Arterial stiffening: Causes and consequences
- DOI
- 10.1016/j.artres.2012.09.001How to use a DOI?
- Keywords
- Aortic stiffness; Pulse pressure; Atherosclerosis; Aortic imaging; Heritability; Aortic calcification; Haemodynamics
- Abstract
Increased arterial stiffness, as measured by pulse wave velocity, is increasingly recognised as an important predictor of future cardiovascular events. At present, the mechanisms leading to stiffening of large arteries and the processes underlying the association between arterial stiffness and cardiovascular disease remain unclear. One suggestion is that stiffening may be a caused by atherosclerosis along the aorta, explaining its association with cardiovascular disease. However, age-related stiffening of large arteries can occur independently of atherosclerosis and development of atherosclerosis does not necessarily contribute to increased aortic stiffness. Vascular calcification, extracellular matrix degradation and inflammation are likely to contribute to arterial stiffening. One consequence of increased large artery stiffness is an increase in pulse pressure, which, in older subjects, is the blood pressure components most closely correlated with cardiovascular events. The association between arterial stiffness and cardiovascular events may be explained, at least in part, by the adverse haemodynamic consequences of increased stiffness. This review summarises the potential mechanisms of arterial stiffening and its haemodynamic consequences.
- Copyright
- © 2012 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Cardiovascular disease is a major cause of morbidity and mortality in Western populations.1 The majority of cardiovascular events occur from complications of atherosclerosis.2 Over the last decade, arterial stiffness has emerged as an important predictor of cardiovascular events including atherosclerotic coronary events.3 The prognostic importance of pulse wave velocity (PWV), a measure of arterial stiffness, was first highlighted in renal patients where the probability of remaining free of a cardiovascular event is greatly reduced in patients with high PWV.4 These findings have been replicated in other cohorts including hypertensive, diabetic and elderly subjects where PWV is a predictor of cardiovascular morbidity and mortality independently of other more established cardiovascular risk factors including blood pressure.3
At present, the mechanisms leading to arterial stiffening and the processes underlying the association between arterial stiffness and cardiovascular disease remain unclear. The aim of this review is to summarise potential mechanisms of arterial stiffening and its haemodynamic consequences.
Mechanisms of arterial stiffness
Does atherosclerosis cause arterial stiffness?
One of the earliest studies to highlight an association between atherosclerosis and arterial stiffness was by Hirai et al.5; they showed abdominal aortic and carotid artery stiffness to be significantly higher in patients with coronary vessel disease when compared to age-matched controls. Furthermore, large artery stiffness increased linearly with progressively higher degrees of coronary disease.5 More recently, these findings have been replicated by large epidemiological studies. The Rotterdam study included >3000 participants and showed a stepwise increase in PWV with progressively higher plaque burden when measured in the carotid artery and abdominal aorta.6 One explanation proposed for these findings is that atherosclerosis causes arterial stiffness and that the prognostic importance of PWV is explained by it being a marker for the degree of atherosclerosis along the aorta. In support of this, both atherosclerosis and age-related artery stiffness are localised to large and medium sized arteries and increase in prevalence after 50 years of age.7,8 However, early work by Avolio et al.9 suggests that this may not be the case. In a Northern Chinese community which is known for its low serum cholesterol, incidence of atherosclerosis and coronary artery disease, age-related increase in PWV was comparable to and even more marked than that of western communities where atherosclerosis is prevalent.9 This suggests that age-related stiffening of large arteries can occur independently of atherosclerosis.
