An updated global picture of cigarette smoking persistence among adults
- DOI
- 10.1016/j.jegh.2012.06.003How to use a DOI?
- Keywords
- Tobacco; Smoking persistence; Meta-analysis
- Abstract
Background: Cross-national variance in smoking prevalence is relatively well documented. The aim of this study is to estimate levels of smoking persistence across 21 countries with a hypothesized inverse relationship between country income level and smoking persistence.
Methods: Data from the World Health Organization World Mental Health Survey Initiative were used to estimate cross-national differences in smoking persistence—the proportion of adults who started to smoke and persisted in smoking by the date of the survey.
Results: There is large variation in smoking persistence from 25% (Nigeria) to 85% (China), with a random-effects meta-analytic summary estimate of 55% with considerable cross-national variation. (Cochran’s heterogeneity Q statistic = 6845; p < 0.001). Meta-regressions indicated that observed differences are not attributable to differences in country’s income level, age distribution of smokers, or how recent the onset of smoking began within each country.
Conclusion: While smoking should remain an important public health issue in any country where smokers are present, this report identifies several countries with higher levels of smoking persistence (namely, China and India).
- Copyright
- © 2012 Ministry of Health, Saudi Arabia. Published by Elsevier Ltd.
- Open Access
- Open access under CC BY-NC-ND license. http://creativecommons.org/licenses/by-nc-nd/4.0/
1. Introduction
Cigarette smoking (hereinafter referred to as ‘smoking’) continues to be one of the leading causes of preventable global mortality [1]. Global surveillance of smoking activity continues to be an important task in an effort to reduce this burden [2]. The Global Youth Tobacco Survey estimated that one third of the world’s 13–15 year olds had ever smoked a cigarette, and an estimated one fourth of these had done so before their 10th birthday [3]. The World Health Organization Framework Convention on Tobacco Control (WHO FCTC) was established to halt the global tobacco epidemic in 2003. This treaty has currently been ratified by 174 parties, 120 of which have adopted or strengthened their tobacco regulation legislation [4,5]. Article 20 of the WHO FCTC places particular emphasis on the need for standardized tobacco use surveillance for the purpose of cross-national comparisons.
In a recent report by Storr et al., cross-national estimates for smoking prevalence were presented based on results from the first 17 sites of the World Mental Health Surveys Initiative (WMHS) [6]. The prevalence of current smoking ranged from 3.9% (Nigeria) to 36.0% (Lebanon) within the countries studied. Here, a more complete view of this global smoking experience is sought, with new analyses and new data from all 21 WMH sites with a focus on smoking persistence.
The two main parameters affecting the prevalence of a condition are the incidents of the condition (the rate at which people are becoming new cases) and the duration of the condition, thus any difference in smoking prevalence might be owing to a difference in smoking initiation, smoking persistence or a combination of the two. This study focused on smoking persistence. Prior research documented a relatively steady increase in smoking prevalence in many low to middle income countries of the world during the twentieth century [2], concurrent with relatively more stable or declining prevalence elsewhere [7,8]. Accordingly, this study was approached with an implicit hypothesis that there might be an inverse relationship between the general income level of a country and its level of smoking persistence (i.e., smokers in lower income countries in this report might be more likely to persist in smoking, once smoking starts, as compared with smokers in the higher income countries).
2. Methods
WMHS methods have been described in detail elsewhere [6,9–11]. In brief, the cross-sectional surveys have a mental health focus, and their main goal has been to estimate the prevalence and impact of psychiatric disorders worldwide using a common survey protocol [10,11]. Adult designated respondents (DRs) at each site were recruited for standardized assessments after multi-stage community probability sampling, with participation levels generally at 70%–80%. Most surveys were nationally representative; however, this was infeasible in certain countries. The samples of Nigeria, Mexico and Colombia were representative of 57%, 73% and 73% of their populations, respectively. In addition, the samples from India, Japan and China were representative of large metropolitan areas. Attempts were made to recruit as many countries as possible; however, the final sample reflects countries with collaborators able to obtain funding for the survey. All surveys were conducted from 2001–2008. Site sample sizes ranged from 2357 (Romania) to 12,992 (New Zealand), with 101,392 DRs in the aggregate sample. A list of all countries as well as each country’s sample size and income level can be found in Table 1. All sites except Japan surveyed subjects ⩾18 years old (Japan surveyed subjects ⩾20 years old). Columbia and Mexico did not survey adults >65 years old while all other sites had no age limit. Language variations across countries prompted methods designed for multi-national and cross-cultural research [12]. All protocols were approved by institutional review boards for protection of human subjects across all sites.
