Gender difference in age-related carotid stiffness: A prospective study in Chinese diabetic patients
- DOI
- 10.1016/j.artres.2012.09.002How to use a DOI?
- Keywords
- Type 2 diabetic mellitus; Gender; Age; Carotid stiffness
- Abstract
Background: The reason for the particularly increased cardiovascular morbidity and mortality in diabetic women remains unclear. The purpose of our study was to prospectively investigate sex difference in carotid stiffness in elder Chinese patients with type-2 diabetes mellitus (T2DM).
Methods: 109 T2DM patients (mean age at baseline, 58.5 years; 65.1% women) were included in the current study. Carotid stiffness index (CSI) was measured using an ultrasonic phase-locked echo-tracking system at baseline (CSI1) and at follow-up (CSI2). Biochemical measurements and clinical information were also measured.
Results: Mean value of CSI1 and CSI2 were 4.49 ± 1.45 and 7.84 ± 2.37, respectively. CSI1 were similar in both sex (CSI1: 4.49 ± 1.38 vs 4.51 ± 1.59), while CSI2 was higher in women than in men (CSI2: 8.22 ± 2.41 vs 7.13 ± 2.13). After adjusted for other cardiovascular risk factors, the difference in CSI2 between women and men was still significant. Age was the main determinant of CSI1 in both genders. However, correlation between age and CSI2 was only found in women. Women had higher CSI progression (ΔCSI) than men (3.74 ± 2.22 vs 2.62 ± 1.73). Furthermore, being female was an independent risk factor of ΔCSI.
Conclusion: Age was the most important risk factor of carotid stiffness. Nevertheless, after a 4 year follow-up, the impact of ageing on carotid stiffness progression only existed in T2DM women.
- Copyright
- © 2012 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Diabetic patients are at higher risk for cardiovascular1,2 and cerebrovascular disease than persons without diabetes.3 In addition, type 2 diabetes weakens the protective impact of female sex and causes greater relative risk in women compared with men.4–7 Diabetic women are more susceptible to coronary heart disease and cerebrovascular complication.4,8 However, the reason for the particularly increased cardiovascular and cerebrovascular morbidity and mortality in diabetic women remains unclear.
Several previous studies showed that increased aortic pulse wave velocity, which was an accepted index of arterial stiffness9–11 only existed in diabetic women (compared with non-diabetic controls), not in diabetic men. However, these studies either recruited subjects with insulin dependent diabetes mellitus, which has different pathophysiological mechanism from type 2 diabetic mellitus, or had a case-control design. Moreover, few longitudinal researches focused on this issue in a Chinese cohort with diabetes. Therefore, the aim of the current study was to evaluate the mechanical property of the common carotid artery in elder Chinese type 2 diabetic patients in a four-year follow-up study and to clarify whether there is a gender difference in arterial stiffness.
Patients and methods
Subjects
Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine approved the study protocol. From October 2006 to February 2007, 547 type 2 diabetic patients (diagnosed according to the 1999 World Health Organization criteria) were recruited via the outpatient clinic of the Department of Endocrine and Metabolism at Ruijin Hospital, Shanghai. All study participants gave written informed consent. We excluded 438 patients because of missing clinical information (n = 121), having a history of ischaemic heart disease or stroke (n = 89) and without carotid stiffness measurement at baseline and/or follow-up (n = 222). Thus, the present analysis included 109 subjects.
Clinical and biochemical assessment
Blood pressure was measured with a standard mercury sphygmomanometer in the sitting position after resting for at least 10 min. Blood samples were obtained after an overnight fast. Plasma HbA1C was measured by high performance liquid chromatography (BRO-RAD Company, USA). Serum concentrations of total cholesterol and triglycerides were measured by the enzymatic method, and high density lipoprotein (HDL) cholesterol was measured using a specific precipitation method (Beckman LX-20, Brea, CA, USA). Low density lipoprotein (LDL) cholesterol was calculated using the Friedewald’s formula.12 Body mass index was calculated as weight in kilogrammes divided by the square of height. We used a questionnaire to collect information on medical history, smoking habits and alcohol intake. All these measurement and information were collected between October 2006 and February 2007 at baseline, and between October 2010 and February 2011 at follow-up.