At a histological level, arteriosclerosis the stiffening and dilation of the arterial wall is distinct from atherosclerosis. Arteriosclerosis is mainly localised to the medial part where there is an abundance of elastin10 which becomes fragmented with age, showing a disorganised structure that may become calcified.10 By contrast, atherosclerosis is primarily a disease of the intimal part of the wall which is characterised by accumulation of lipids accompanied by inflammation, fibrosis and calcification at the most advanced stages.10 Furthermore, arteriosclerosis and atherosclerosis are likely to have different regulatory processes. Although established cardiovascular risk factors including hypercholesterolemia, diabetes mellitus, male sex and smoking have been associated with increased arterial stiffness, we recently showed in a systematic review that adjustment for age and blood pressure in cross-sectional studies often renders these associations non-significant.11 These findings have been substantiated by the limited number of longitudinal follow-up studies. Benetos et al. 12 found a significant increase in PWV in both hypertensive and normotensive subjects over a 6-year follow-up period. However baseline measures of serum cholesterol, triglycerides, glucose or body mass index were not significant predictors of PWV progression. More recently, data from the Caerphilly prospective study showed only a modest relationship between PWV and cardiovascular risk factors measured over a 20 year follow-up period.13 In animal studies of atherosclerosis, early deposition of plaque does not necessarily lead to increased arterial stiffening14 and autopsy studies show only a modest relationship between arterial stiffness and plaque burden.15 In postmenopausal women, we have shown a lack of association between PWV and lipid-rich non-calcified plaque assessed by ultrasound16 or total aortic plaque burden as measured by magnetic resonance imaging.17 Moreover, Paini et al.18 observed increased local arterial compliance at the level of carotid plaques when compared to adjacent plaque free areas. These findings suggest that development of atherosclerotic plaque alone does not necessarily contribute to increased stiffness of arteries and that measures of arterial stiffness are unlikely to accurately reflect the extent of atherosclerosis along the aorta.
Calcification and arterial stiffness
Calcification within the central arteries can occur at two distinct sites: in the media of the arterial wall where it associates with elastin fibers or in the intima where it localizes within atherosclerotic plaque. In animal models, induced medial calcification leads to increased arterial stiffening independently of atherosclerotic plaque.19 In humans, vascular calcification is increased in diabetic and end-stage renal disease patients, and in these patients arterial stiffness is greater compared to control subjects.20–22 In healthy subjects, arterial stiffness relates to intimal calcification independently of non-calcified plaque assessed using ultrasound.16,23 Furthermore, aortic calcification as measured by computed tomography is associated with increased aortic stiffness, independently of total aortic plaque burden.17 As there is often an overlap between medial and intimal calcification, and because current imaging techniques are not able to distinguish between the two types of calcification, their individual associations to arterial stiffness are unknown. Indeed, intimal calcification may simply be a marker for minor degrees of medial calcification, undetectable by CT/ultrasound imaging but which alter the elastic properties of the media. Alternatively, because medial calcification co-localises to areas of elastin fracture, its association to arterial stiffness could be secondary to or it may be a marker for degeneration of elastin.
Extracellular matrix and arterial stiffness
Elastin and collagen are the main loading bearing components of the arterial wall.10 A rise in blood pressure passively increases wall stiffness by transferring the pressure load from elastin to stiffer collagen fibers10 and, reduction in arterial pressure leads to a reduction in arterial wall stiffness.24 Prolonged increase in arterial pressure may additionally lead to structural changes within the arterial wall resulting from increased mechanical stimuli. In this instance, acute blood pressure lowering would not be able to normalise arterial stiffness.24 The inability to lower arterial stiffness with anti-hypertensive drugs in end-stage renal disease patients is associated with worse prognosis.25
Age-related structural change of arterial extracellular matrix includes elastin fragmentation, change in elastin-collagen ratio, matrix cross-links from advanced glycation end-products and calcification.26–30 Matrix metalloproteinases (MMP) are members of the zinc-dependent endopeptidase family capable of degrading components of the extracellular matrix including elastin and collagen.28 In healthy individuals, higher circulating levels of MMP-2 and 9 are associated with increased PWV.31,32 Furthermore, MMP gene polymorphisms are associated with increased stiffness and coronary artery calcification.33,34 MMP-2 and-9 activity can be induced by an increase in transmural pressure resulting in enhanced elastin degradation in animal models,35 which is retarded in the presence of MMP inhibitors.30 MMP-mediated elastin degradation closely associates with medial calcification which may further contribute to increased arterial stiffness.36 Furthermore, markers of elastin degradation predict change in stiffness in pre-dialysis patients.29 Changes in mechanical stimuli resulting from a rise in blood pressure also lead to proliferation of vascular cells and extracellular matrix synthesis of elastin and collagen in vitro.28 In animal models an increase in aortic pulse pressure increases the collagen-to-elastin ratio leading to reduced elasticity of the wall.26 Accumulation of advanced glycation end-products (AGEs) in the extracellular matrix is another potential mechanism contributing to increased stiffness. Breaking of AGEs using ALT-711 in humans, a non-enzymatic breaker of collagen cross-links, reduces arterial stiffening.27 Although changes in large artery extracellular matrix are likely to be an important determinant of wall stiffness, the contribution of elastin and collagen to normal vascular aging and its role in pathological conditions remains unknown.