| Country (sample size; n of ever smokers) | Birth Cohort | Elapsed time since smoking onset | Sex | |||
|---|---|---|---|---|---|---|
| Pre-1945 | Post-1945 | >10 years | ⩽10 years | Male | Female | |
| Nigeriac (n = 6752; 1137) | 13 (8, 18) | 31 (27, 35) | 21 (15, 26) | 32 (12, 51) | 27 (23, 31) | 24 (6, 42) |
| Colombiab (n = 4426; 2087) | 22 (14, 30) | 40 (37, 43) | 39 (36, 43) | 38 (32, 45) | 39 (35, 44) | 36 (31, 40) |
| Mexicob (n = 5782; 3276) | 30 (22, 38) | 44 (41, 47) | 46 (42, 49) | 42 (38, 47) | 48 (44, 51) | 35 (30, 39) |
| New Zealanda (n = 12,992; 6855) | 23 (21, 25) | 54 (52, 55) | N/A | N/A | 46 (43, 48) | 47 (44, 49) |
| United Statesa (n = 9282; 4835) | 28 (26, 30) | 55 (53, 56) | 51 (47, 55) | 63 (52, 75) | 48 (46, 50) | 47 (45, 49) |
| Northern Irelanda (n = 4340; 2074) | 33 (29, 37) | 60 (57, 63) | N/A | N/A | 48 (44, 52) | 57 (53, 60) |
| Belgiuma (n = 2419; 1234) | 34 (28, 40) | 59 (54, 64) | N/A | N/A | 53 (49, 58) | 52 (46, 58) |
| Brazilb (n = 5211; 2120) | 35 (31, 39) | 57 (54, 60) | 52 (48, 56) | 67 (48, 86) | 51 (48, 55) | 55 (53, 58) |
| Francea (n = 2894; 1502) | 27 (21, 33) | 61 (59, 63) | N/A | N/A | 52 (49, 56) | 55 (51, 59) |
| Netherlandsa (n = 2372; 1453) | 35 (30, 40) | 61 (57, 65) | N/A | N/A | 51 (47, 55) | 56 (52, 60) |
| Japana (n = 3417; 1623) | 39 (34, 44) | 63 (59, 67) | N/A | N/A | 57 (53, 60) | 50 (45, 57) |
| Italya (n = 4712; 2295) | 35 (30, 40) | 67 (65, 69) | N/A | N/A | 54 (51, 57) | 65 (61, 68) |
| Germanya (n = 3555; 1833) | 33 (26, 40) | 71 (68, 74) | N/A | N/A | 59 (55, 63) | 64 (59, 69) |
| Romaniab (n = 2357; 866) | 47 (39, 55) | 68 (63, 72) | 65 (60, 71) | 71 (60, 82) | 62 (57, 68) | 68 (61, 75) |
| Spaina (n = 5473; 2796) | 36 (31, 41) | 74 (72, 76) | N/A | N/A | 58 (55, 62) | 75 (73, 78) |
| Ukrainec (n = 4725; 1911) | 46 (42, 50) | 77 (74, 80) | 74 (69, 80) | 66 (55, 78) | 73 (70, 76) | 63 (60, 66) |
| South Africab (n = 4315; 1375) | 49 (42, 56) | 76 (73, 79) | 84 (80, 87) | 66 (60, 72) | 73 (70, 77) | 70 (66, 74) |
| Bulgariab (n = 5318; 2610) | 46 (41, 51) | 80 (78, 82) | 73 (69, 76) | 85 (79, 92) | 72 (69, 75) | 77 (74, 80) |
| Indiac (n = 2992; 369) | 71 (61, 81) | 76 (70, 82) | 68 (58, 78) | 82 (55, 100) | 77 (72, 82) | 55 (36, 74) |
| Lebanonb (n = 2857; 1439) | 56 (49, 63) | 83 (80, 86) | 79 (72, 85) | 92 (83, 100) | 77 (73, 81) | 78 (73, 83) |
| Chinac (n = 5201; 2001) | 55 (48, 62) | 87 (85, 89) | 80 (76, 84) | 86 (72, 100) | 83 (81, 85) | 77 (70, 85) |
| Meta-analytic summary: | 37 (33, 42)1 | 64 (58, 70)2 | 61 (51, 71)3 | 66 (55, 77)4 | 57 (60, 64)5 | 58 (52, 63)6 |
Here, the estimates are for the proportion of all smokers who were also current smokers on the date of assessment. Sites are listed in ascending order according to their unadjusted persistence estimates. Data from Israel are not included owing to a difference in assessment of smoking. Elapsed time since smoking onset stratification is not available for sites that did not ask about the age of smoking onset.
