The Malay version of the brief questionnaire on smoking urge: Translation and psychometric properties of the questionnaire
- DOI
- 10.1016/j.jegh.2014.10.006How to use a DOI?
- Keywords
- Smoking urge; QSU-Brief; Translation; Factor analysis; Malaysia
- Abstract
This study aimed to evaluate the psychometric properties of Malay translated version of the brief questionnaire of smoking urges (QSU-Brief). The translation procedure was done following the standard guidelines. The reliability and validity of the Malaysian version scale were evaluated based on the data collected from 133 Malaysian smokers. The internal consistency was calculated to assess the reliability. Factor analysis and construct validity were performed to validate psychometric properties of the scale. Total Cronbach’s alpha of the scale was 0.806. The exploratory factor analysis revealed two factors that accounted for 66.15% of the explained total variance. The first component consisted of items 1, 3, 6, 7, and 10, while the second component included the rest. The QSU-Brief total score had a significant positive relationship with exhaled CO level (r = 0.24; P = 0.005), number of cigarettes smoked per day (r = 0.30; P < 0.001) and other clinical factors. Items 2 and 5 loaded strongly on factor 2, whereas both items loaded ambivalently on two factors in the previous studies. This discrepancy might be clarified by language differences. The Malaysian QSU-Brief is a good candidate for evaluating urge to smoke in both clinical practice and clinical trials.
- Copyright
- © 2014 Ministry of Health, Saudi Arabia. Published by Elsevier Ltd.
- Open Access
- This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Craving is often described as an important concept in smoking dependence and the most noticeable and bothersome symptom experienced during the quitting attempt [1]. According to an expert group meeting organized by the United Nations International Drug Control Programme (UNDCP) and WHO, craving is defined as “the desire to experience the effect(s) of a previously experienced psychoactive substance [2]”.
Several studies have concluded that craving hinders successful smoking cessation and that it correlates with relapse after periods of abstinence [3–6]. Moreover, the effects of positive outcome expectations of smoking on relapse appear to be completely mediated by craving [7]. Accordingly, the assessment of withdrawal symptoms with the urge to smoke form an integral part of assessing health and quality of life in smokers in order to predict relapse, understand the nature of nicotine dependence and improve cessation treatment [8,9].
The decision to translate the brief questionnaire on smoking urge (QSU-Brief) was made because there was no translated scale to evaluate craving to smoke in Malay language for research and clinical practice. The current study aimed to subject the QSU-Brief to translation and validation processes for future use by clinicians and researchers.
2. Material and methods
2.1. Study design and setting
A cross-sectional study design was adopted to conduct the study. It was carried out at the Quit Smoking Clinic in the Pulau Pinang Hospital, Penang State, Malaysia. The Pulau Pinang Hospital is the largest public tertiary hospital in the State of Penang.
2.2. Participants
Smokers who attended the Quit Smoking Clinic were included in the study subjects and were either referred from the outpatient clinics of the hospital or outside clinics and/or walk-in smokers. Furthermore, all outpatient clinics of the Pulau Pinang Hospital were contacted to refer any smoker patient willing to quit to the Quit Smoking Clinic. Adult smokers (male or female) aged more than 18 years, who were able to read/understand and complete the Malay language measurement tool independently were included.
The subject was excluded if he/she had a past or present history of mental illness, used concomitant antidepressant, antianxiety medication or sedatives, suffer from alcohol or drug abuse or were subjects who, in the researchers’ opinion, would be unlikely to commit to the study.
2.3. Sample size
In general, it is highly recommended to use at least 10 subjects for each item of a questionnaire or an instrument scale for the validity evaluation [10,11]. However, a target sample size of 100 patients was estimated to give a better precision to the reliability and validity of the study [12]. Others suggest that five subjects for each item are adequate in most cases [13].
