Reducing arterial stiffness and wave reflection – Quest for the Holy Grail?
First presented at Artery 6, Athens, Greece, 22–23 September 2006
- DOI
- 10.1016/j.artres.2007.03.001How to use a DOI?
- Keywords
- Arterial stiffness; Wave reflection; Pulse wave velocity; Augmentation index; Anti-hypertensive drugs; Vasodilators
- Abstract
Arterial stiffness and wave reflection are fast emerging as therapeutic targets in their own right. While thiazide diuretics have little or no effect on either arterial stiffness or wave reflection, vasodilators including nitrates and phosphodiesterase type-5 inhibitors e.g., sildenafil, reduce wave reflections and aortic pressures but not aortic stiffness. β-blockers have the opposite effect; they reduce aortic stiffness but increase aortic pulse pressure and wave reflections while calcium antagonists and α-blockers show varying effects on the vascular wall. Drugs targeting the renin–angiotensin–aldosterone system, namely angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARBs) and aldosterone antagonists have been shown as the most effective in reducing both arterial stiffness and wave reflection, and in some cases, to a greater extent than predicted from the extent of blood pressure (BP) reduction. Also, there is evidence of an additive effect on arterial stiffness with combined ACEI and ARBs. Exploring further the synergistic effects of anti-hypertensive drugs on arterial stiffness, a polypill containing a low-dose combination of a thiazide diuretic, calcium antagonist, β-blocker and an ACEI, decreased arterial stiffness more than the individual drugs in standard doses. However, beyond the dynamic effects of anti-hypertensive drugs, future therapies may directly target vascular structural alterations including collagen degradation, advanced glycation end-products, the matrix-metalloproteinases and vascular inflammation. Finally, one can speculate about the role of pharmacogenomics which may help tailor ‘de-stiffening therapy’ in individuals with stiff arteries.
- Copyright
- © 2007 Published by Elsevier B.V. on behalf of Association for Research into Arterial Structure and Physiology.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
“Nothing is now or ever will be, more significant in medical science, no more necessary to it, than the observation of the pulse”
Throughout history the study of the pulse has captured the minds of many great physicians in every civilization, but it was not until the nineteenth century that sphygmography provided the first graphic recording of the arterial pressure pulse.1 Abnormalities in the pulse wave contour were considered indicative of stiff arteries and subsequently used to diagnose hypertension and demonstrate the effects of drugs such as nitrates.1 With the advent of the cuff sphygmomanometer, the pulse lost its central place in medicine. However, waveform analysis experienced a rebirth when arterial tonometry became more accurate and changes in the pulse waveform could be described in numeric terms.
The most commonly used parameters of arterial stiffness are pulse wave velocity (PWV) and augmentation index (AIx), while closely related are not the same entity. Whereas PWV is a measure of aortic stiffness, AIx is a far more complex parameter, reflecting the adverse effects of arterial stiffening on the heart and central vessels due to early wave reflection from smaller arteries and arterioles secondary to endothelial dysfunction and/or elevated vascular smooth muscle tone. While the main determinants of PWV are age and blood pressure (BP), AIx depends not only on age and BP but gender, heart rate and body habitus are also major determinants.2 It is important to measure both parameters in clinical studies as the correlation between these two parameters is quite weak3 and some drugs can influence one parameter independent of the other.4 Longitudinal studies have firmly established PWV and AIx as independent prognosticators of cardiovascular events in different disease populations5 and obviously drugs that reduce either or preferably both are of great interest. However, the key question is whether arterial stiffness and wave reflection is a therapeutic target in its own right. This review aims at discussing the role of drugs in reducing arterial stiffness with emphasis on those that show benefits beyond BP lowering and those potential drugs that may target the ‘root causes’ of arterial stiffness.
