Insulin-like Growth Factor Administration Stimulates Wound Healing on Colonic Anastomosis in Hypoxic Rat Model
- DOI
- 10.2991/dsahmj.k.200529.001How to use a DOI?
- Keywords
- Cytokine; gastrointestinal anastomosis; hypoxia; insulin-like growth factor-1; TGF-β
- Abstract
Objective: Intestinal anastomosis is among the most commonly performed major abdominal procedures; however, leaks are an ongoing problem. The aim of this study was to investigate the effect of Insulin-like Growth Factor (IGF-1) on colonic healing under hypoxia.
Methods: Thirty-two Sprague–Dawley male rats underwent laparotomy with colonic transection and anastomosis. Rats were divided into four groups—Group A: normoxic (FiO2 21%) with saline [2 mg/kg, Intraperitoneally (IP)] at days 0, 2, 4, and 6; Group B: hypoxic (FiO2 11%) with saline injection; Group C: normoxic with IGF-1 injection at a dose of 2 mg/kg at days 0, 2, 4, and 6; Group D: hypoxic with IGF-1 treatment. On day 7, all animals were sacrificed, and intestine weight and bursting pressure were measured; anastomotic tissues were analyzed for mRNA level of collagen I and Matrix Metalloproteinase-13 (MMP-13) and cytokines analysis by enzyme-linked immunosorbent assay.
Results: There was significant increase in intestinal length in normoxic group treated with IGF-1 as compared to control (129.9 cm vs. 99.38 cm, p < 0.05); the length increased significantly in hypoxic group treated with IGF as compared to hypoxic control (123.1 cm vs. 82.25, p < 0.01). In the normoxic groups, a 6.7% decrease in mean body weight was found; however, the normoxic IGF-1 group sustained a weight gain of 0.5% (p < 0.01). The average weight decreased in the hypoxic control group (28.3%) and in the hypoxic IGF-1 treated group (16.6%, p < 0.05). Anastomotic bursting pressure increased significantly (p < 0.05) after IGF-1 treatment. Collagen I and MMP-13 mRNA expressions increased significantly (p < 0.001) in the IGF-1 treated group. Interleukin-10 and tumor necrosis factor alpha levels decreased under hypoxia with IGF-1 (p < 0.05). Vascular endothelial growth factor (p < 0.001), transforming growth factor beta (p < 0.05), and IGF-1 (p < 0.001) were significantly elevated in response to IGF-1.
Conclusion: IGF-1 stimulates the healing of colonic anastomosis under hypoxia. Factors promoting neoangiogenesis, collagen deposition, and paracrine expression were increased after IGF-1 treatment resulting in wound healing.
- Copyright
- © 2020 Dr. Sulaiman Al Habib Medical Group. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Despite significant improvements in perioperative care and the development of novel surgical techniques, colonic anastomotic leakage remains the colorectal surgeon’s most feared complication [1]. Anastomotic leaks are associated with high rates of morbidity and mortality and increased healthcare costs [1]. Among the multiple risk factors considered, hypoxia–ischemia is considered a primary cause of intestinal anastomotic leakage [2].
Understanding the mechanism and physiology of gastrointestinal healing is of great significance to the surgeon. Multiple factors are responsible for impaired would healing. Local factors that play an important role in wound healing include oxygenation, infection, and venous sufficiency. Age and sex, sex hormones, stress, ischemia, diabetes, fibrosis, jaundice, uremia, obesity, cancer, AIDS, glucocorticoid steroids, nonsteroidal anti-inflammatory drugs, chemotherapy, diets, alcoholism, and smoking constitute systemic factors that control wound healing [2,3]. Despite the improvement of both systemic and local factors that affect wound healing, healing failure and anastomotic dehiscence remains about 1–26% [3]. Anastomotic healing failure may lead to devastating and severe complications and higher costs [4]. It has been shown that after colorectal surgery, at least one-third of mortality can be attributed to anastomotic leak [5], and clinically observed leaks are responsible for two to threefold increase in hospital stay and perioperative mortality, respectively [6–8].