Inflammation and arterial stiffness
Arterial stiffness is increased in chronic inflammatory disorders such as rheumatoid arthritis and large vessel vasculitis37,38 and correlates positively with circulating levels of CRP, a recognised inflammatory biomarker.13 Acute induction of an inflammatory response with Salmonella typhi vaccination increases arterial stiffness, an effect inhibited by aspirin pre-treatment.39 Together, these findings suggest that inflammation has an important role in large artery stiffening. Several possible mechanisms have been suggested by which inflammation may contribute to increased arterial stiffening.40 Inflammation plays a major role in atherosclerosis.41 However, lack of association between arterial stiffness and early stages of atherosclerosis makes this mechanism unlikely.14,16,17 Calcification is a process thought to be driven by inflammation, occurring either systemically or within atherosclerotic plaque, and which is associated with arterial stiffening.16,40,42 Inflammatory signalling may also enhances the activity of MMPs and cathepsins which may contribute to increased extracellular matrix degradation and arterial stiffening.40,43
Genetics and arterial stiffness
The relative contribution of genotype and environment to arterial stiffness can be quantified by studying monozygotic (MZ) and dizygotic (DZ) twins. The twin design compares the degree of correlation for a particular phenotype between genetically identical MZ twins and DZ twins that share approximately 50% of their genes. A greater correlation between MZ twin pairs for a particular phenotype compared to DZ twin pairs suggests a genetic influence.44 Using this classical twin model, we have shown heritability of carotid-femoral PWV to be 38%,16 similar to that reported from family studies where heritability is estimated between 19 and 40%.45–48 Similarly, vascular calcification also has a significant genetic component. In our twin study heritability of intimal calcification detected in the carotid and femoral arteries using ultrasound techniques was 61%.16 Furthermore, over 90% of the correlation between PWV and calcified plaque was explained by genetic factors common to both phenotypes.16 By contrast, heritability of non-calcified atheroma was only 5%,16 in agreement with low heritability of carotid plaque score reported by the Erasmus Rucphen Family study.47 Whether there is a causal pathway between PWV and vascular calcification, or whether they share a common etiology remains to be determined. As well as investigating the nature of this relationship, future studies should seek to identify underlying genetic mechanisms that account for the observed phenotypic correlation.
Haemodynamic consequences of arterial stiffening
