Achieving high seroprevalence against polioviruses in Sri Lanka-Results from a serological survey, 2014
- DOI
- 10.1016/j.jegh.2015.06.004How to use a DOI?
- Keywords
- Immunization; Poliomyelitis; Seroprevalence; Sri Lanka
- Abstract
The immunization program in Sri Lanka consistently reaches >90% coverage with oral poliovirus vaccines (OPV), and no polio supplementary vaccination campaigns have been conducted since 2003. We evaluated serological protection against polioviruses in children. A cross-sectional community-based survey was performed in three districts of Sri Lanka (Colombo, Badulla, and Killinochi). Randomly selected children in four age groups (9–11 months, 3–4 years, 7–9 years, and 15 years) were tested for poliovirus neutralizing antibodies. All 400 enrolled children completed the study. The proportion of seropositive children for poliovirus Type 1 and Type 2 was >95% for all age groups; for poliovirus Type 3 it was 95%, 90%, 77%, and 75% in the respective age groups. The vaccination coverage in our sample based on vaccination cards or parental recall was >90% in all age groups. Most Sri Lankan children are serologically protected against polioviruses through routine immunization only. This seroprevalence survey provided baseline data prior to the anticipated addition of inactivated poliovirus vaccine (IPV) into the Sri Lankan immunization program and the switch from trivalent OPV (tOPV) to bivalent OPV (bOPV).
- Copyright
- © 2015 Ministry of Health, Saudi Arabia. Published by Elsevier Ltd.
- Open Access
- This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
With polio eradication making rapid progress, only three countries remain in 2014 which had never eradicated wild poliovirus (Afghanistan, Nigeria, and Pakistan) [1]. In this context, a high priority is assigned to the preparations for the posteradication era. The key policy changes described in the Polio Eradication and Endgame Strategic Plan 2013–2018 [2] are global cessation of the Type 2 component of the oral poliovirus vaccine (OPV) (meaning the switch from trivalent OPV [tOPV] to bivalent OPV [bOPV]); and the global introduction of at least one dose of inactivated poliovirus vaccine (IPV) into every country’s routine immunization schedule. The strategy will allow for cessation of circulation and emergence of vaccine-derived poliovirus Type 2 (VDPV 2) while the last endemic foci of wild poliovirus are cleared [3].
The last case of poliomyelitis in Sri Lanka was reported in 1993 [4]. The routine immunization program in Sri Lanka is considered to be performing well and includes five doses of OPV administered at 2, 4, and 6 months of age as a primary series followed by revaccinations at 18 months and 5 years of age. The vaccination coverage with the third OPV dose has consistently exceeded 95% nationwide [5]. Supplementary immunization activities have been conducted to address known population immunity gaps from 1995 to 2003, and there has been no polio vaccination campaign since 2003.
Sri Lanka plans to introduce one dose of IPV into its routine immunization schedule in 2015. Following the recommendation of the Strategic Advisory Group of Experts, one dose of IPV will be introduced into the routine immunization schedule of all OPV-using countries in 2015; this IPV dose will be co-administered with a dose of OPV after 14 weeks of age [6]. The switch from tOPV to bOPV is planned for April 2016.
This study provides baseline data on seroprevalence of polio neutralizing antibodies in targeted age groups in Sri Lanka in anticipation of the IPV introduction and tOPV to bOPV switch.
2. Methods
We performed a cross-sectional community-based survey in three districts of Sri Lanka: Colombo, Badulla, and Killinochi. The study area purposefully represented the lower socioeconomic strata of the Sri Lankan society: in Colombo, children were selected from urban slum areas; in Badulla children of tea plantation workers were selected; and the district of Killinochi is considered less developed overall. Children in four age groups were selected: 9–11 months, 3–4 years, 7–9 years, and 15 years of age. The age groups were selected to assess serological protection achieved after primary immunization series as well as after revaccinations with OPV at 18 months and 5 years. The oldest age group was selected to assess serological protection in older children.
The participants were selected using simple random sampling from field level health registers kept with the Medical Officers of Health. Parents of eligible children were approached during regular visits of Public Health Midwives, consented, and enrolled. On the same day, the participants were transported to the nearest health center where a 1 mL blood sample was collected and a short questionnaire administered.