Anti-hypertensive drugs and arterial stiffness
The effects of anti-hypertensive drugs on arterial stiffness are complex and can vary with the duration of treatment, the arterial territory being studied and the distending pressure in the arteries. Also, the arterial pressure–volume relationship is curvilinear, arteries being stiffer at high pressure, and arterial stiffness may decrease with BP reduction. Therefore it is often difficult to ascertain whether the improvement in PWV and AIx with anti-hypertensive therapy is the “passive” result of BP reduction or is a consequence of pressure-independent alterations of the arterial wall. Drugs like angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARBs) and aldosterone antagonists improve large artery compliance independently of BP changes, probably acutely by functional changes of vascular smooth muscular relaxation and in the long-term by decreased arterial wall thickness, collagen content, and reversion of smooth muscle cell hypertrophy.
Table 1 summarises the effect of the major anti-hypertensive drug groups on arterial stiffness as measured by PWV and wave reflection. A more comprehensive discussion can be found in our previous review.2
| PWV | Wave reflection | |
|---|---|---|
| Diuretics | ||
| Hydrochlorthiazide | ↔ | ↔ |
| Indapamide | ↔ | ↔ |
| Bendroflumethiazide | ↔ | ↔ |
| β-blockers | ||
| Propranolol | ↓ | |
| Bisoprolol | ↓ | |
| Dilevalol | ↓ | |
| Atenolol | ↓ | ↔/↑ |
| Metoprolol | ↔ | |
| Nebivolol | ↓ | ↔/↑ |
| Calcium channel blockers | ||
| Amlodipine | ↔ | ↓ |
| Nitrendipine, isradapine | ||
| Lacidipine, nifedipine, felodipine | ↓ | ↓ |
| Verapamil | ↓ | |
| α-blockers | ||
| Doxazosin | ↔ | ↓ |
| Aldosterone antagonists | ||
| Canreonate | ↔ | |
| Spironolactone | ↔/↓ | ↓ |
| Eplernone | ↓ | |
| ACE Inhibitors | ||
| Captopril | ↓ | ↓ |
| Ramipril, lisinopril, cilazapril | ↓ | |
| Trandolopril | ↓ | ↓ |
| Quinapril | ↓ | ↓ |
| Fosinopril | ↓ | |
| Perindopril | ↓ | ↓ |
| Ramipril | ↓ | ↓ |
| Angiotensin receptor blockers | ||
| Losartan | ↓ | ↓ |
| Telmisartan | ↓ | |
| Valsartan | ↓ | ↓ |
| Candesartan | ↓ | |
↔ = no change; ↓ = decreased; ↑ = increased.
Summary of the effect of major anti-hypertensive drug groups on arterial stiffness adapted from Ref. 2 (see text for details) as assessed by pulse wave velocity (PWV) and other methods
Diuretics
As salt intake contributes to arterial stiffness,2 the expectation was that diuretics would be effective in reducing arterial stiffness but the results so far have been disappointing. Both placebo-controlled and comparative studies have shown diuretics to have no effect on arterial stiffness (Table 1). We have shown that previously6 in a randomised controlled trial comparing losartan 50 mg to hydrochlorthiazide 25 mg daily in mild to moderate hypertension that the latter had no effect on PWV or arterial wave reflection. It was argued that this lack of effectiveness may be due to the potassium loss; however both indapamide and canreonate, two drugs with opposite effects on potassium levels did not show any beneficial effect on arterial stiffness.7 Benetos et al. in a randomised study comparing hydrochlorthiazide 50 mg plus amiloride 5 mg to a hydrochlorthiazide 25 mg/captopril 50 mg combination showed the ACEI-diuretic combination decreased arterial wave reflection despite similar BP reduction with the two regimens.8 This may suggest a role for renin–angiotensin–aldosterone system (RAAS) and sympathetic nervous system activation secondary to salt and water depletion favouring arterial constriction and increased arterial stiffness with diuretics.
β-blockers
Most studies have shown that β-blockers reduce PWV (Table 1).2 They, however, slow heart rate, leading to an increase in PP and AIx.9,10 Kelly et al. showed that vasodilating β-blockers may have a beneficial effect on wave reflection.11 However, we were not able to find any difference between atenolol and nebivalol, a vasodilating β-blocker in reference to their effect on AIx.12 The jury is still out on whether the confounding effects of heart rate reduction should be taken into account when considering β-blockers.
