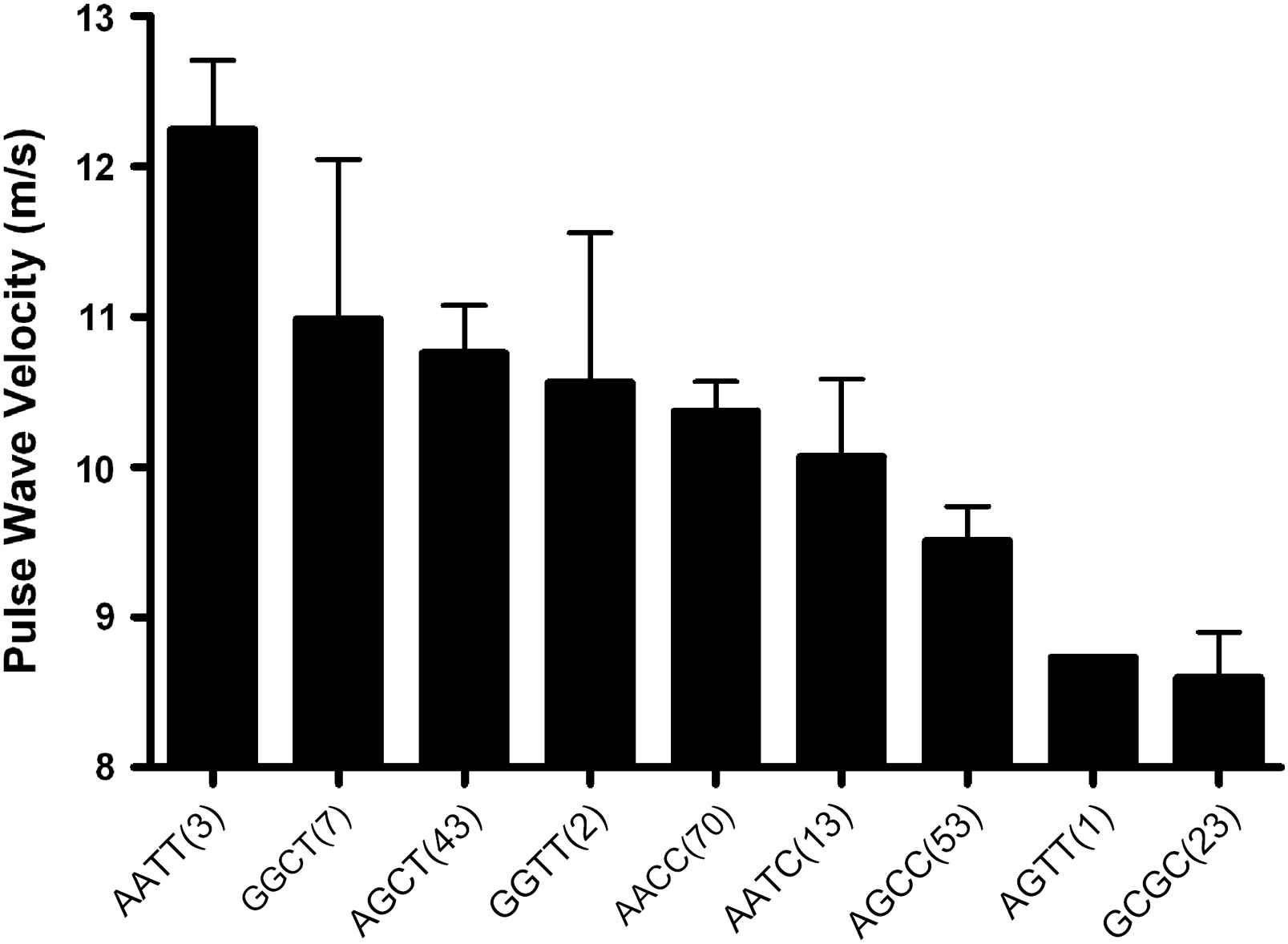
MMP-9 whole genotypes influence on aortic pulse wave velocity in a hypertensive population. The MMP-9 genotypes have a significant dose-dependent effect on pulse wave velocity (p < 0.001) independent of confounding factors. Numbers in parenthesis indicate number of subjects in each genotype (mean ± SEM).