CSI measurement
This measurement were described in details elsewhere.13,14 Briefly, in 2006 (baseline) and 2010 (follow-up), the same trained sonographer performed CSI measurements using an ultrasonic phase-locked echo-tracking system equipped with a high-resolution, real-time 10-MHz linear scanner (Esaote Picus, Italy). Ultrasound examinations of the common carotid arteries were performed in the supine position with slight hyperextension of the neck. CSI were measured at 1.5 cm proximal to the common carotid bifurcation. CSI was calculated using the blood pressure and diameter of the artery as follows: CSI = ln (Ps/Pd)/(As/Ad − 1), where Ps and Pd are systolic and diastolic blood pressures and As and Ad are the systolic and diastolic area of the artery, respectively. Blood pressures are brachial cuff measurements. The mean value of the right and left common carotid CSI was used for analysis, recorded as CSI1 in 2006 and CSI2 in 2010. ΔCSI was calculated as the difference between CSI1 and CSI2. The variation of variability was 3.3%.
Statistical analysis
For database management and statistical analysis, we used SAS software (SAS Institute, Cary, NC, USA), version 9.2. We compared means and proportions, using Student’s t-test and Chi-square test, respectively and correlated proportions by McNemar’s test. Our statistical methods also included multiple linear regressions. We searched for possible covariables of CSI, using a stepwise regression procedure with the P-values for independent variables to enter and to stay in the model set at 0.15. As covariables, we considered sex, age, body mass index, blood pressure measurements, HbA1c, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, and design variables (0, 1), coding for current smoking and alcohol intake.
Results
Characteristics of participants at baseline and follow-up
The recent study contains 71 (65.1%) women, with a mean age of 58.8 years (range from 45 to 74). Among the total subjects, 21 (19.3%) are current smokers and 9 (8.2%) report regular alcohol intake at baseline. Compared with women, men had lower systolic and pulse pressure at baseline, and higher diastolic and mean blood pressure at follow-up (Table 1). At follow-up, women had higher levels of total cholesterol, HDL and LDL cholesterol than men. Other characteristics were similar in women and men. The prevalence of smoking and alcohol intake do not change significantly during the 4 year period.
| Characteristic | Women (n = 71) | Men (n = 38) | ||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | Change | Baseline | Follow-up | Change | |
| Anthropometrics measurements | ||||||
| Age (years) | 57.9 ± 6.8 | 62.3 ± 6.7 | 4.39 (2.15–6.63)§ | 59.5 ± 8.2 | 63.9 ± 8.1 | 4.37 (0.64–8.11)† |