The haemodynmic consequence of arterial stiffening most often cited is an increase in arterial pulse pressure (PP) and it is notable that, in older subjects, pulse pressure is the blood pressure component most closely associated with cardiovascular events.49 PP varies along the arterial tree being amplified in the brachial artery compared to the aorta (Fig. 1). In terms of cardiovascular outcome, initial data suggested that aortic or central PP (cPP) may predict cardiovascular risk above that of traditionally measured brachial PP.50–54 A systematic review suggests that the difference in prognostic value may be marginal.55 Nevertheless, measuring or estimating cPP provides at least as good an indication of cardiovascular risk as brachial PP. Furthermore, since amplification is greater in younger subjects its predictive value relative to brachial PP could be greater in young subjects with cPP thus being one of the best predictors of cardiovascular events at all ages. In the CAFÉ study a higher reduction in cPP with amlodipine and perindopril based therapy in comparison to atenolol and bedroflumethiazide based therapy translated into reduced cardiovascular risk, despite similar reduction in brachial PP.54
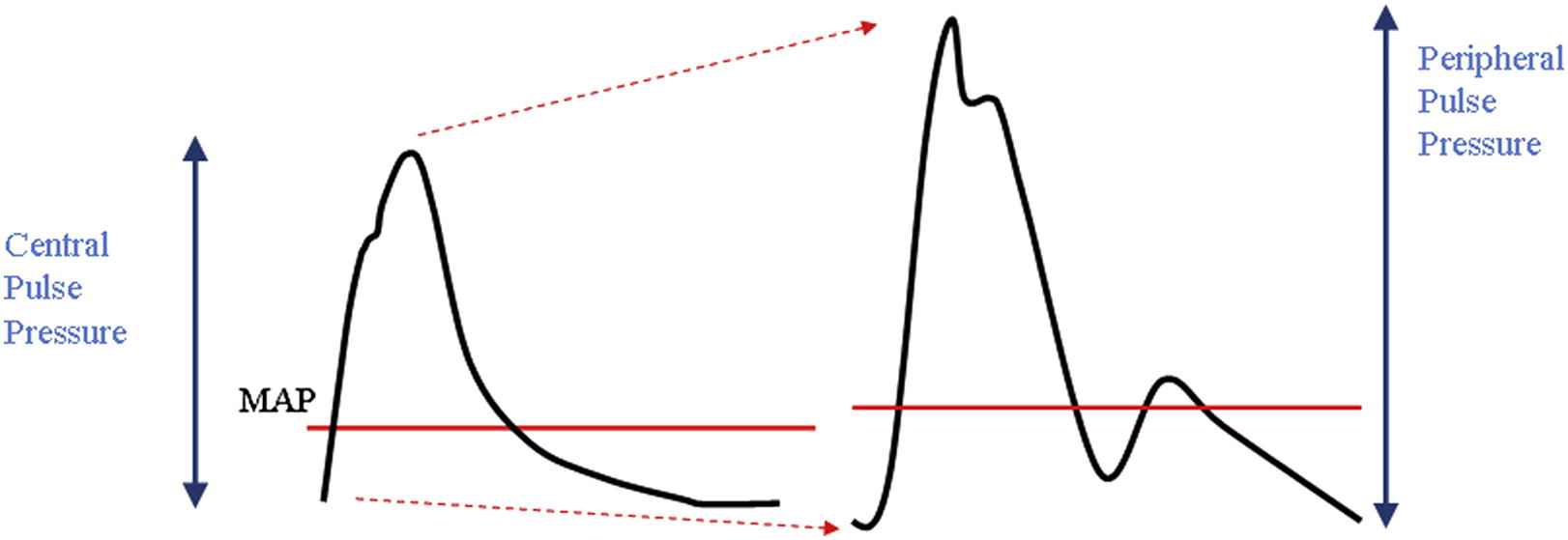
Pressure wave amplification. The arterial pressure pulse differs in contour and amplitude as it propagates along the arterial tree. In young individuals systolic blood pressure increases between the aortic (central) and brachial (peripheral) artery where as diastolic and mean arterial pressure remains relatively constant.
The aortic pressure waveform can be separated into two components. Firstly, the pressure of the forward wave, generated by left ventricular contraction and defined by the first systolic shoulder (P1, Fig. 2), is dependent on stroke volume and aortic stiffness.10 This forward travelling pressure wave may further be augmented above the height of P1, by an amount called augmentation pressure (AugP, Fig. 2). This is thought to results from reflection of the forward wave at peripheral sites but may also be influenced by aortic reservoir pressure.10,56 We recently showed that the contribution of P1 and AugP to age-related increase in cPP is driven mainly by AugP increase in women under 50 years of age (Fig. 3).57,58 By contrast, the contribution of P1 becomes increasingly prominent after the age of 50 years and becomes comparable to the increase in AugP explaining the observed plateau in augmentation index (calculated as AP/(AP + P1)*100) increase after 50 years of age (Fig. 3).58 It has been hypothesised that PWV may associate with AugP by reducing the time it takes for the reflected wave to arrive back to the aorta or through reduced compliance affecting the reservoir pressure.10,56 However, we have found no association between PWV and AugP in women across a wide age-range.57,58 AugP does associate with aorto-femoral tapering and dilation of muscular arteries with nitroglycerine effectively reduces AugP independently of any change in P1 or PWV.57,58 Thus, although arterial stiffening is an important determinant of cPP, it is not the only determinant of cPP and even when arterial stiffening contributes to a raised cPP, cPP can be effectively reduced by strategies which lower AugP.