Hypoxia is an important factor that occurs frequently in postoperative settings as a result of acute conditions such as pneumonia, pulmonary embolism, congestive heart failure, and atelectasis or chronic conditions such as chronic obstructive pulmonary disease [9]. Oxygen becomes of paramount importance during wound healing as the metabolic demands of cells increase to aid in cellular proliferation and collagen synthesis [10,11]. Several clinical trials examined the usefulness of recombinant growth factors on wound healing under hypoxic environment. Transforming Growth Factor Beta (TGF-β), Platelet-derived Growth Factor-BB (PDGF-BB), basic Fibroblast Growth Factor (FGF)–FGF-2, keratinocyte growth factor, and Vascular Endothelial Growth Factor (VEGF) [12] showed improved healing process under ischemic and hypoxic environments by elevating the epithelialization and breaking strength and granulation tissue formation.
Researchers continue to evaluate cytokines and growth factor’s effect on anastomotic healing in order to find agents that might help in the anastomotic healing process especially in high-risk patients with anastomotic problems. Insulin-like Growth Factor (IGF-1) is a single-chain polypeptide that resembles proinsulin in its structure with pleotrophic effects on the gut [13]. In many tissues, such as intestine, IGF-1 is also synthesized acting in an autocrine or paracrine manner to aid in different and multiple functions including the process of wound healing in its early phases. IGF-1 has been studied in humans and has been proven safe in many clinical trials under different endocrine and neurological indications; however, studies on its role in anastomotic healing per se were done mainly using animal models with promising results. IGF-1 has been suggested to improve impaired wound healing in the animal model for colonic anastomosis [14,15], from diabetes [16], chemotherapy [17], immunosuppressive therapy [18], and cortisone [19]. The effect of glucagon-like peptide-2 receptor agonists has been studied in anastomotic wound healing [20]. Research efforts had been carried out to improve colonic anastomatosis in Saudi Arabia. Upregulation of the proinflammatory cytokine-induced chemokines in rat’s intestinal anastomotic wound healing has also been reported [21]. Zubaidi et al. [22] reported that certain cytokines play an important role in faster healing of intestinal and colonic anastomatic wounds. However, the effect of exogenous IGF-1 has not been explored on colonic anastomosis under hypoxic condition. Therefore, the aim of this study was to understand the effect of IGF-1 on colonic healing under hypoxia as well as to define the mechanism by which IGF-1 acts locally in the intestine after exogenous administration.
2. MATERIALS AND METHODS
Ethical approval for this study was granted by the Institutional Review Board of King Saud University College of Medicine. A total of 32 Sprague–Dawley male rats (200–400 g) were selected for laparotomy with colonic transection and immediate anastomosis. After the procedure, all animals were housed individually in a standard animal room with light/dark cycle (12 h/12 h) and controlled temperature. All animals were divided equally and randomly by a coinvestigator who was blinded to the procedure into four groups: animals were grouped into four categories; each group consisted of eight rats; Group A (Normoxic control) were kept under a normoxic condition (FiO2 21%) and were given Intraperitoneal (IP) saline injections (2 mg/kg) on days 0, 2, 4, and 6. Group B (Hypoxic control) rats were exposed to hypoxic condition (FiO2 11%) and were given saline injection at the same dose and time interval used in Group A. Group C (Normoxic treated) rats were kept under normoxic condition and were given an IGF-1 injection (2 mg/kg, IP) (Tercica, San Francisco, CA, USA) on days 0, 2, 4, and 6. Group D (Hypoxic treated) were exposed to hypoxia along with IGF-1 treatment as reported [15]. All animals were fed with normal rat pellet food. On day 7, all animals were sacrificed; analysis parameters were animal weight, intestine length, bursting pressure, and anastomotic tissues for mRNA level of collagen I and Matrix Metalloproteinase-13 (MMP-13) and cytokine content by enzyme-linked immunosorbent assay (ELISA).