Tests for heterogeneity: Cochran’s Q (df = 20) = 410; p < 0.001.
Tests for heterogeneity: Cochran’s Q (df = 20) = 2133; p < 0.001.
Tests for heterogeneity: Cochran’s Q (df = 11) = 240; p < 0.001.
Tests for heterogeneity: Cochran’s Q (df = 11) = 912; p < 0.001.
Tests for heterogeneity: Cochran’s Q (df = 20) = 2276; p < 0.001.
Tests for heterogeneity: Cochran’s Q (df = 20) = 869; p < 0.001.
High income country.
Upper-middle income country.
Lower-middle income country.
Estimated smoking persistence among adults, by site, stratified by birth cohort and elapsed time since smoking onset (estimated % with 95% confidence intervals).* Data from the World Mental Health Surveys Consortium, 2001–2008.
Assessments required ever-smokers to characterize themselves as current or former smokers. For each site, the proportion of ever-smokers who qualified as current smokers at the assessment (i.e., ‘persistent’ smokers’) was estimated, while addressing survey weighting and complex sampling design features (SAS v9.1.3). This was ascertained by the question: “Are you a current, former or never-smoker?” The question was only asked in reference to cigarette smoking. The smoking persistence proportion was defined as the number of current smokers divided by the number of (current smokers plus former smokers). The point of interest with respect to this study was the differences in smoking persistence for the following reason: smoking prevalence changes in relation to smoking incidents and average smoking duration (which is a function of both smoking persistence and smoking mortality). As described above, much is known about the global distribution of smoking prevalence, however, less is known about whether high smoking prevalence in an area might be owing to a high number of persons starting to smoke or a high proportion of smokers persisting in smoking.
Observed levels of smoking persistence might differ owing to how recently smoking had become a phenomenon within each country. It was assumed that an individual who started to smoke 5 years prior to assessment would be more likely to be a current smoker than an individual who started to smoke 25 years prior to the assessment. As a control for this, the researchers for this study stratified the elapsed time since the onset of smoking (>10 years versus ⩽10 years). Unfortunately, information on the age of first cigarette was only available for 12 countries. In an attempt to control this problem across all sites, the researchers also stratified across birth cohorts (post- vs. pre-Second World War). Here, the assumption is that older smokers have had a greater elapsed time since smoking onset than younger smokers, as smoking generally begins at adolescence and early adulthood [13,14].
The researchers also tested for global gender differences in smoking persistence to complement recent estimates of global gender differences in smoking prevalence with the hypothesis that there are no male/female differences on a global scale [6].
In addition, a series of meta-regressions were conducted in order to evaluate whether a country’s birth cohort distribution or how recent the onset of distribution might serve as useful predictors of the cross-national heterogeneity in smoking persistence estimates.
Homogeneity in summary estimates was sought by stratifying across high- and low-income countries (as defined by the World Bank) [15]. In addition, income as a covariate in the meta-regressions mentioned above was tested.
Meta-analyses were conducted in STATA using the ‘meta’ command. Random effects models were used in order to address between-study heterogeneity and to reduce variations owing to unequal sample size [16]. Cross-national heterogeneity was gauged via the Q statistic where a significant test indicates significant heterogeneity [17]. In the meta-analysis, each country’s estimate was weighted by the inverse of its variance.
Meta-regressions were accomplished in STATA using the ‘metareg’ command. In these analyses, the additive between study variance (T2) statistics was estimated using an iterative restricted maximum likelihood procedure [18].
3. Results
Table 1 is a display of each site’s total sample size as well as the sample size of ever-smokers. The overall sample sizes ranged from 2357 (the Netherlands) to 12,992 (New Zealand) with an aggregate sample of 101,392 persons across all sites. The sample size of ever-smokers across sites ranged from 369 (India) to 6855 (New Zealand) with a total of 45,691 ever-smokers used in this analysis; 45% of the individuals from the total sample were ever-smokers and the focus of the analysis. Nonsmokers were included in the analysis for variance estimation.