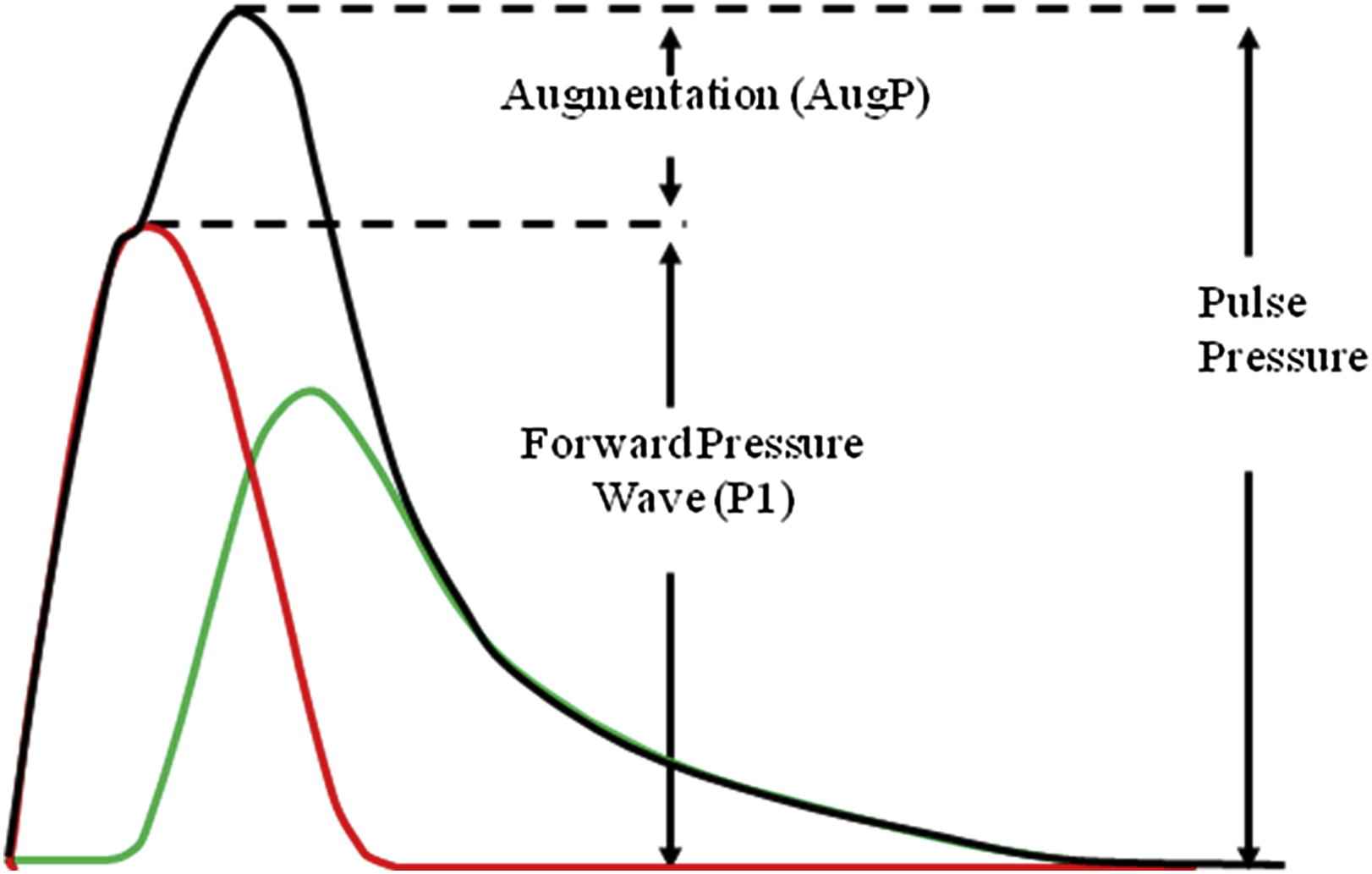
The aortic pressure waveform can be separated into two components; the pressure of the forward wave, generated by left ventricular contraction and defined by the first systolic shoulder augmentation pressure (Aug), traditionally attributed to pressure wave reflection.