| Duration (years) | 9.00 ± 5.45 | 13.2 ± 5.4 | 4.25 (2.45–6.05)§ | 10.9 ± 4.67 | 12.3 ± 6.0 | 4.40 (1.66–7.14)‡ |
| Body mass index (kg/m2) | 25.0 ± 3.3 | 24.9 ± 3.7 | −0.134 (−1.29 to 1.02) | 24.7 ± 2.5 | 24.4 ± 2.2 | −0.299 (−1.38 to 0.78) |
| Systolic pressure (mm Hg) | 127.6 ± 14.7 | 131.9 ± 15.4 | 4.29 (−0.71 to 9.28) | 121.8 ± 10.0* | 136.1 ± 16.2 | 14.2 (8.1–20.3)§ |
| Diastolic pressure (mm Hg) | 76.8 ± 10.0 | 72.9 ± 9.5 | −4.25 (−7.50 to −0.99)‡ | 78.1 ± 6.3 | 76.9 ± 9.7* | −1.21 (−4.96 to 2.54) |
| Pulse pressure (mm Hg) | 50.8 ± 10.1 | 59.4 ± 14.9 | 8.53 (4.30–12.8)§ | 43.7 ± 8.3* | 59.1 ± 14.4 | 15.4 (10.0–20.8)§ |
| Mean arterial pressure (mm Hg) | 93.7 ± 10.8 | 92.3 ± 9.5 | 1.40 (−4.78 to 1.97) | 92.7 ± 6.7 | 96.6 ± 10.2* | 3.93 (−0.01 to 7.87) |
| Biochemical measurements | ||||||
| HbA1c (%) | 6.81 ± 1.16 | 7.72 ± 1.20 | 0.908 (0.516–1.301)§ | 6.48 ± 1.05 | 7.49 ± 1.31 | 1.02 (0.47–1.56)§ |
| Total cholesterol (mmol/l) | 5.15 ± 0.81 | 5.27 ± 0.98 | 0.120 (−0.179 to 0.420) | 5.06 ± 0.94 | 4.88 ± 0.82* | −0.185 (−0.592 to 0.222) |
| HDL cholesterol (mmol/l) | 1.55 ± 0.24 | 1.47 ± 0.32 | −0.083 (−0.176 to 0.010) | 1.56 ± 0.30 | 1.31 ± 0.36* | −0.242 (−0.393 to −0.091)‡ |
| LDL cholesterol (mmol/l) | 3.14 ± 0.68 | 3.32 ± 0.91 | 0.171 (−0.096 to 0.439) | 3.10 ± 0.66 | 2.95 ± 0.75* | −0.151 (−0.479 to 0.178) |
| Triglycerides (mmol/l) | 1.37 ± 0.75 | 1.60 ± 0.88 | 0.230 (−0.043 to 0.503) | 1.47 ± 1.25 | 1.70 ± 1.51 | 0.234 (−0.403 to 0.871) |
| Questionnaire data | ||||||
| Current smoking (n (%)) | 13 (18.3) | 13 (18.3) | 0 | 8 (21.0) | 6 (15.8) | −0.028 (−0.067 to 0.011) |
| Drinking alcohol (n (%)) | 4 (5.63) | 3 (4.22) | −0.014 (−0.042 to 0.014) | 5 (13.2) | 5 (13.2) | 0 |
Data are mean ± (SD), number of subjects (%) or mean changes from baseline to follow-up (95% confidence interval).
Abbreviations: HbA1c: glycosylated haemoglobin A1c; HDL: high density lipoprotein; LDL: low density lipoprotein.
An asterisk indicates a significant difference (P < 0.05) with women.
Significance of the change from baseline to follow-up:
P < 0.05;
P < 0.01;
P < 0.001.
Characteristics of participants at baseline and at follow-up.
CSI measurements
At baseline, women had similar CSI1 with men (Table 2). However, after a 4-year follow-up, CSI2 was significantly higher in women than in men. Moreover, women had a larger ΔCSI than men. After adjustment for other covariables listed in Table 1, ΔCSI remained higher in women (Fig. 1).
| Characteristics | Women | Men | P |
|---|---|---|---|
| CSI1 | 4.49 ± 1.38 | 4.51 ± 1.59 | 0.93 |
| CSI2 | 8.22 ± 2.41 | 7.13 ± 2.13 | 0.0210 |
| ΔCSI | 3.74 ± 2.22 | 2.62 ± 1.73 | 0.0079 |
Data are mean ± (SD).
Abbreviations: CSI1, carotid stiffness index measured at baseline; CSI2, carotid stiffness index measured at follow-up; ΔCSI = CSI2 − CSI1.
Carotid stiffness measurements.