Vasodilators
Nitrates
Nitrates were shown as far back as the 1800s to have a pronounced effect on the arterial pressure waveform. Nitrates do not affect stiffness of the proximal aorta yet they reduce PP more effectively than other vasodilators by causing little or no change in mean arterial pressure probably by both venodilation13 and attenuation of peripheral wave reflections during systole.14 Long-term effects have been limited by the development of nitrate tolerance. Nitrates enhance vascular cGMP synthesis and this can also be accomplished by blocking cGMP catabolism using phosphodiesterase-5 inhibitors, such as sildenafil which can also reduce central PP15 and wave reflections probably without causing tolerance from long-term nitrate exposure. Atrial natriuretic peptide (ANP) and brain natriuretic peptide by elevating cyclic GMP levels through activation of a receptor-coupled guanylate cyclase, have been shown to reduce arterial stiffness in vitro and in animal studies.16
Calcium channel blockers
It has been shown in the past that both the dihydropyridine and non-dihydropyridine group of calcium channel blockers (CCBs) reduce PWV.2 A recent comparison of amlodipine 5 mg to hydrochlorthiazide 12.5 mg daily in a randomised cross-over trial showed that neither HTCZ nor amlodipine reduce PWV,17 while the latter reduced AIx, a feature it shares with the other CCBs.17,18
α-blockers
While one study reported an improvement in PWV and endothelial dysfunction with doxazosin in patients with essential hypertension19; the study was not randomised. We compared doxazosin 5 mg to bendroflumethiazide 2.5 mg daily in a randomised controlled one-month study and observed that while both drugs did not reduce aortic PWV, only doxazosin had a significant, albeit modest, beneficial effect on AIx.4
Drugs acting on the renin–angiotensin–aldosterone axis
The RAAS plays a central role in both short-term and chronic BP control and adaptive responses. One of the mechanisms by which it may alter arterial stiffness is through the potent vasoconstrictor angiotensin II (AII). In healthy volunteers, AII infusion increases PWV which is only partly BP dependent.20 It also increases AIx and aortic PP without any concomitant change in peripheral PP.21 There is also evidence that the detrimental effect of AII on arterial stiffness is mediated by the AT1 receptor.22 Enhanced AII activity is also associated with collagen degradation, smooth muscle proliferation and the development of fibrosis.23 Substantial evidence has emerged to show that aldosterone also plays an independent role in cardiac and vascular remodelling, regulating collagen turnover and fibrous tissue formation23 but may also exert acute vasoconstriction in blood vessels.24 Patients with hyperaldosteronism have greater cardiovascular risk, left ventricular hypertrophy and end organ damage.25 Immunohistochemical evidence has suggested the presence of aldosterone receptors in the aorta.25 Experimentally it increases arterial stiffness in salt-fed rats through changes in the elastin and collagen densities, an effect that is prevented by the specific aldosterone antagonist, eplerenone.26
Blacher et al.27 have shown a relationship between increased plasma levels of aldosterone and decreased systemic compliance in patients with long standing hypertension. Resnick et al. demonstrated a significant inverse relationship between plasma renin activity and the baseline level of both large and small artery compliance in normotensive subjects.28 We found a significant positive correlation between the aldosterone–renin ratio (ARR) and aortic systolic BP, aortic PP and AIx, but not PWV and a negative correlation with PP amplification in untreated hypertensive patients.29
ACE inhibitors and angiotensin receptor blockers
There is extensive literature on the effect of ACEI and ARBs on arterial stiffness and wave reflection and the studies are summarised in Table 1.