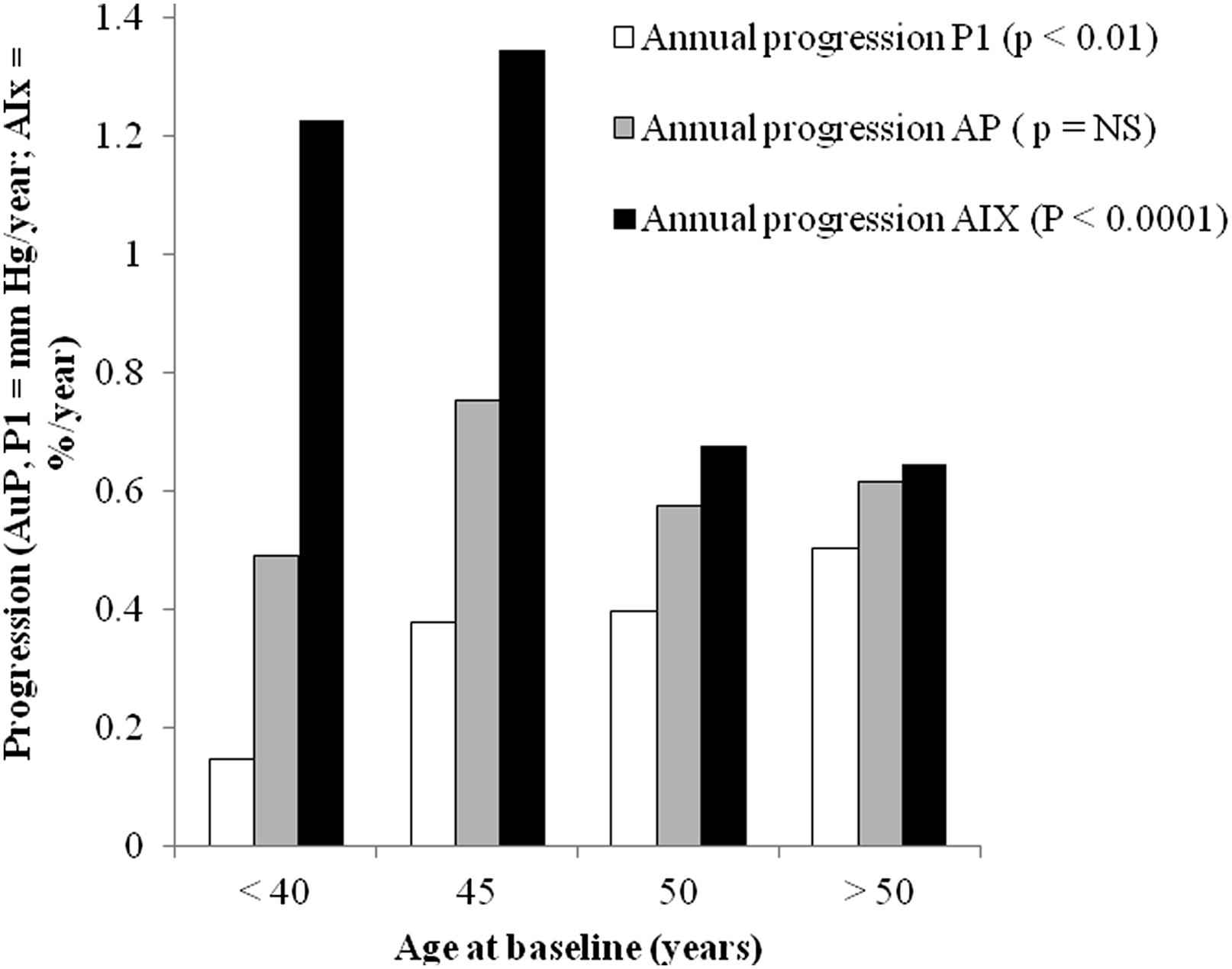
Annual progression of P1, augmentation pressure (AP) and augmentation index (AIx) over a 10 year follow-up period according to age in women. Adapted from Cecelja et al. J Am Coll Cardiol 2012; 59: 475–83.
Conclusion
The prognostic importance of PWV is unlikely to it being a marker of atherosclerosis in the aorta. The exact mechanisms of arterial stiffening still remain unknown but inflammation, calcification and extracellular matrix degradation are likely to be important. Heamodynamic consequences of aortic stiffening include increased central (and peripheral pulsitility). In addition to preventing or reversing arterial stiffening, this haemodynamic consequence may be reversed by reducing AugP which can be reduced independent of any effect on PWV.
Disclosures
None.
References
Cite this article
TY - JOUR AU - Marina Cecelja AU - Phil Chowienczyk PY - 2012 DA - 2012/09/22 TI - Arterial stiffening: Causes and consequences JO - Artery Research SP - 22 EP - 27 VL - 7 IS - 1 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2012.09.001 DO - 10.1016/j.artres.2012.09.001 ID - Cecelja2012 ER -