The main estimates of this study are shown in Fig. 1. This forest plot shows each country’s estimated proportion of persistent smokers and the 95% confidence interval of those estimates. Estimates ranged from 27% in Nigeria to 84% in China with a random-effects summary estimate of 55%. Considerable variation in smoking persistence was found across countries (Cochran’s heterogeneity for Fig. 1. Q = 6845; p < 0.001).
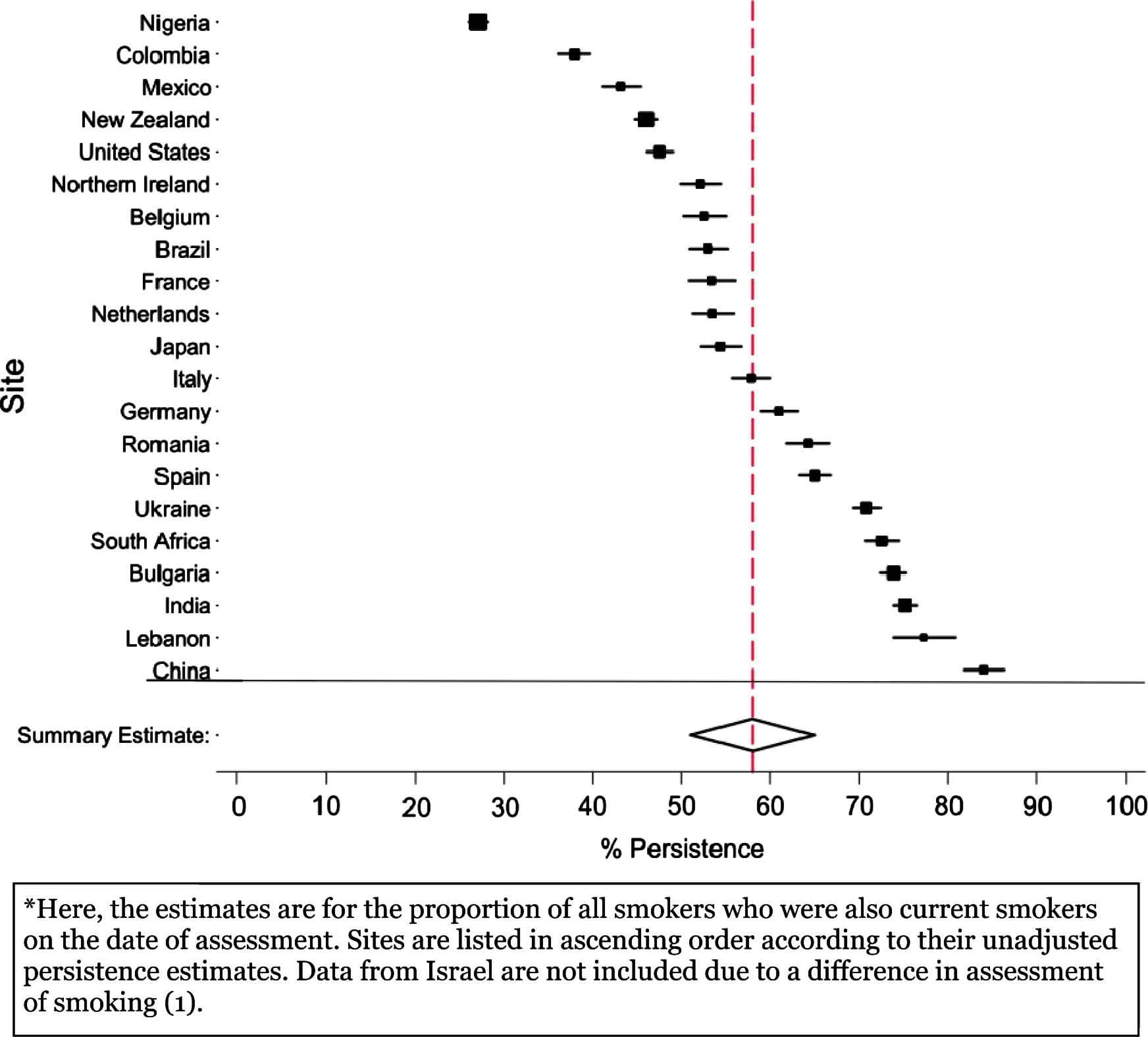
Forest plot for smoking persistence estimates among adults, by site.* Data from the World Mental Health Surveys Consortium, 2001–2008.