ACE inhibitors have been shown to have a favourable effect on arterial stiffness, when compared with other anti-hypertensive agents. This is a class effect and appears to be partly BP independent. ACE inhibition, however, appears less effective in preventing experimental vascular fibrosis and stiffening over ARBs or aldosterone antagonists.16
Given the favourable effects of ACEI, it was not surprising that ARBs would have beneficial effects on arterial stiffness. The ARB valsartan significantly reduced the AIx when added to the current regimen consisting of at least 3 anti-hypertensive drugs including an ACE inhibitor for two weeks in poorly controlled hypertensive patients.30 In a 4-week cross-over study with the AT1 receptor antagonist losartan compared to hydrochlorthiazide in patients with essential hypertension, for the same BP reduction, only losartan decreased PWV and the aortic AIx and enhanced pulse pressure amplification6 and the reduction in PWV and AIx appeared to be independent of BP reduction. Most interesting are the additive effects of ACEI and ARBs on arterial stiffness; in a randomised cross-over trial in hypertensive patients comparing valsartan 160 mg with captopril 100 mg per day, both therapies produced a similar reduction in PWV and AIx which remained significant when corrected for the BP reduction and the effects on arterial stiffness were additive when the two drugs were used in combination (Fig. 1).31 Omapatrilat, a dual ACE and neutral endopeptidase inhibitor, in a 12-week double blind study in hypertensive patients was significantly more effective in reducing BP and aortic PWV than enalapril16; however, the incidence of angioedema with the drug has proved to be a problem precluding clinical use for the moment.
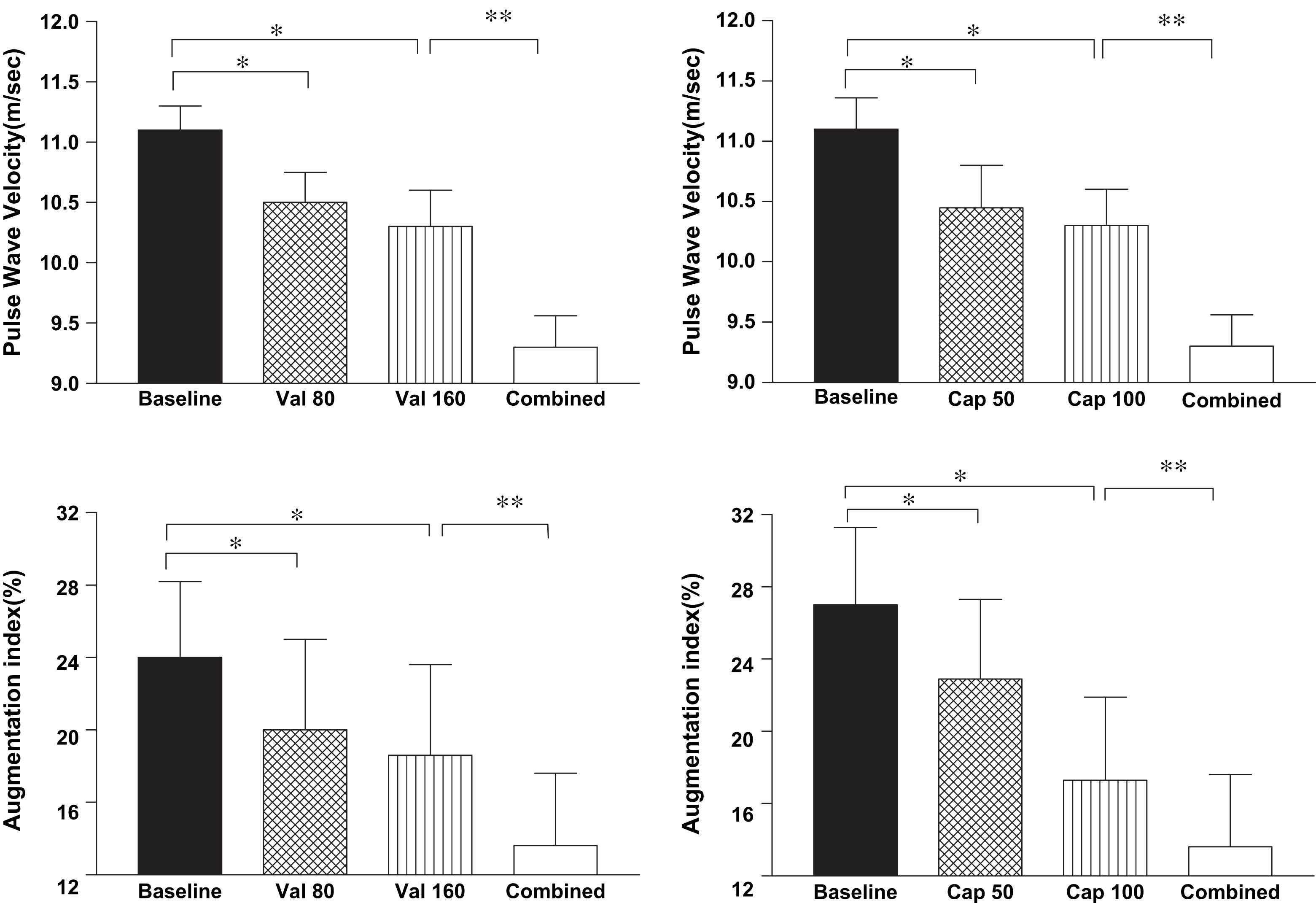
Pulse wave velocity and augmentation index following treatment with valsartan (val) 80 and 160 mg and captopril (cap) 50 and 100 mg and their combination in 12 hypertensive subjects. *p < 0.001 from baseline, **p < 0.05 monotherapy versus combined therapy (mean ± SEM).