In this study, it was decided to depend on the recommendation of at least 10 subjects for each item of a questionnaire or an instrument scale for the validity evaluation [11]. The QSU-Brief consisted of 10 items, and it was estimated that 100 smokers were needed for the purpose of validation. An additional 30% of drop outs were considered to be necessary for the study to overcome the erroneous results and to increase the reliability of the conclusion. A convenience sample of (total = 133) smokers who attended the Quit Smoking Clinic was collected. In addition, only 75 subjects agreed to participate in a test–retest reliability analysis. There is no evidence available to aid in the selection of the time interval between questionnaire administrations for a study of test–retest reliability of health status instruments, and an interval ranging from 10 min to 1 month was selected. Therefore, a month interval was chosen for the purpose of subjects’ feasibility.
2.4. Ethical approval
This study was conducted after it was approved by the Ethics Committee of the Institute of Public Health (IPH), the National Institutes of Health (NIH) and the Medical Research and Ethics Committee (MREC) of the Ministry of Health, Malaysia. Before starting the interviews by an expert counselor, a written consent form was provided to all of the participants. All participants were assured that their personal information would be kept confidential. The counselor interview for each participant to explain the study aims and procedures took about 15–20 min.
2.5. Instruments
A structured questionnaire was used for the collection of data that was needed for the validation study and it consisted of three sections: (1) participants’ socio-demographic information, patient’s smoking status history, and carbon monoxide (CO) concentration value measured by the Smokerlyzer MicroCO meter, which is made by the Micro Medical Limited Company. This device measures the concentration of CO on the breath, and considers the subject a smoker when the CO level is more than 6 parts per million (ppm) (<6 ppm = non-smoker, 7–10 ppm = light smoker, 11–20 = smoker, and >20 = heavy smoker); (2) Malay version of the Fagerstrom Test for Nicotine Dependence (FTND-M); (3) Malay version of 10-item QSU-Brief.
2.6. Linguistic validation process
In order to develop or translate any patient-reported outcome measures such as QSU-Brief for cross-cultural comparisons, it was necessary to achieve “conceptual equivalence” between the original scale and the target language version of the scale [14,15]. In the present study, the conceptual equivalence occurs when the differences in meaning and content of the context between the source language (English) of the QSU-brief and the translated version (Malay) are absent [14]. This is achieved through a procedure called linguistic validation and cultural adaptation [15]. This process includes two essential and complementary steps: a translation step to achieve linguistic validity of the instrument in the desired language and to assess the underlying structure of the translated version. Permission was taken from the copyright owners of the original instrument to translate the questionnaires into Malay language. Moreover, the translation was done according to the standard guidelines as follows [15,16]:
- 2.6.1.
A forward translation: one-way translation into the target language was carried out by two qualified independent linguistic translators from the School of Language, Literacies and Translation, Universiti Sains Malaysia who are experts in linguistic validation procedure to create a version that was semantically and conceptually as close as possible to the original scale. They are both native Malaysian speakers and proficient in English. Each translator formed a forward translation version without any mutual consultation. During this step, two translated Malay versions which contained words and sentences that cover both the medical and usual Malay speaking language with its culture nuances were generated. Comparison and reconciliation of the two forward translations was done by two native Malaysian researchers who resolved any existing ambiguities and discrepancies. Thereafter, a single preliminary-initial translated version was evolved based on the two forward translations and reconciliation.
- 2.6.2.
Blind back-translation: translation back of the first reconciled translated Malay version into the original language was undertaken by a third translator who is fluent in both the languages. The translator was completely blind to the original version of the instrument. This aimed to obtain a translation that was free of bias and expectation, but may have revealed unexpected but important meanings or interpretation in the final version. Subsequently, a back translation review was done by comparison of the back-translated version with the original to highlight and investigate discrepancies between the original and the reconciled translation. Inconsistencies were resolved in a consensus meeting and a pre-final Malay version, ready for a pilot testing, was generated.
- 2.6.3.
Pilot testing: the pre-final version of the instruments was pretested on 20 smokers who were native Malay speakers at the Quit Smoking Clinic of Pulau Pinang Hospital. The participants were asked to complete the questionnaire and were interviewed by a counselor to identify if they had any difficulty in comprehending any question. Then, reviews of participants’ feedback were discussed by the researchers.