The blood specimens were allowed to clot. Sera were separated and transported to Colombo, where they were stored at −20 °C until the shipment to the Centers for Disease Control and Prevention (CDC) in Atlanta, GA, USA. The sera were tested for the presence of poliovirus neutralizing antibodies using standard neutralization assays at the virological laboratory at the CDC [7,8]. Seropositivity was defined as reciprocal titers of poliovirus neutralizing antibodies ⩾8.
The vaccination history of the enrolled children was recorded from vaccination cards when available; otherwise the history was obtained through parental recall.
A sample size of 100 children in each age group (to a total of 400 children) was calculated to be sufficient to detect, at the 95% confidence level, a seroprevalence point estimate with a precision of approximately ±5% assuming ⩾90% seroprevalence.
Ethical clearance was obtained by the Ethical Committees of the Ministry of Health, Sri Lanka and of the World Health Organization, Geneva, Switzerland.
Statistical analysis was performed using EpiInfo 3.5.3., point estimates and 95% confidence intervals were calculated for seroprevalence proportions and for median titers. The Chi-square uncorrected two-tailed p value was calculated to assess statistically significant differences in baseline characteristics between districts; we considered any p < 0.05 as an indicator of statistical significance.
This study was approved by the Ethics Review Committee of the Ministry of Health, Sri Lanka and by the WHO’s Ethics Review Committee in Geneva.
3. Results
We enrolled 400 eligible children and 400/400 (100%) completed the study. We did not encounter any dropout because enrollment and the blood collection occurred on the same day. All collected blood samples were of sufficient quantity, sera were obtained, and transportation to the CDC, Atlanta was carried out under proper cold chain conditions. All samples were analyzed for the presence of poliovirus neutralizing antibodies at the CDC, Atlanta.
The female to male ratio in the sample was 1:1.3; there were more families living with an average monthly income of USD <75 in Killinochi than in Badulla or Colombo; and the vaccination history with OPV indicated >90% coverage in all areas and for all age groups (Table 1).
| 9–11 mo | 3–4 y | 7–9 y | 15 y | Total | |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Enrolled | 100 | 100 | 100 | 100 | 400 |
| Colombo | 37 | 34 | 33 | 33 | 137 |
| Badulla | 30 | 32 | 33 | 34 | 129 |
| Killinochi | 33 | 34 | 34 | 33 | 134 |
| Sex (% female); n/N (%) | |||||
| Colombo | 20/37 (54%) | 14/34 (41%) | 13/33 (39%) | 11/33 (33%) | 58/137 (42%) |
| Badulla | 11/30 (37%) | 15/32 (47%) | 17/33 (52%) | 15/33 (44%) | 58/129 (45%) |
| Killinochi | 12/33 (36%) | 15/34 (44%) | 14/41 (41%) | 14/33 (42%) | 55/134 (41%) |
| Fully vaccinated with OPV (%) according to age; n/N (%) | |||||
| Colombo | 37/37 (100%) | 34/34 (100%) | 33/33 (100%) | 33/33 (100%) | 137/137 (100%) |
| Badulla | 29/30 (97%) | 32/32 (100%) | 33/33 (100%) | 34/34 (100%) | 128/129 (99%) |
| Killinochi | 33/33 (100%) | 34/34 (100%) | 34/34 (100%) | 30/33 (91%) | 131/134 (98%) |
| Average monthly income per household <$75 (%);n/N (%) | |||||
| Colombo | 0/37 (0%) | 0/34 (0%) | 1/33 (3%) | 0/33 (0%) | 1/137 (1%) |
| Badulla | 1/30 (3%) | 0/32 (0%) | 3/33 (9%) | 2/34 (6%) | 6/129 (5%) |
| Killinochi | 12/33 (36%) | 6/34 (18%) | 0/34 (0%) | 3/33 (9%) | 21/134 (16%) |
| Serological results | – | – | – | – | |
| Poliovirus Type 1 | |||||
| % Positive (95% CI) | 96 (90.1, 98.9) | 99 (94.6, 100) | 99 (94.6, 100) | 97 (91.5, 99.4) | 98 (95.6, 98.9) |
| Median titer, (95% CI) | ⩾1448 (⩾1448, ⩾1448) | 724.1 (455, 1152) | 325 (181, 455) | 90.5 (56, 144) | 455 (455, 724) |
| Poliovirus Type 2 | |||||
| % Positive (95% CI) | 98 (93, 99.8) | 97 (91.5, 99.4) | 99 (94.6, 100) | 100 (96.4, 100) | 99 (96.6, 99.4) |
| Median titer, (95% CI) | ⩾1448 (⩾1448, ⩾1448) | 576 (408, 724) | 227 (144, 455) | 113 (90, 181) | 455 (362, 576) |
| Poliovirus Type 3 | |||||
| % Positive (95% CI) | 95 (88.7, 98.4) | 90 (82.4, 95.1) | 77 (67.5, 84.8) | 75 (65.3, 83.1) | 84 (80.3, 87.7) |
| Median titer, (95% CI) | 910 (576, 1152) | 113 (64, 227) | 36 (18, 90) | 28 (11, 45) | 91 (72, 144) |
CI = confidence interval; OPV = oral poliovirus vaccine.