Aldosterone antagonism
In hypertensive patients, two-week treatment with spironolactone did not reduce brachial artery stiffness23 and similar results were seen with canreonate.7 We have shown randomised controlled one-month study in a hypertensive population that spironolactone 50 mg alone reduced PWV and AIx even after adjustment for BP reduction when compared with bendroflumethiazide 2.5 mg.33 The more selective aldosterone antagonist eplerenone has also been shown to reduce aortic PWV in patients with systolic hypertension and wide PP.32
The polypill for arterial stiffness?
As low-dose anti-hypertensive combinations are increasingly being used in the first-line treatment of hypertension, the possibility of targeting arterial stiffness with similar approach seems intriguing. We were able to show, using a low-dose combination of a thiazide diuretic, CCB, ACEI and β-blocker that the reduction in BP and aortic PWV was significantly greater than each individual drug in a one-month randomised controlled trial suggesting that synergistic therapy may be the way forward.33
Lowering arterial stiffness – does it matter?
For patients with end stage renal disease the improvement in PWV in response to ACEI therapy was associated with decreased mortality and survival.34 Thus a lack of an effect on aortic PWV despite significant reduction in brachial BP was not associated with a significant reduction in cardiovascular death. Trials such as HOPE5 and LIFE5 raised the possibility that drugs like ACEI and ARBs may have effects independent of BP reduction. The recent CAFÉ study,35 a sub-study of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) has shown a greater benefit with a perindopril/amlodipine combination than an atenolol/diuretic regimen for the same fall in cuff pressures but with a significantly greater reduction in aortic BP and AIx, suggesting a clear benefit for reducing aortic BP beyond the reduction in the brachial artery.
Blood pressure-independent reduction in arterial stiffness – what does the future hold?
Whereas most anti-hypertensive drugs are directed at the dynamic vasoconstrictive component of arterial stiffness, newer therapies are targeting the underlying causes of arterial stiffness such as vascular inflammation and structural causes such as collagen degradation and advanced glycation end-products (AGEs).