- 2.6.4.
The final form of the Malay version of the questionnaire was accomplished and prepared for the reliability and validity study. The measurement scales took approximately 10 min to complete.
2.7. Statistical analysis
All statistical analyses were conducted by using SPSS version 18.0 (SPSS Inc., Chicago, IL). The significance level was set at a P value less than 0.05. Descriptive statistics were used to describe demographic and smoking-related characteristics of the subjects in the QSU-Brief sample separately. Descriptive analyses were performed for quantitative (continuous) variables by calculating mean ± standard deviation (SD), while percentages and frequencies were determined for qualitative (categorical) variables.
Cronbach’s alpha coefficient was used to measure the internal consistency and homogeneity of the items and the total score for the questionnaire. Intraclass correlation (ICC) for each item and for the total score of the Malay version of QSU-Brief was estimated to evaluate test–retest reliability. The internal consistency and test–retest reliability were used in order to assess the reliability of the scale. In order to assess the validity of the scale, factor analysis and concurrent validity were employed to validate the psychometric properties of the scale. Exploratory Factor analysis with orthogonal rotation was conducted on the items of the scale to determine the factor structure of the translated scale. To verify that the data set is suitable for factor analysis, the Kaiser–Meyer–Olkin Measure of Sampling Adequacy (KMO) [17] and the Bartlett’s test of sphericity [18] were applied. The criteria used to select the number of factors and the number of items within a factor of exploratory factor analysis included: eigenvalue greater than 1; item-factor loading of at least 0.4 [19]. Concurrent validity was used to support the validation of the scale by administering the FTND-M with the translated QSU-Brief to assess the association between these two tools. Construct validity is established when there is a correlation between the results of a desired measure and the results of a validated measure that are obtained at approximately the same point in time [20,21]. In addition, scale validation was assessed through the association of scale total score with several variables using Spearman Rank Correlation Coefficient test.
3. Results
The mean age for our participants’ study was 48 years. About ninety nine percent of them were males (Table 1).
| Minimum–Maximum | ||
|---|---|---|
| Age (M ± SD) (years) | 47.7 ± 14.0 | 18–76 |
| Gender [N (%)] | ||
| Male | 132 (99.2%) | |
| Female | 1 (0.8%) | |
| Race [N (%)] | ||
| Malay | 50 (37.6%) | |
| Chinese | 52 (39.1%) | |
| Indian | 31 (23.3%) | |
| Educational status [N (%)] | ||
| No formal education | 4 (3.0%) | |
| Primary | 59 (44.4%) | |
| Secondary | 62 (46.6%) | |
| Collage/University | 8 (6.0%) | |
| Marital status [N (%)] | ||
| Single | 19 (14.3%) | |
| Married | 114 (85.7%) | |
| Age starting smoking (M ± SD) | 18.43 ± 5.4 | 8–54 |
| Number of cigarettes smoked/day (M ± SD) | 14.92 ± 9.1 | 2–40 |
| Duration of smoking (M ± SD) (years) | 29.26 ± 13.2 | 2–60 |
| Previous quit attempts [N (%)] | ||
| Yes | 30 (22.6%) | |
| No | 103 (77.4%) | |
| FTND total score | 1.97 ± 1.33 | 0–7 |
| Exhaled CO level | 13.83 ± 5.26 | 4–28 |
M ± SD = Mean ± standard deviation, CO = carbon monoxide.
Data were presented as (M ± SD) with minimum to maximum values unless otherwise indicated.
Socio-demographic and smoking-related information characteristics for the study participants (N = 133).