Baseline characteristics and serological results.
There were no significant differences in the serological results between the three districts. The seroprevalence to all three serotypes was >90% for the 9–11 months and 3–4 years age groups. For the 7–9 years and 15 years age groups, the proportion of seropositive children for poliovirus Type 1 and Type 2 remained >90%, however for serotype 3 it dropped to 77% for 7–9-year-olds and to 75% in 15-year-olds (Table 1, Figs. 1 and 2). The median titers of poliovirus neutralizing antibodies were high for all three serotypes in the youngest age groups, and decreased with increasing age (Table 1, Figs. 1 and 2). No children were found to be seronegative to all three poliovirus types, and there were 7/400 (1.8%) children seronegative for two of the three serotypes. There was no statistically significant difference observed in the vaccination history among seropositive versus seronegative children; the seronegative children in our survey received 100% OPV doses according to their age. Further, there was no statistical difference observed in seroprevalence between children from families living with an average monthly income of <USD 75 and children from families living with higher monthly income.
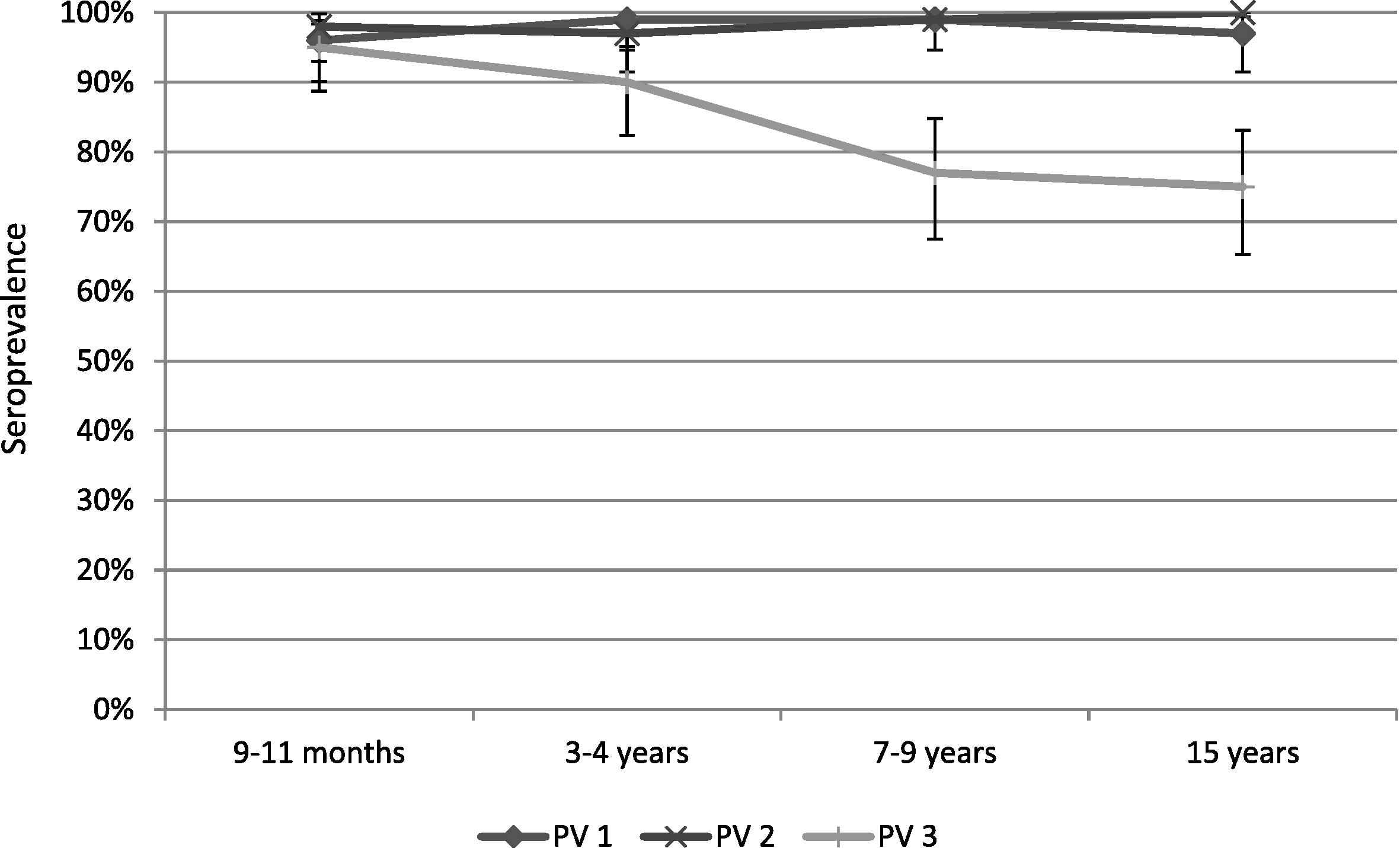
Seroprevalence in the selected age groups with a 95% confidence interval.
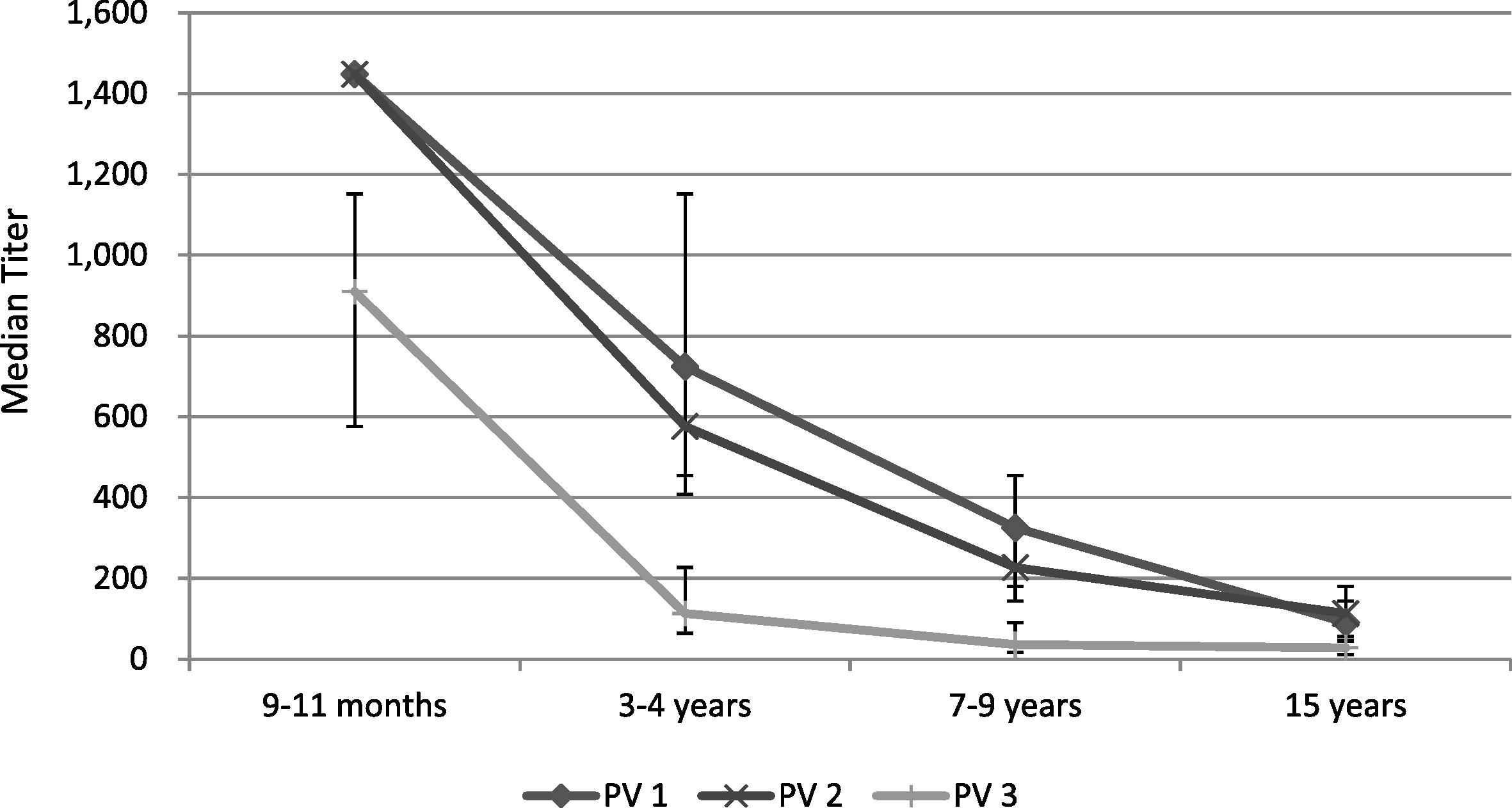
Median reciprocal antibody titers in the selected age groups with a 95% confidence interval. PV1 = poliovirus serotype 1; PV2 = poliovirus serotype 2; PV3 = poliovirus serotype 3.