2.1. Surgery Procedure
Prior to surgery, all animals were fed a standard laboratory pellet diet until the day of the procedure. Weight measurements were done at the start of the experiment and when animals were sacrificed. A single prophylactic, cefazolin (30 mg/kg; Novopharm Limited, Toronto, ON, Canada) was injected subcutaneously; the animals were anesthetized with 5% inhalational isoflurane at induction followed by 1–2% maintenance. The animals’ abdomen was shaved, prepared with alcohol solution (70%), and entered through a laparotomy incision (3 cm). Transverse colon transection with immediate anastomosis was performed in the following manner: identification of the transverse colon and finding a suitable site at the midpoint for the anastomosis. At the site the mesenteric marginal vessel was carefully dissected away from the colon and ligated using 5–0 Silk (Ethicon, Cincinnati, OH, USA). The fecal contents were milked out, and the colon was divided. A standard end-to-end anastomosis was performed with eight to 10 interrupted inverting sutures using 6–0 Prolin suture (Ethicon) depending on the diameter of the colon. The abdomen was washed out with sterile saline and closed with a running suture using 4–0 Vicryl™ (Ethicon). After colonic anastomoses had been performed, the rats in the hypoxic control group were intraperitoneally injected with 3 mL of 0.9% NaCl solution and were repeated on postoperative days 2, 4, and 6. The hypoxic IGF-I group was given intraperitoneally recombinant human IGF-I (2 mg/kg; IPSEN, Cambridge, MA, USA) after the colonic anastomosis was performed and on days 2, 4, and 6. The rats recovered and were provided with normal pellets and water 24 h after the procedure. Each animal was given the same amount of food (20 g/day), and the intake was recorded daily. On a daily basis, the animals were monitored for their food intake, signs of sepsis, and dehydration. The animals were reanesthetized with 5% inhalational isoflurane on postoperative day 7. The laparotomy incision was opened, the colonic anastomosis was identified and gently dissected away from the surrounding tissues; small and large bowel length and weight were recorded, and the colonic anastomosis was harvested intact with a 5-mm perianastomotic tissue and carefully avoiding disrupting adhesions as much as possible.
2.2. Measurement of Anastomotic Bursting Pressure
Bursting pressure is defined as a measure of the resistance of the intestinal wall to increasing intraluminal pressure. ABP is considered a good mechanical parameter to monitor the anastomotic repair as long as the rupture takes place within the anastomosis. The bursting pressure is the pressure at which disruption occurs when the segment of bowel (containing the anastomosis) is progressively distended with gas experimentally [15,20,23]. The apparatus for bursting pressure consisted of an air-filled 60-mL syringe placed in an infusion pump (Harvard Apparatus, Holliston, MA, USA) connected through plastic tubing to an in-line sphygmomanometer (Welch Allyn Tycos, Skaneateles Falls, NY, USA). Plastic tubing from the distal end of the sphygmomanometer was secured to the proximal end of the harvested colon with three ties of 5–0 silk suture (Ethicon). The isolated colon from the mesentery and surrounding tissues and transected at two points 3 cm in each direction from the anastomosis. The colon was milked free of fecal contents and gently flushed with saline. The distal end of the colon was closed off with three ties of 5–0 silk suture (Ethicon). The colon was submerged in saline and infused with air at a rate of 5 mL/min. The appearance of air bubbles was considered evidence of anastomotic disruption, and the pressure at which this occurred was read from the sphygmomanometer and recorded as the bursting pressure. The perianastomotic tissue was cut longitudinally into strips that included 2 cm of normal tissue on either side of the anastomosis were then gently washed with normal saline. One strip of tissue was analyzed for mRNA level of collagen I and MMP-13 and a second strip was analyzed for tissue cytokine quantification [Interleukin (IL)-4, IL-1β, IL-10, IL-12, Tumor Necrosis Factor Alpha (TNF-α), Interferon-Gamma (IFN-γ), TGF-β, IGF-1, and VEGF) using ELISA by luminex™ (Luminex Corporation, Austin, TX, USA).