3.1. Reliability of the questionnaire
The internal consistency estimate for the total score of the QSU-Brief was 0.806. Therefore, the Malay version of the QSU-Brief has a good internal consistency [22,23]. Item-to-total correlation for each item ranged from 0.29 to 0.71 (Table 2). The ICC value for each single item ranged from 0.97 to 0.98 and the questionnaire’s total score was 0.99.
| Corrected Item-Total Correlation | Cronbach’s Alpha if Item Deleted | ICC | 95% confidence interval | P value | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Question 1 | 0.71 | 0.76 | 0.98 | 0.97 | 0.99 | <0.001 |
| Question 2 | 0.37 | 0.80 | 0.97 | 0.95 | 0.98 | <0.001 |
| Question 3 | 0.61 | 0.77 | 0.98 | 0.98 | 0.99 | <0.001 |
| Question 4 | 0.33 | 0.80 | 0.98 | 0.98 | 0.99 | <0.001 |
| Question 5 | 0.44 | 0.79 | 0.97 | 0.96 | 0.98 | <0.001 |
| Question 6 | 0.46 | 0.79 | 0.98 | 0.97 | 0.99 | <0.001 |
| Question 7 | 0.69 | 0.76 | 0.98 | 0.98 | 0.99 | <0.001 |
| Question 8 | 0.46 | 0.79 | 0.98 | 0.98 | 0.99 | <0.001 |
| Question 9 | 0.29 | 0.80 | 0.98 | 0.98 | 0.99 | <0.001 |
| Question 10 | 0.42 | 0.79 | 0.98 | 0.98 | 0.99 | <0.001 |
| Total | – | – | 0.995 | 0.99 | 0.99 | <0.001 |
ICC: intra-class correlation; The Cronbach’s Alpha for the questionnaire was 0.81.
Reliability and test–retest analysis for the Malay version of QSU-Brief.
3.2. Validation of the Malay version of QSU-Brief
All items of the Malay version of the QSU-Brief were subjected to EFA with orthogonal rotation to assess the questionnaire structure. Bartlett’s test of sphericity revealed that data were suitable for factor analysis (P < 0.001). KMO measure of sampling adequacy for the QSU-Brief was 0.779 (above 0.6) indicating that a sample of 133 subjects was adequate for factor analysis. Examination of the scree plot showed that the first scree cut-off fell after the second factor. Both factors had an eigenvalue above the traditional cut-off of 1.0 (3.69 and 2.91 for the first and second factors, respectively) (Fig. 1).