4. Discussion
In our study we observed high serological protection against polioviruses in all age groups and for all three serotypes. The Sri Lankan immunization program has achieved a high level of population immunity through its routine immunization program and without polio supplementary immunization activities. Our study purposefully selected areas with higher proportions of children from lower socioeconomic strata; we therefore hypothesize that seroprevalence in other populations of Sri Lanka would likely be equal to or higher than our findings.
We observed declining seroprevalence of poliovirus Type 3 neutralizing antibodies in the older age groups while seroprevalence against poliovirus Type 1 and Type 2 remained unchanged with age. Further, as expected, the median titer of poliovirus neutralizing antibodies declined for all three serotypes with interval from the last vaccination, which in this study, corresponds with increasing age [9,10]. This decline, including decline to nondetectable titers (i.e., <1:8) for some individuals, does not imply loss of protection against paralytic disease, as demonstrated by rapid anamnestic responses following revaccination in older adults [11,12]. However, because of rapidly waning mucosal immunity, these individuals would likely excrete polioviruses in stools and therefore participate in the chain of transmission if exposed to live or VDPVs [13].
This study had some limitations. The selection of participants was based on the knowledge of the area Public Health Midwife and available field level registers kept with the Medical Officers of Health. This may have meant that some households were excluded from participation because they were not included in the registers. However, the Sri Lankan health system provides registration of all births. In Sri Lanka, very few children are unregistered with the Medical Officers and therefore we believe that this bias was unimportant. Further, the sample size calculation assumed seroprevalence level of ⩾90%, however, for serotype 3, the seroprevalence was lower and this resulted in a slightly wider 95% confidence interval than originally anticipated.
Sri Lanka is well positioned to implement the changes to its immunization program recommended by the Strategic Advisory Group of Experts of the WHO-the introduction of one dose of IPV and the switch from tOPV to bOPV. Our results provide important baseline data prior to the switch; repeating a similar survey after the switch will allow assessment of population immunity to polioviruses achieved with the new polio immunization schedule.
Conflicts of interest
The authors do not declare any conflict of interest.
Role of the funding source
Funding for this study was provided by the World Health Organization, Geneva.
Role of medical writer or editor
No medical writer or editor was engaged.
Ethics committee approval
This study was approved by the Ethics Review Committee of the Ministry of Health, Sri Lanka and by the WHO’s Ethics Review Committee in Geneva.
Acknowledgment
Funding for this study was provided by the World Health Organization, Geneva.
References
Cite this article
TY - JOUR AU - Deepa Gamage AU - Paba Palihawadana AU - Ondrej Mach AU - William C. Weldon AU - Steven M. Oberste AU - Roland W. Sutter PY - 2015 DA - 2015/07/09 TI - Achieving high seroprevalence against polioviruses in Sri Lanka-Results from a serological survey, 2014 JO - Journal of Epidemiology and Global Health SP - S67 EP - S71 VL - 5 IS - Supplement 1 SN - 2210-6014 UR - https://doi.org/10.1016/j.jegh.2015.06.004 DO - 10.1016/j.jegh.2015.06.004 ID - Gamage2015 ER -