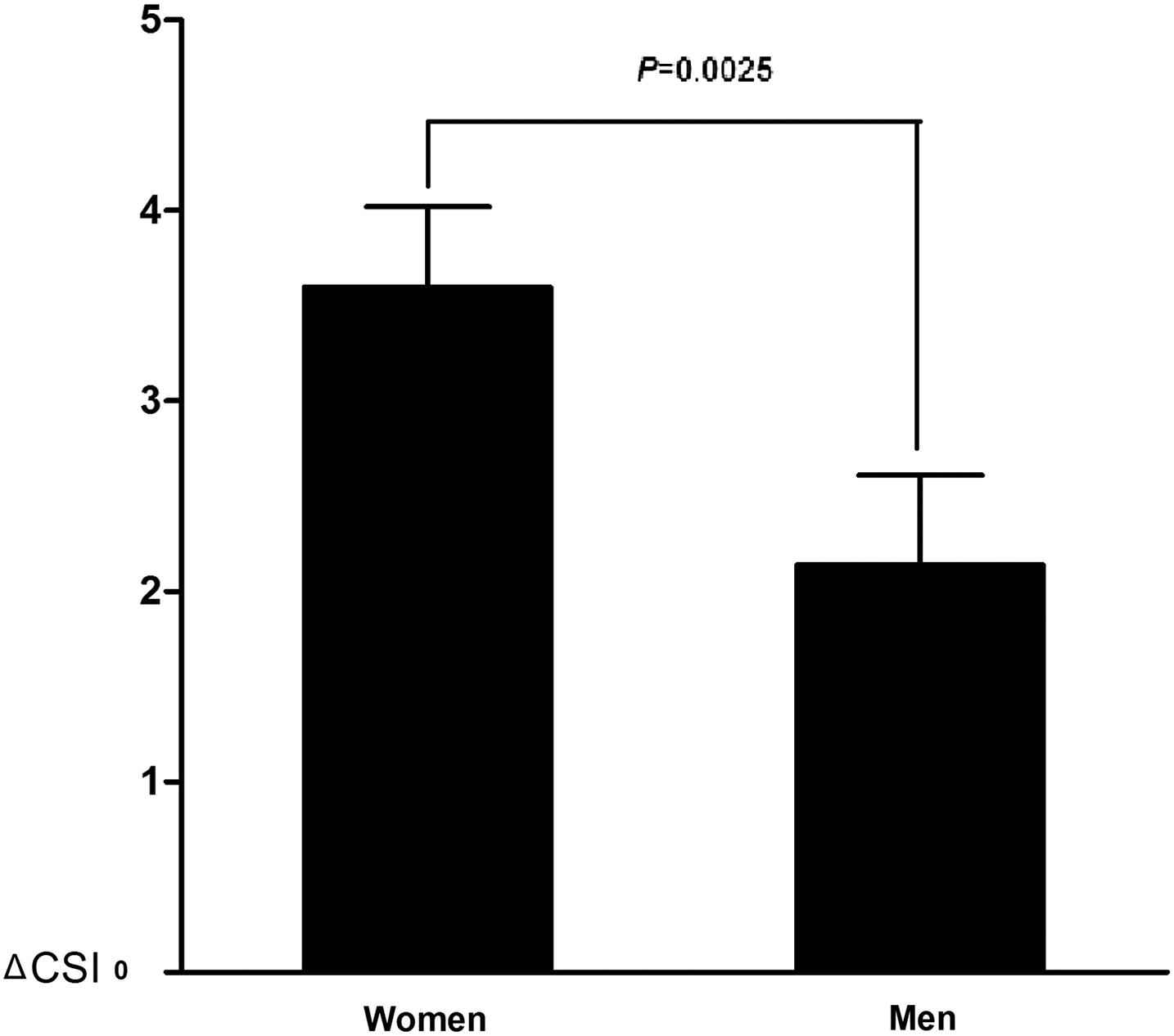
Difference in carotid stiffness progression (ΔCSI) between T2DM women and men with adjustment for other cardiovascular risks (listed in Table 1).
Correlates of CSI at baseline and follow-up
Age and pulse pressure are independent risk factors of CSI1, while ΔCSI increased by 1.17 for being female (Table 3). Figure 2 showed that CSI1 increased across age groups in both genders, however, similar trend was only found in women for CSI2.
| Characteristics | CSI1 | ΔCSI | ||||
|---|---|---|---|---|---|---|
| β ± SE | r2 | P | β ± SE | r2 | P | |
| Being female | – | – | – | 1.17 ± 0.41 | 0.0640 | 0.0079 |
| Age, +10 years | 0.777 ± 0.172 | 0.170 | <0.0001 | – | – | – |
| Pulse pressure, +10 mm Hg | 0.292 ± 0.127 | 0.0321 | 0.0413 | – | – | – |
Data are mean ± (SD) or mean (95% confidence interval).
Abbreviations: CSI1, carotid stiffness index measured at baseline; CSI2, carotid stiffness index measured at follow-up; ΔCSI = CSI2 − CSI1.
Correlates of carotid stiffness.