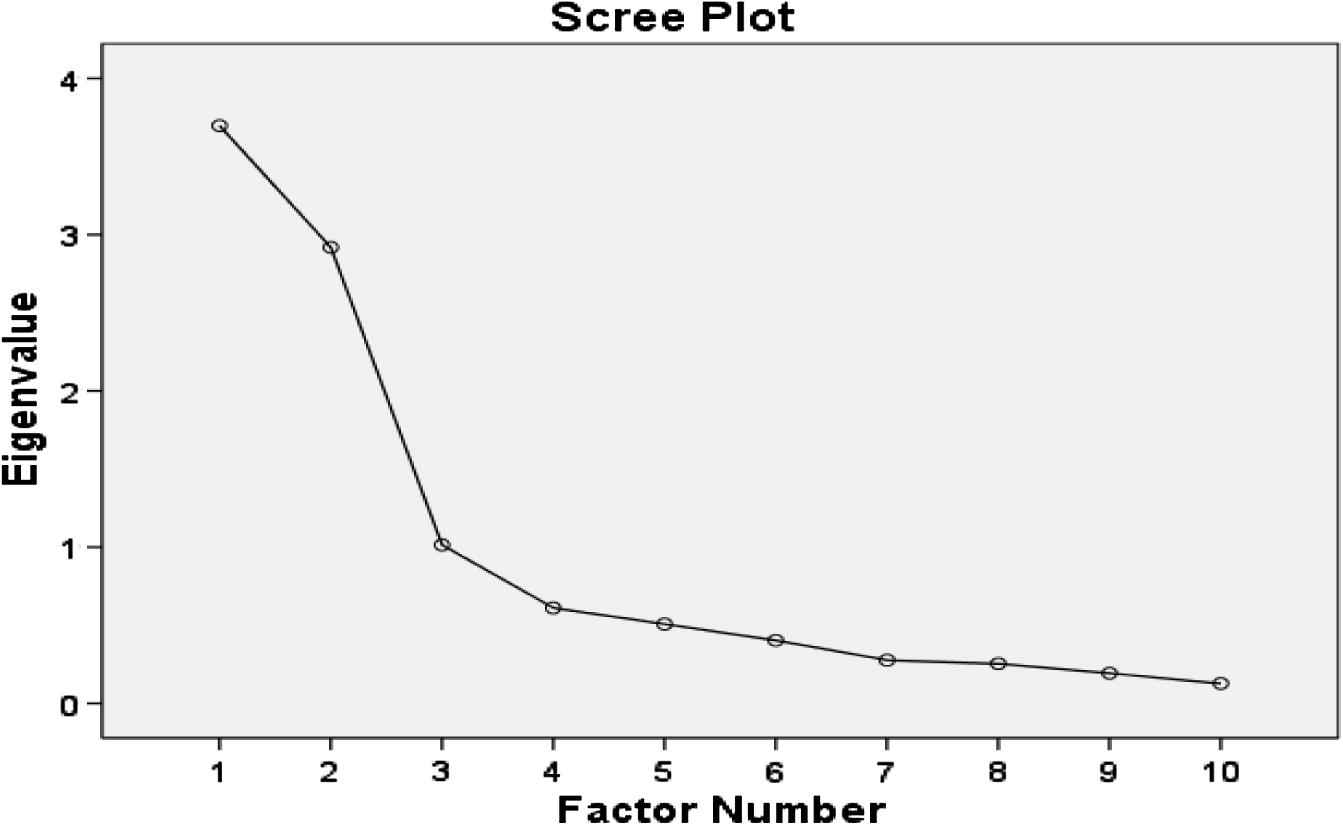
Scree Plot test for the Malay QSU-Brief.
These two factors accounted for 66.15% of the explained total variance (Table 3). The first factor included 5 items (item numbers 1, 3, 6, 7, and 10), which had a loading of more than 0.40 on the first factor and reflect the desire and intention to smoke with an anticipation of pleasure from smoking. Similarly, the other 5 items (item numbers 2, 4, 5, 8, and 9) had high loading on the second factor, which represents an anticipation of relief from negative affect with an urgent desire to smoke, as described in Cox et al. [26].
| Original and translated items | Rotated factor loading | |
|---|---|---|
| Factor I | Factor II | |
| 1. I have a desire for a cigarette right now Saya terasa ingin merokok sekarang |
0.896 | |
| 2. Nothing would be better than smoking a cigarette right now Sekarang ini, tiada apa yang lebih hebat melainkan saya dapat merokok |
0.685 | |
| 3. If it were possible, I probably would smoke now Jika boleh, saya mahu merokok sekarang juga |
0.874 | |
| 4. I could control things better right now if I could smoke Saya dapat mengawal sesuatu dengan lebih baik sekarang, jika saya dapat merokok |
0.682 | |
| 5. All I want right now is a cigarette Yang saya inginkan sekarang adalah sebatang rokok |
0.794 | |
| 6. I have an urge for a cigarette Saya mempunyai kehendak untuk merokok |
0.602 | |
| 7. A cigarette would taste good now Rokok akan rasa bagus sekarang |
0.912 | |
| 8. I would do almost anything for a cigarette right now Saya sanggup lakukan apa sahaja untuk merokok sekarang ini |
0.816 | |
| 9. Smoking would make me less depressed Merokok membuatkan saya kurang tertekan |
0.602 | |
| 10. I am going to smoke as soon as possible Saya akan merokok seberapa segera yang mungkin |
0.633 | |
| % of total variance | 66.15 | |
Principle component analysis for the Malay QSU-Brief (N = 133).