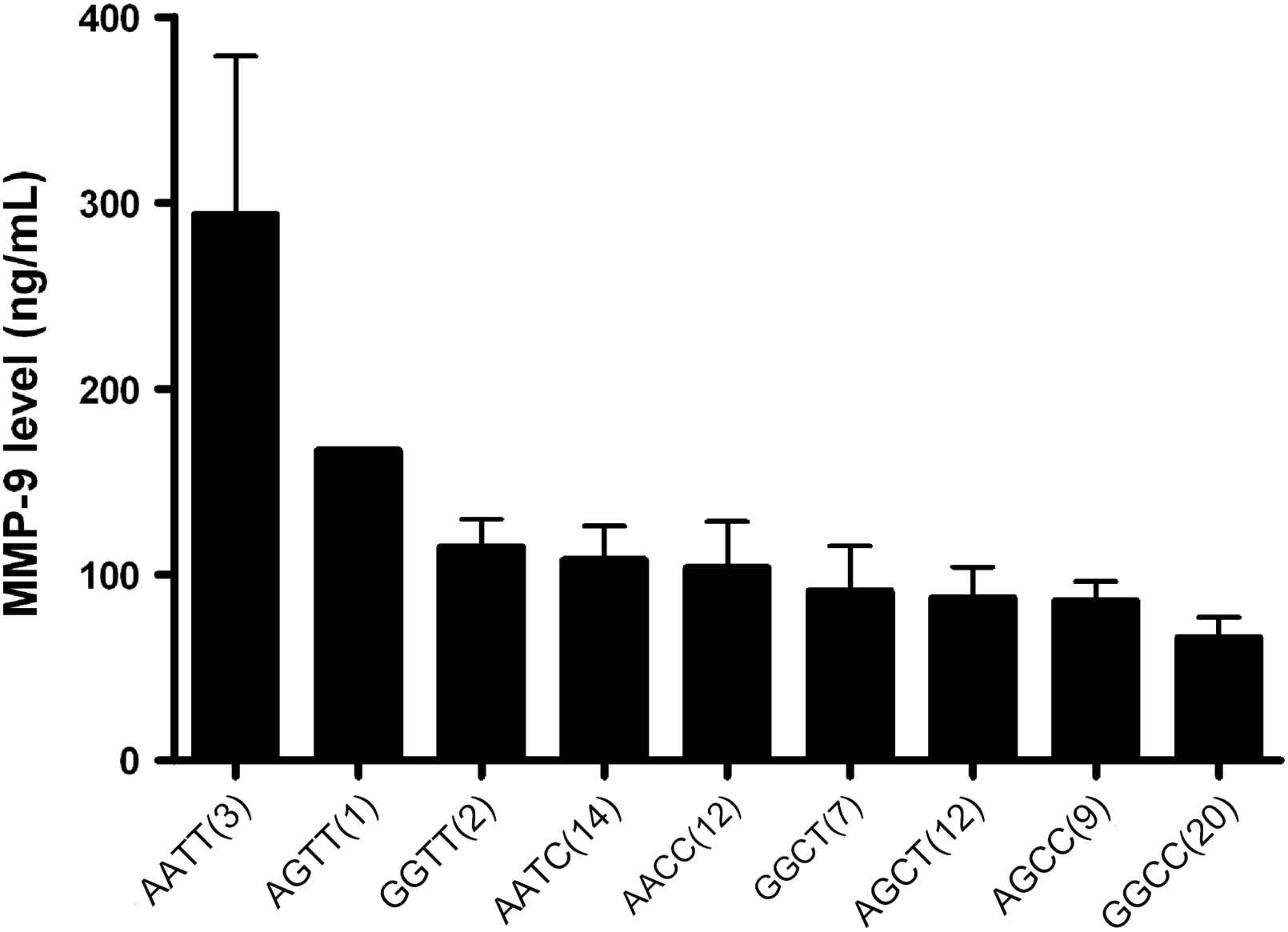
MMP-9 whole genotypes influence on serum MMP-9 levels in a hypertensive population. The MMP-9 genotypes have a significant dose-dependent effect on pulse wave velocity (p < 0.001) independent of confounding factors. Numbers in parenthesis indicate number of subjects in each genotype (mean ± SEM).
Discussion
The present study is the first to explore the relationship between haplotypes of the MMP-9 gene −1562C > T promoter region and 836G > A (coding region), BP, arterial stiffness, and MMP-9 levels in never-treated hypertensive patients. We found that PWV and BP were influenced by both polymorphisms in the MMP-9 gene with a significant gene–dose effect. Furthermore, both polymorphisms were independent predictors of both systolic and diastolic BP in these patients with additive effects seen for carriers of both at-risk alleles. In addition, the −1562C > T polymorphism was an independent determinant of serum MMP-9 levels in our study population, with higher MMP-9 levels seen in −1562-T allele carriers with a significant gene–dose effect.
Aortic stiffness, measured as PWV, is an independent prognosticator of cardiovascular morbidity and mortality in healthy1 and hypertensive populations.2,3,26 Circulating MMP-9 levels are increased in hypertension27 and predict cardiovascular risk.15 Genetic variation in the MMP-9 gene is associated with severity of vascular disease15,17 and large artery stiffness.18,19 Medley et al showed increased aortic stiffness, measured as aortic input and characteristic impedance, pulse pressure and MMP-9 expression in T allele carriers of the −1562C > T polymorphism in patients with CAD.18 Yasmin et al also showed increased PWV and MMP-9 levels in T allele carriers, however, they did not observe any significant difference in BP between the three genotypes in a normotensive population.19 In contrast, we have shown a significant gene–dose effect of both the MMP-9 polymorphisms not only on aortic PWV but also on BP. Both the −1562C > T and 836G > A polymorphisms were independent predictors of arterial stiffness after adjustment for factors known to influence PWV.