2.3. Isolation and Quantification of RNA
Total RNA was purified and quantified from all tissue samples according to the TRIspin method [20,24]. Briefly, the frozen tissue (15–45 mg) was powdered (Mikro-Dismembrator S, B. Braun Biotech International, Allentown, PA, USA) and mixed with Trizol (1 mL; Sigma Chemical Co., St. Louis, MO, USA). Total RNA was further purified using the RNeasy spin extraction kit (Qiagen Inc., Mississauga, ON, Canada) with the addition of a DNAse step (RNAse free DNAse, Qiagen Inc.) after the initial wash. The quantification of RNA was performed using SYBR Green II dye (Molecular Probes Inc., Eugene, OR, USA) on a Turner Model 450 Fluorometer (Barnstead/Thermolyne Corp., Dubuque, IA, USA) with excitation at 468 nm and emission at 525 nm using a standard curve of rRNA (Sigma Chemical Co.). RNA was stored at –80°C until experimental analysis.
2.4. Semiquantitative Reverse Transcription-Polymerase Chain Reaction Assays
RNA (1 µg) was used for reverse transcription using the Omniscript RT kit (Qiagen Inc.) using primers and Omniscript reverse transcriptase. To avoid variability, Polymerase Chain Reaction (PCR) was performed on all samples at the same time as described previously [20], using 15 µL volume of RT reaction per sample: addition of 1.25 U of Taq DNA polymerase (Rose Scientific Ltd, Edmonton, AB, Canada) per reaction. PCR amplification was performed with an initial 5 min denaturation step at 94°C, followed by repeated cycles of denaturation for 30 s at 94°C, annealing for 30 s, and extension for 30 s at 72°C, concluded by a final extension of 7 min at 72°C. After PCR was performed under optimized conditions for each molecule (cycle number, annealing temperature, buffers), 20 µL of the PCR product was electrophoresed on a 2% agarose gel (Amersham Pharmacia Biotech, Baie d’Urfe, QC, Canada) at 10 V/cm for 1 h. All samples were in the linear zone of detection by the image analysis system. For each mRNA of interest (collagen I and MMP-13), the integrated density of the PCR product was normalized to the integrated density of the PCR product of the housekeeping gene 18 S. The densitometric values for the full-thickness wounds were compared to those for normal tissue to determine the relative expression levels at each time point.
2.5. Statistical Analysis
Two-tailed unpaired Student’s t-test was used to compare the means between hypoxic control and hypoxic treated animals for each of the parameters measured. The difference in the means and a 95% Confidence Interval (CI) were calculated. A p-value <0.05 was considered significant. All statistical analyses were performed using Graph Pad Prism® Version 3.03 for Windows (Graph Pad Software Inc., San Diego, CA, USA), and graphs were also created using this program.
3. RESULTS
3.1. Effect of IGF-1 on Intestinal Length, Body Weight, and Bursting Pressure
No deaths, anastomotic leak, intra-abdominal abscess and no abdominal wall infection were observed among all four study groups. The mean small bowel length in the normoxic group that received IGF-1 treatment was statistically higher than that in the normoxic control group (129.9 ± 2.89 cm vs. 99.38 ± 1.82 cm, p < 0.05). Similarly, the mean small bowel length in hypoxic control group was 82.25 ± 2.13 cm, and in the hypoxic group treated with IGF-1, the mean length was increased to 123.1 ± 2.42 cm (p < 0.01) (Figure 1a). In the normoxic group, rats had a 6.7% decrease in mean body weight; however, the normoxic IGF-1 group sustained a weight gain of 0.5% (p < 0.01). Meanwhile, body weight of the hypoxic group was significantly decreased postoperatively compared to preoperative levels; the average weight in the hypoxic control group decreased to 28.3% postoperatively, where the average weight in the hypoxic IGF-1 treated group was decreased to 16.6% (p < 0.05) (Figure 1b and Table 1). Bursting pressure measurement was performed in all animals. The mean ABP was significantly increased in the IGF-1 treated group (180 ± 3.89, n = 8) as compared to the hypoxic control group (114.1 ± 6.45, n = 8; p < 0.05). No difference was noted in bursting pressure between the normoxic control group and the normoxic IGF-1 treated group [181.9 ± 4.219 (n = 8) vs. 194.4 ± 4.950 (n = 8); p < 0.07] (Figure 1c).