Table 1 also displays persistence estimates and 95% confidence intervals, with stratifications by birth cohort, elapsed time since smoking onset, and sex. The countries are listed from the lowest to the highest level of persistence before stratification (as in Fig. 1). Within each stratum, the rank ordering of sites (in order of persistence) is well preserved. For example, Nigeria has the lowest persistence estimate across all strata; China has among the highest persistence estimate across all strata. As with the un-stratified meta-analysis, there is a considerable amount of cross-national heterogeneity within each stratum.
It was tested whether improved homogeneity might be attained by collapsing countries into their income categories (as defined by the World Bank). Among the 10 high-income countries, the smoking persistence estimates ranged from 46% in New Zealand to 65% in Spain; there was a random-effects summary estimate of 54% (95% confidence interval, CI = 50%, 59%; Cochran’s heterogeneity Q = 431; p < 0.001). Among the seven upper-middle income countries, the corresponding smoking persistence estimates ranged from 38% in Colombia to 77% in Lebanon, with a random-effects summary estimate of 60% (95% CI = 48%, 72%; Cochran’s heterogeneity Q = 1529; p < 0.001). Among the 4 lower-middle income countries, the smoking persistence estimates ranged from 28% in Nigeria to 84% in China, with a random-effects summary estimate of 64% (95% CI = 36%, 93%; Cochran’s heterogeneity Q = 4637; p < 0.001).
The meta-regression results can be found in the Web-only Appendix. Table A2 presents the results of the meta-regression. Country income level, birth cohort distribution, and how recent the onset of smoking distribution were not found to be statistically significant predictors of the smoking prevalence values observed for each country that participated in the WMHSI. (The distribution of these covariates for each country can be found in Table A1.)
4. Discussion
These findings indicate considerable cross-national variation in smoking persistence estimates. The rank ordering of high to low persistence did not change appreciably with stratification by country income level, birth cohort, elapsed time since smoking onset, or sex (i.e., countries who were ranked high or low in terms of persistence prior to stratification remained high or low respectively within each of the stratifications). Hence, within study evidence limits, investigation of other sources of variation is needed (e.g., cigarette nicotine content, taxation policies, and access to effective prevention and smoking cessation aids) [19–22]. Cross-national differences of the effectiveness of cigarette-warning labels are also a possibility [23].
Several limitations deserve mention before a detailed discussion of the study results. These include the use of self-report assessments, a limited smoking status question (e.g., “Are you a current, former or never-smoker?”), exclusion of non-cigarette smoked tobacco products (e.g., water pipes, ‘bidis’) and this study’s use of proportions to summarize a time-to-event process because age at quitting values was not assessed. In addition, there were no data on how recently an individual had become a smoker in each country, and this ‘elapsed time since onset’ stratification was limited to 12 countries. However, the researchers attempted to address this across all countries by stratifying by birth cohort. It is also possible that cross-national differences in reporting bias could also exist (where smokers in some countries are more likely to report on their smoking than smokers in other countries). While these limitations are worth mentioning, they are common to most studies on this topic.
With respect to the birth cohort stratification, there is a clear selection bias among the pre-Second World War cohorts (many individuals from these cohorts died by the time these surveys were conducted—some from tobacco-related diseases). This will obviously bias comparisons between the pre- and post-Second World War strata. However, the goal was instead to compare countries within strata.
A counterbalancing strength is the ability to assess smoking persistence on a global scale, among adults in countries with different income levels. While the Global Youth Tobacco Survey and the Global Adult Tobacco Survey (both Centers for Disease Control Prevention [CDC] initiatives part of the Global Tobacco Surveillance System [GTSS]) are both valuable resources, the former is limited to 13–15 year olds and the later was only conducted in 14 lower-income countries [23,24]. Other strengths include epidemiological sampling of adults which addresses the bias found in clinical research on help-seeking smokers, as well as the use of a well-translated standardized assessment. These findings add to a growing body of research on smoking persistence in a context that seeks a more complete global view of the epidemiological dynamics of smoking [6,9,25,26].
In terms of the public health implications of this work, it is important to consider the primary finding of this study: the large amount of cross-national variation in smoking persistence found by the Q-statistic.
Also, our meta-analytic summary estimates indicate no male–female variation in smoking persistence, but given the heterogeneity across countries, it may be that male–female differences may exist in individual countries (such as Mexico and the Ukraine as reported in this study). On a global scale, it may be difficult to identify high-risk populations (such as male vs. female) for interventions that can be considered high risk across all countries. Still, the absence of a global male–female difference in smoking persistence is consistent with similar research on the topic among youth [3].