The majority of the proposed relationships with the QSU-Brief total score showed moderate to good correlation. The QSU-Brief total score had a significant positive relationship with exhaled CO level (r = 0.24; P = 0.005), FTND total score (r = 0.24; P = 0.005) and number of cigarettes smoked per day (r = 0.30; P < 0.001). In addition, the QSU-Brief total score was not correlated with the duration of smoking (P = 0.503) and previous quit attempts (P = 0.077) (Table 4).
| Malay QSU-Brief total score Correlation coefficient |
P value* | |
|---|---|---|
| CO-level | 0.24 | 0.005 |
| FTND total score | 0.24 | 0.005 |
| Number of cigarettes smoked/day | 0.30 | <0.001 |
| Duration of smoking | 0.06 | 0.503 |
| Chances for quitting | −0.29 | 0.001 |
| Previous quit attempts | 0.15 | 0.077 |
Spearman rank correlation coefficient test.
Correlations between the total score of Malay QSU-Brief with characteristics variables.
4. Discussion
This is the first study that systematically translates and validates the 10 items of the QSU-Brief into the Malay language. The translated questionnaire showed a good reliability (internal consistency estimate for the total score of the QSU-Brief was 0.806) according to rule of thumb by George and Mallery [22].
The factor analysis revealed that the translated questionnaire consists of two dimensions, which is largely consistent with the findings of the exploratory and confirmatory factor analyses of the original English version of the QSU and the original English version of the 10-item QSU-Brief [24,25]. The first component consisted of the items 1, 3, 6, 7, and 10, while items 2, 4, 5, 8 and 9 comprised component 2.
In addition, items 2 and 5 loaded strongly on factor 2, whereas both items loaded ambivalently on two factors in previous studies. This discrepancy might be clarified by language differences. However, this result was consistent with findings of the Dutch version [26]. Items 2 and 5 convey extreme utterances, especially if they were exactly translated into the Malay language. Because item numbers 4 and 9 have the same extreme sounds, it is not surprising that they all loaded on the same factor.
As expected, the total score of the Malaysian version of the QSU-Brief was significantly correlated with the CO level, number of cigarettes smoked per day and the total score of one questionnaire, i.e., cigarette dependence (FTND) (Table 4). Similarly, significant correlations between the total score of the Dutch version of the QSU-Brief and the total score of FTND, r = 0.14, P < 0.05 and number of cigarettes smoked/day, (r = 0.14, P < 0.05 and r = 0.25, P < 0.01, respectively) were found [26].
There were a few limitations to the study. First, there was only one female in the cohort (corresponding to seven females who attended the clinic during the period of data collection).
This limitation might be related to different reasons, such as social and cultural restriction issues. Numerous studies reported that while cigarette smoking remains acceptable for males, smoking by women is not socially sanctioned in Malaysia and other Asian countries in general [27–29]. In addition, it might be related to the fact that the majority of Malaysian tobacco users (80.3%) tried to stop smoking by themselves without seeking professional assistance [30]. Another limitation is that approximately 80% of the recruited participants were classified as having a very low level of nicotine dependence upon the score of FTND-M. Therefore, the generalizability of these results with other Malay speaking females or those with the higher nicotine dependence levels might be compromised.
5. Conclusion
The Malaysian QSU-Brief is a good candidate for evaluating the urge to smoke in both clinical practice and clinical trials.
Conflict of interest
There was no conflict of interest to be declared.
Acknowledgment
Authors are grateful to the staff of the Quit Smoking Clinic at Pulau Pinang Hospital for their support and cooperation in data collection during the period of the study.
References
Cite this article
TY - JOUR AU - Ali Qais Blebil AU - Syed Azhar Syed Sulaiman AU - Mohamed Azmi Hassali AU - Juman Abdulelah Dujaili AU - Alfian Mohamed Zin PY - 2014 DA - 2014/12/01 TI - The Malay version of the brief questionnaire on smoking urge: Translation and psychometric properties of the questionnaire JO - Journal of Epidemiology and Global Health SP - 15 EP - 22 VL - 5 IS - 1 SN - 2210-6014 UR - https://doi.org/10.1016/j.jegh.2014.10.006 DO - 10.1016/j.jegh.2014.10.006 ID - Blebil2014 ER -