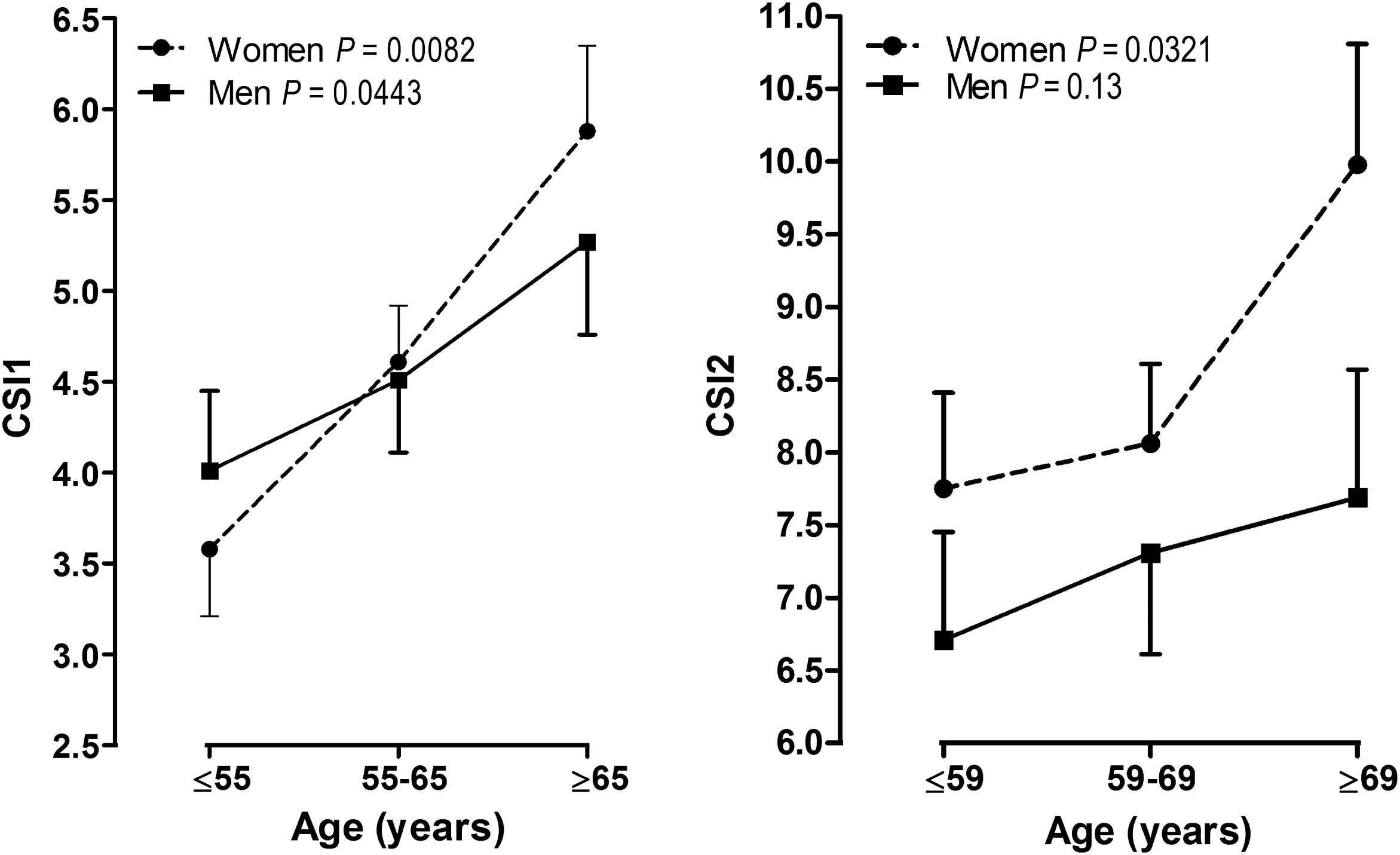
Carotid stiffness by sex and age at baseline (CSI1) and at follow-up (CSI2). P-values are for linear trend across the age groups.
Discussion
The key finding of our study was that age was the most important risk factor of carotid stiffness in elder Chinese diabetes, and female diabetic patients were more sensitive to the age-related arterial stiffness.