This report can, perhaps, shed light on which countries are in the greatest need of smoking cessation initiatives. For example, China and India, two of the most populous countries in the world each with emerging economies, had alarmingly high rates of smoking persistence. While smoking persistence is an important public issue in any country where smokers may be found, perhaps it is important for public health professionals to pay particular attention to certain countries identified in this report. It is difficult to determine from this study why countries, such as China and India, have such high levels of persistence. As shown by the overlapping meta-analytic summary estimates for each income level, it can be concluded that a country’s income level is not a valid predictor of its smoking persistence. Future analyses could instead consider the income level of the individual smokers instead of the overall income level of the country. Previous research on the high rates of smoking persistence in China has suggested one problem that few individuals in China plan on quitting smoking and only do so after the onset of adverse health outcomes [27,28]. Higher persistence in these countries could also be owing to limited access to smoking cessation aides [7], although data from the American Cancer Society’s Tobacco Atlas suggest that Chinese smokers have access to nicotine replacement therapy and some clinical cessation services with costs covered [29]. Still, it is important to remember that this study only measured cigarette smoking. Particularly in countries such as India, this is a limitation because of the added complexity of other types of tobacco use, such as ‘bidis’, water-pipe tobacco and smokeless tobacco [30].
These findings may also provide useful insight in conjunction with a conceptual model of smoking epidemics developed by Lopez and colleagues [31]. Briefly, this model posits a three to four decade lag between the peak in smoking prevalence and a subsequent peak in smoking-related mortality. The model divides this process into four stages of development in this shift. In the context of this report, India had a low rate of smoking prevalence (13%; data not presented in a table), but among the highest rate of smoking persistence. Smoking may not be currently common in India, but given the low likelihood of an individual quitting smoking once they start (i.e., high persistence), there may be a large smoking epidemic years down the line. High persistence indicates individuals are not quitting, and the main changes in smoking prevalence that are expected would be upward. A marked gender difference in smoking prevalence in India (data not presented in a table) is also consistent with Lopez’s model, that is, increases in male smoking prevalence precede increases in female smoking prevalence.
5. Conflicts of interest
None.
6. Support
The World Health Organization World Mental Health (WMH) Survey Initiative is supported by the National Institute of Mental Health (NIMH; R01 MH070884), the John D. and Catherine T. MacArthur Foundation, the Pfizer Foundation, the US Public Health Service (R13-MH066849, R01-MH069864, and R01 DA016558), the Fogarty International Center (FIRCA R03-TW006481), the Pan American Health Organization, Eli Lilly and Company, Ortho-McNeil Pharmaceutical, GlaxoSmithKline, and Bristol-Myers Squibb. We thank the staff of the WMH Data Collection and Data Analysis Coordination Centers for assistance with instrumentation, fieldwork, and consultation on data analysis. The work of the MSU-based authors (JT, DB, JCA) in preparing this report was additionally supported by the National Institute on Drug Abuse (K05DA015799; R01DA016558; T32DA021129). None of the funders had any role in the design, analysis, interpretation of results, or preparation of this paper. This report was prepared under the auspices of the World Health Organization ICD-11 Chapter 5 (Mental and Behavioral Disorders) epidemiology working group, which is co-chaired by Chatterji and Kessler. The views and opinions expressed in this report are those of the authors and should not be construed to represent the views of the sponsoring organizations, agencies, or governments.