The role of infection in arterial stiffness was shown recently where vaccination in healthy individuals led to increased PWV and plasma levels of inflammatory markers prevented in subjects pre-treated with aspirin.36 We have previously shown a significant relationship between both PWV and AIx with plasma markers of vascular inflammation including high-sensitivity C-reactive protein (hs-CRP), tumour necrosis factor-α and interleukin-6 in patients with essential hypertension.37 Another novel target may be the anti-inflammatory adipocytokine adiponectin; its levels are reduced in patients with hypertension, obesity, type II diabetes38 and at least in hypertensive patients, it is also inversely associated with aortic stiffness.38 Arterial stiffness related to insulin resistance can be modified with ligands of peroxisome proliferator activated receptor (PPAR)-γ receptors, such as the thiazolidinediones (Table 2). In type II diabetics, one-month treatment with pioglitazone reduced aortic PWV while increasing adiponectin and lowering hs-CRP and these effects were unrelated to improved diabetic control.16 While there is evidence that ARBs increase plasma adiponectin levels38 it remains to be seen whether increasing adiponectin concentrations per se can reduce arterial stiffness. Drugs such as the 3-hydroxy-3-methylglutaryl-coenzyme A inhibitors (Table 2) decrease arterial stiffness which in part may be attributable to a reduction in LDL cholesterol but may occur in the absence of hyperlipidemia, probably related to their anti-inflammatory and anti-proliferative actions.16
| Vasodilators | Nitrates, phosphodiesterase-5 inhibitors (sildenafil), atrial natriuretic peptide, brain natriuretic peptide |
| HMG Co A inhibitors | Statins |
| Anti-inflammatory agents | Aspirin, prostacyclin analogues, corticosteroids |
| PPAR-γ ligands (thiazolidinediones) | Pioglitazone, rosiglitazone, troglitazone |
| Advanced glycation end-products | Prevent formation: aminoguanidine |
| Link breakers: alagebrium (ALT-711) | |
| Soluble receptors for advanced glycation end-products (sRAGES) |
The effect of non-anti-hypertensive agents on arterial stiffness and wave reflection
The collagen in the vessel wall is probably the most important structural determinant of arterial stiffness. We have shown previously a very strong association between collagen turnover and both PWV and AIx in an untreated hypertensive population.39 However, collagen turnover is a slow process making it a difficult therapeutic target. Probably more promising is the pharmacological interference with AGEs which accumulate in the vascular wall in conditions like ageing, diabetes and hypertension.16 We have shown a close association between accumulation of plasma AGEs and both PWV and AIx in hypertensive subjects independent of age and BP.40 Drugs that block the formation of AGEs (aminoguanidine, pyridoxamine, OPB-9195), those that nonenzymatically cleave existing AGE cross-linking (alagebrium (ALT-711)), and drugs that either serve as sham receptors for advanced glycation end-products (RAGEs) or block RAGEs are undergoing development.16 Although aminoguanidine reduces aortic PWV, clinical trials have shown glomerulonephritis at high doses.16 The AGE cross-link breaker, ALT-711 has been shown in man to reduce PP and arterial stiffness and is being investigated for a possible therapeutic role in isolated systolic hypertension and diastolic heart failure.
Arterial stiffness – is the answer in the genes?
Arterial stiffness has a genetic component, largely independent of the influence of BP, heart rate, height and age and other cardiovascular risk factors. Variations in arterial stiffness have been related to gene polymorphisms in the ACE or AT1 receptor, endothelin A and B receptor, polymorphisms specifically linked with collagen, elastin, or the reduction of telomere with age.2 The matrix-metalloproteinases (MMPs) modulate collagen and elastin turnover in the vessel wall and the polymorphism in the MMP-9 genotype determined BP and arterial stiffness in a hypertensive population.41
There are a limited number of studies where response to anti-hypertensive treatment is studied in relation with genetic polymorphisms. The ACEI were associated with greater reduction in arterial stiffness in the presence of a certain AT1 receptor gene polymorphism.2
While we continue to identify various genes that are associated with arterial stiffening, the question whether the genotype can in any way help us tailor ‘de-stiffening’ treatment in individual hypertensive patients remains unanswered.
The ideal ‘de-stiffening drug’ – the quest goes on
As we live longer, arterial stiffness is emerging as an endemic problem and in light of the recent CAFÉ study is well established as a therapeutic target in its own right. While certain anti-hypertensives such as the ACEI, ARBs and aldosterone antagonists reduce arterial stiffness, at least in part independently of BP reduction, they have not proved to be the ideal ‘de-stiffening’ agents. Drugs such the AGEs cross-link breakers while targeting arterial stiffness independent of BP are associated with serious side-effects. Therefore, we need a clearer understanding of the ‘root causes’ of arterial stiffness to develop specifically targeted therapeutic interventions to reduce stiffness and wave reflection beyond reduction in cuff pressures. The perfect ‘de-stiffening’ therapy still remains the Holy Grail in hypertension.
References
Cite this article
TY - JOUR AU - Azra Mahmud PY - 2007 DA - 2007/06/06 TI - Reducing arterial stiffness and wave reflection – Quest for the Holy Grail? JO - Artery Research SP - 13 EP - 19 VL - 1 IS - 1 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2007.03.001 DO - 10.1016/j.artres.2007.03.001 ID - Mahmud2007 ER -