The higher aortic stiffness and BP in the T-allele carriers of −1562C > T and G alleles carriers of 836G > A may be secondary to excessive degradation of the arterial elastin matrix. The stiffness of the vascular wall is determined by the relative concentrations of collagen and elastin which are kept tightly regulated by a balance between production and degradation in the extracellular matrix. MMP-9 is thought to be involved in destruction of the arterial media and plaque growth.28 Moreover, targeted deletion of the MMP-9 gene in mice attenuates collagen accumulation and enhanced expression of other MMPs after myocardial infarction, suggesting that MMP-9 plays a prominent role in extracellular matrix remodeling.29
MMP-9 genotypes may influence BP and aortic stiffness either through enhancing plasma MMP-9 levels or by altering the qualitative function of the MMP-9 gene product. Because of its location in the promoter, i.e., regulatory region, of the MMP-9 gene, the −1562 polymorphism might be expected to influence levels of gene expression. This was born out in the present study, which found that the T allele was associated with significantly higher plasma levels of MMP-9. This finding is consistent with previous studies, in which the presence of the T-allele was associated with increased plasma MMP-9 levels in CAD and healthy subjects.18,19 In these studies, higher MMP-9 expression was also associated with higher aortic stiffness.18,19 We have shown in a hypertensive population that the presence of the T-allele conferred increased BP and arterial stiffness associated with higher plasma MMP-9 levels with a significant gene–dose effect, with heterozygotes exhibiting levels intermediate between those of CC and TT homozygotes.25
In contrast, the 836 exonic variant is more likely to have a qualitative effect on MMP-9 function, which might explain the lack of association with plasma MMP-9 levels in this study, despite a clear influence on blood pressure and arterial stiffness. The G-836A mutation leads to the substitution of a positively charged amino acid (arginine) by an uncharged amino acid (glutamine) at position 279 within the MMP9 active site. The substitution site is located in the catalytic domain of the MMP-9 gene, particularly in the fibronectin type II domains which confers MMP-9 with high affinity binding to type IV collagen, type I gelatin and elastin.2,3 The digestion of type IV collagen in the epithelial basement membrane has been suggested to be a key regulatory event in the initiation of fibrosis. A plausible explanation of these results is that G-836A represents a partial higher activity mutation within the proteinase whose presence accelerate the development of fibrosis; these findings implicate MMP-9 as a key molecule in the pathogenesis of arterial stiffness. The higher expression and/or activity of MMP-9 associated with these gene variants may be directly involved in promoting stiffness of arteries through vascular remodeling. Animal studies using MMP-9-deficient carotid artery cells have demonstrated that MMP-9 may influence arterial remodeling not only through matrix degradation but also through reorganization.30 These theories provide a potential explanation for the observed increased aortic PWV and BP in the T and A allele carriers.
Recently, haplotype association analysis has been proposed as a more powerful tool for detecting genetic variation than individual SNPs in complex clinical conditions,31,32 as in this case of essential hypertension. In our study, the two polymorphisms were in linkage and both the genotypes influenced the BP and PWV with evidence of a gene–dose effect. The combined effect of the two genotypes was additive with homozygotes for both the T and A alleles having the highest BP and arterial stiffness. Although both genotypes were associated with similar absolute differences in BP and PWV, the higher frequency of the 836-A allele (63%) vs. the −1562-T allele(18%), means that at a population level, the 836-A allele is likely to be of greater significance. Looked at another way, the haplotype analysis revealed a protective GC haplotype (for which one in 10 of our population were homozygous) that was associated with significantly lower BP and PWV. Our findings support the notion that genetic variations in the MMP-9 gene can influence the MMP-9 activity, which can lead to higher BP and arterial stiffness.
There are certain limitations in this study. The results have to be interpreted with caution as the numbers are small. While we have observed an association between MMP-9 genetic variants and both BP and arterial stiffness, this does not suggest a causal relationship. Although we have adjusted for known confounding factors, the influence of those unknown cannot be ruled out. Finally, this study was conducted in a hypertensive population and the results may not be applicable to other populations.
However, despite these caveats, this is the first study to observe a direct relationship between MMP-9 gene polymorphisms and their haplotypes with MMP-9 levels and arterial stiffness in untreated hypertensive patients. While Yasmin et al19 showed that a MMP-9 haplotype is associated with increased PWV but similar BP in healthy individuals, our results demonstrate that in hypertensive patients, presence of the T allele of −1562 and A allele of −836 polymorphism conferred increased BP and arterial stiffness.
References
Cite this article
TY - JOUR AU - Azra Mahmud AU - Sixiang Zhou AU - Anthony W. Ryan AU - Paula Jerrard-Dunne AU - John Feely PY - 2009 DA - 2009/02/08 TI - A haplotype at the MMP-9 locus is associated with high-blood pressure and arterial stiffness in patients with essential hypertension JO - Artery Research SP - 17 EP - 23 VL - 3 IS - 1 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2009.01.002 DO - 10.1016/j.artres.2009.01.002 ID - Mahmud2009 ER -