The São Paulo Megacity Mental Health Survey is supported by the State of São Paulo Research Foundation (FAPESP) Thematic Project Grant 03/00204-3. The Bulgarian Epidemiological Study of common mental disorders EPIBUL is supported by the Ministry of Health and the National Center for Public Health Protection. The Chinese World Mental Health Survey Initiative is supported by the Pfizer Foundation. The Shenzhen Mental Health Survey is supported by the Shenzhen Bureau of Health and the Shenzhen Bureau of Science, Technology, and Information. The Colombian National Study of Mental Health (NSMH) is supported by the Ministry of Social Protection. The ESEMeD project is funded by the European Commission (Contracts QLG5-1999-01042; SANCO 2004123), the Piedmont Region (Italy), Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Spain (FIS 00/0028), Ministerio de Ciencia y Tecnología, Spain (SAF 2000-158-CE), Departament de Salut, Generalitat de Catalunya, Spain, Instituto de Salud Carlos III (CIBER CB06/02/0046, RETICS RD06/0011 REM-TAP), and other local agencies and by an unrestricted educational grant from GlaxoSmithKline. The WMHI was funded by WHO(India)and helped by Dr. R Chandrasekaran, JIPMER. Implementation of the Iraq Mental Health Survey (IMHS) and data entry were carried out by the staff of the Iraqi MOH and MOP with direct support from the Iraqi IMHS team with funding from both the Japanese and European Funds through United Nations Development Group Iraq Trust Fund (UNDG ITF). The Israel National Health Survey is funded by the Ministry of Health with support from the Israel National Institute for Health Policy and Health Services Research and the National Insurance Institute of Israel. The World Mental Health Japan (WMHJ) Survey is supported by the Grant for Research on Psychiatric and Neurological Diseases and Mental Health (H13-SHOGAI-023, H14-TOKUBETSU-026, H16-KOKORO-013) from the Japan Ministry of Health, Labor and Welfare. The Lebanese National Mental Health Survey (LEBANON) is supported by the Lebanese Ministry of Public Health, the WHO (Lebanon), Fogarty International, anonymous private donations to IDRAAC, Lebanon, and unrestricted grants from Janssen Cilag, Eli Lilly, GlaxoSmithKline, Roche, and Novartis. The Mexican National Comorbidity Survey (MNCS) is supported by The National Institute of Psychiatry Ramon de la Fuente (INPRFMDIES 4280) and by the National Council on Science and Technology (CONACyT-G30544-H), with supplemental support from the PanAmerican Health Organization (PAHO). Te Rau Hinengaro: The New Zealand Mental Health Survey (NZMHS) is supported by the New Zealand Ministry of Health, Alcohol Advisory Council, and the Health Research Council. The Nigerian Survey of Mental Health and Wellbeing (NSMHW) is supported by the WHO (Geneva), the WHO (Nigeria), and the Federal Ministry of Health, Abuja, Nigeria. The Northern Ireland Study of Mental Health was funded by the Health & Social Care Research & Development Division of the Public Health Agency. The Romania WMH study projects “Policies in Mental Health Area” and “National Study regarding Mental Health and Services Use” were carried out by National School of Public Health & Health Services Management (former National Institute for Research & Development in Health), with technical support of Metro Media Transilvania, the National Institute of Statistics-National Center for Training in Statistics, SC. Cheyenne Services SRL, Statistics Netherlands and were funded by Ministry of Public Health (former Ministry of Health) with supplemental support of Eli Lilly Romania SRL. The South Africa Stress and Health Study (SASH) is supported by the US National Institute of Mental Health (R01-MH059575) and National Institute of Drug Abuse with supplemental funding from the South African Department of Health and the University of Michigan. The Ukraine Comorbid Mental Disorders during Periods of Social Disruption (CMDPSD) study is funded by the US National Institute of Mental Health (RO1-MH61905). The US National Comorbidity Survey Replication (NCS-R) is supported by the National Institute of Mental Health (NIMH; U01-MH60220) with supplemental support from the National Institute of Drug Abuse (NIDA), the Substance Abuse and Mental Health Services Administration (SAMHSA), the Robert Wood Johnson Foundation (RWJF; Grant 044708), and the John W. Alden Trust.
A complete list of all within-country and cross-national WMH publications can be found at http://www.hcp.med.harvard.edu/wmh/.
Appendix A
| Country | % Persistent smokers | SE (% Persistent Smokers) | % in Pre WWII cohorts | % With onset ⩽10 years1 | Income level2 |
|---|---|---|---|---|---|
| Nigeria | 27.1 | 0.5 | 20.0 | 12.3 | Lower-middle |
| Colombia | 37.9 | 0.9 | 11.7 | 21.2 | Upper-middle |
| Mexico | 43.2 | 1.1 | 15.8 | 32.7 | Upper-middle |
| New Zealand | 46.0 | 0.6 | 25.9 | N/A | High |
| United States | 47.5 | 0.8 | 28.7 | 11.8 | High |
| Northern Ireland | 52.1 | 1.2 | 28.6 | N/A | High |
| Belgium | 52.6 | 1.2 | 26.2 | N/A | High |
| Brazil | 53.0 | 1.1 | 18.2 | 11.0 | Upper-middle |
| France | 53.4 | 1.3 | 22.7 | N/A | High |
| Netherlands | 53.5 | 1.2 | 28.7 | N/A | High |
| Japan | 54.4 | 1.2 | 32.9 | N/A | High |
| Italy | 57.8 | 1.1 | 29.1 | N/A | High |
| Germany | 61.0 | 1.1 | 26.0 | N/A | High |
| Romania | 64.2 | 1.2 | 18.6 | 14.1 | Upper-middle |
| Spain | 65.0 | 0.9 | 22.6 | N/A | High |
| Ukraine | 70.8 | 0.8 | 20.1 | 17.7 | Lower-middle |
| South Africa | 72.5 | 1.0 | 13.1 | 35.2 | Upper-middle |
| Bulgaria | 73.8 | 1.8 | 19.0 | 11.3 | Upper-middle |
| India | 75.1 | 0.7 | 22.0 | 17.4 | Lower-middle |
| Lebanon | 77.3 | 1.8 | 23.1 | 21.8 | Upper-middle |
| China | 84.0 | 1.2 | 18.7 | 17.4 | Lower-middle |
Elapsed time since smoking onset stratification is not available for sites that did not ask about the age of smoking onset.