In the study of T2DM patients with hypertension, De Angelis11 and colleagues found that aortic pulse wave velocity, which highly predicts cardiovascular mortality, was higher in diabetic women than non-diabetic controls. However, diabetic and control men had similar aortic pulse wave velocity. Furthermore, this finding cannot be explained by hypertension.11 Rydén Ahlgren et al.9 confirmed this observation in patients with insulin dependent diabetes mellitus. In their study, aortic and carotid stiffness were measured using ultrasound, which was similar with our measurement. They found that stiffness of the aorta and the common carotid artery were increased in diabetic women but not in diabetic men. These results were in line with ours. However, neither of these studies was prospectively designed, and in both studies, diabetic patients were compared with age matched non-diabetic subjects. Therefore, the nature change of arterial stiffness related to diabetes cannot be estimated in these studies.
Although gender difference in arterial stiffness in diabetics was proved by several studies, however, the mechanism remains unknown. Previous studies found that carotid stiffness was correlated with insulin resistance in non-diabetic women but not in men.15 In the Atherosclerosis Risk in Communities Study, the correlation of glucose and insulin with carotid stiffness was higher in women than in men.16 However, metabolic factors did not seem to play a role in determining the present findings. We found no association between BMI, HbA1c, total cholesterol, HDL and LDL cholesterol, and triglycerides and CSI. These observations also complied with previous findings.11,17,18 However, we found an interesting phenomenon related to age. In our study, CSI1 did not show gender difference at baseline. Nevertheless, after a 4-year follow-up, women had significantly higher CSI2 than men. After adjustment for other cardiovascular risk factors, age remained significantly associated with CSI1 in both genders, however, at follow-up, this association only existed in women. Moreover, the progression of CSI (ΔCSI) was higher in women, and being women was an independent risk factor of ΔCSI. According to these findings, T2DM women seemed to be more susceptible to the ageing effect on elastic arteries. Therefore, we supposed that age, which is a well-known risk factor of arterial stiffness, might have impact on the diabetic related gender difference in cardiovascular disease.
Our study had a number of limitations. First, the sample size was small, especially for T2DM men. Thus, we cannot exclude a type II error with respect to the weak association between CSI and age in male subjects. Second, we did not categorized anti-diabetic treatment, which might have impact on the results. Third, the blood pressure, which was used to calculate CSI, was measured at brachial artery rather than carotid artery. There is an amplification of pulse pressure from carotid to brachial artery. However, amplification of pulse pressure between central and peripheral artery decrease with age.19,20 In our study, the patients are elder person with a mean age of 58.5 years. The difference in pulse pressure might be relative small. Thus, the CSI measurements could be slightly overestimated, but this probably had no significant impact on the differences in CSI between male and female patients. Finally, we did not measure heart rate at the ultrasonographic examination. Thus, we cannot assess the effect of heart rate on CSI. However, Kostis et al. found that age had effect on maximal rather than average heart rate.21 Then, heart rate may have limited impact on the change in CSI during a 4 year follow-up period.
In conclusion, gender difference in elastic artery existed in T2DM patients. Age, the main risk factor of arterial stiffness, had more significant influence on women diabetics than men. This might be a simple explanation of gender difference in diabetic-related cardiovascular mortality and morbidity.
Disclosure summary
None of the authors has a conflict of interest.
Acknowledgements
The present study would not have been possible without the participation of the patients. We sincerely appreciated Shanghai Clinical Center for Endocrine and Metabolic Diseases for collecting and providing clinical and laboratory results of these patients.
References
Cite this article
TY - JOUR AU - Bo Zhao AU - Yan-Ping Liu AU - Yifei Zhang AU - Yuhong Chen AU - Zhifang Yang AU - Ying Zhu AU - Weiwei Zhan PY - 2012 DA - 2012/10/01 TI - Gender difference in age-related carotid stiffness: A prospective study in Chinese diabetic patients JO - Artery Research SP - 42 EP - 47 VL - 7 IS - 1 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2012.09.002 DO - 10.1016/j.artres.2012.09.002 ID - Zhao2012 ER -