Income level defined by the World Bank.
Description of each country by covariates used in a meta regression testing for sources of between-country heterogeneity of smoking persistence. Data from the World Mental Health Surveys Consortium, 2001–2008.
| No. of countries | Coefficient | SE(Coefficient) | Z-score | P-value | |
|---|---|---|---|---|---|
| Crude | |||||
| Lower-middle income | 21 | Ref | Ref | Ref | Ref |
| Upper-middle income | 21 | −4.97 | 9.00 | −0.44 | 0.659 |
| High | 21 | −9.89 | 8.50 | −1.16 | 0.244 |
| Birth cohort1 | 21 | −0.23 | 0.58 | −0.40 | 0.689 |
| Recency of onset2 | 12 | 0.19 | 0.71 | 0.27 | 0.786 |
| Adjusted 1 | |||||
| Lower-middle income | 21 | Ref | Ref | Ref | Ref |
| Upper-middle income | 21 | 1.09 | 9.68 | −0.11 | 0.910 |
| High income | 21 | −16.24 | 11.33 | −1.43 | 0.152 |
| Birth cohort1 | 21 | 0.90 | 1.07 | 0.86 | 0.392 |
| Adjusted 2 | |||||
| Lower-middle income | 12 | Ref | Ref | Ref | Ref |
| Upper-middle income | 12 | 2.22 | 13.87 | 0.16 | 0.873 |
| High income | 12 | −39.57 | 28.19 | −1.40 | 0.160 |
| Birth cohort1 | 12 | 3.04 | 2.25 | 1.35 | 0.176 |
| Recency of onset2 | 12 | 0.70 | 0.89 | −0.16 | 0.877 |
NOTE: Meta regressions were first estimated with each covariate as a bivariate predictor. Multivariable analyses were separated into two analyses. This was done because the onset was only available for 12 countries. The first adjusted regression provides estimates for income level and birth cohort among all 21 countries; the second adjusted regression provides estimates for income level, birth cohort and onset of smoking (among the 12 countries with data on the onset).
Income level was defined by the World Bank.
Percent of smokers in Pre-Second World War irth cohorts.
Percent of smokers with onsets within 10 years of survey assessment.
Summary of meta regressions on the effect of country level covariates on the cross-national variation in smoking persistence. Data from the World Mental Health Surveys Consortium, 2001–2008.
References
Cite this article
TY - JOUR AU - Jonathan P. Troost AU - David A. Barondess AU - Carla L. Storr AU - J. Elisabeth Wells AU - Ali Obaid Al-Hamzawi AU - Laura Helena Andrade AU - Evelyn Bromet AU - Ronny Bruffaerts AU - Silvia Florescu AU - Giovanni de Girolamo AU - Ron de Graaf AU - Oye Gureje AU - Josep Maria Haro AU - Chiyi Hu AU - Yueqin Huang AU - Aimee N. Karam AU - Ronald C. Kessler AU - Jean-Pierre Lepine AU - Herbert Matschinger AU - Maria Elena Medina-Mora AU - Siobhan O’Neill AU - Jose Posada-Villa AU - Rajesh Sagar AU - Tadashi Takeshima AU - Toma Tomov AU - David R. Williams AU - James C. Anthony PY - 2012 DA - 2012/08/11 TI - An updated global picture of cigarette smoking persistence among adults JO - Journal of Epidemiology and Global Health SP - 135 EP - 144 VL - 2 IS - 3 SN - 2210-6014 UR - https://doi.org/10.1016/j.jegh.2012.06.003 DO - 10.1016/j.jegh.2012.06.003 ID - Troost2012 ER -