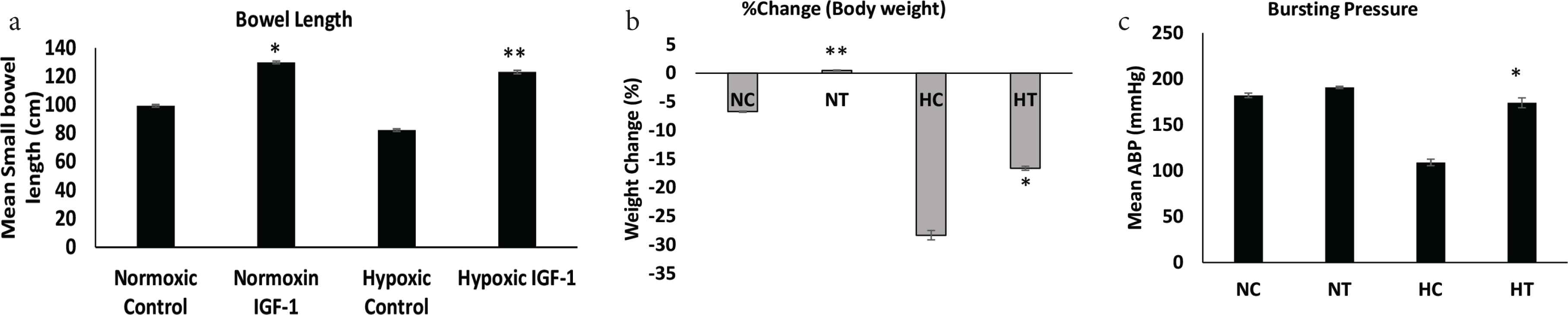
Bowel length, % weight change, and Anastomotic Bursting Pressure (ABP). (a) Small bowel length measurement was performed in all groups (data: mean ± SD; n = 8). *p < 0.05 NT vs. NC; **p < 0.01 HT vs. HC. (b) Percent change in body weight for all groups. **p < 0.01 NT vs. NC and p < 0.05 HT vs. HC. (c) Anastomotic bursting pressures in NC, NT, HC, and HT. Isolated segments of intestine insufflated with air under saline: pressure at which the first bubbles appear is recorded (data: mean ± SD; n = 8). *p < 0.05 HT vs. HC. Student’s t-test was used for significance. NC, normoxic control; NT, normoxic treated with IGF-1; HC, hypoxic control; HT, hypoxic treated with IGF-1.
| Group | n | Mean | 95% CI | Range | p* |
|---|---|---|---|---|---|
| Hypoxic control | |||||
| Before | 8 | 285.78 | 250.2 to 346.3 | ||
| After | 8 | 203.4 | 57.38 to 107.4 | 178.4 to 215.8 | |
| Δ % | 8 | 28.31 | −34.0 to −22.5 | −20.3 to −39.2 | <0.01 |
| Hypoxic IGF | |||||
| Before | 8 | 355.9 | 323.5 to 379.6 | ||
| After | 8 | 296.55 | 43.69 to 75.01 | 280.2 to 311.1 | |
| Δ % | 8 | 16.61 | –18.4 to –14.8 | –13.3 to –18.9 | <0.05 |
Student’s t-test.
Body weight (g)
3.2. IGF-1 Treatment Increases Collagen I and MMP-13 Expression
Collagen I mRNA expression was increased significantly (p < 0.0001) in hypoxic IGF-1 treated animals compared with hypoxic control animals [2.22 ± 0.21 (n = 8) vs. 0.56 ± 0.06 (n = 8)]. IGF-1 treatment resulted in increased collagen I expression as well in normoxic animals [2.042 ± 0.2469 (n = 8) vs. 1.363 ± 0.1746 (n = 8); p < 0.05] (Figure 2a). MMP-13 mRNA expression was significantly increased (p < 0.0001) in the hypoxic IGF-1 treated group compared to hypoxia alone [6.313 ± 0.5640 (n = 8) vs. 0.6909 ± 0.2344 (n = 8)] (Figure 2b). No statistical significance in MMP-13 expression was observed between normoxic controls or IGF-1 treated groups.
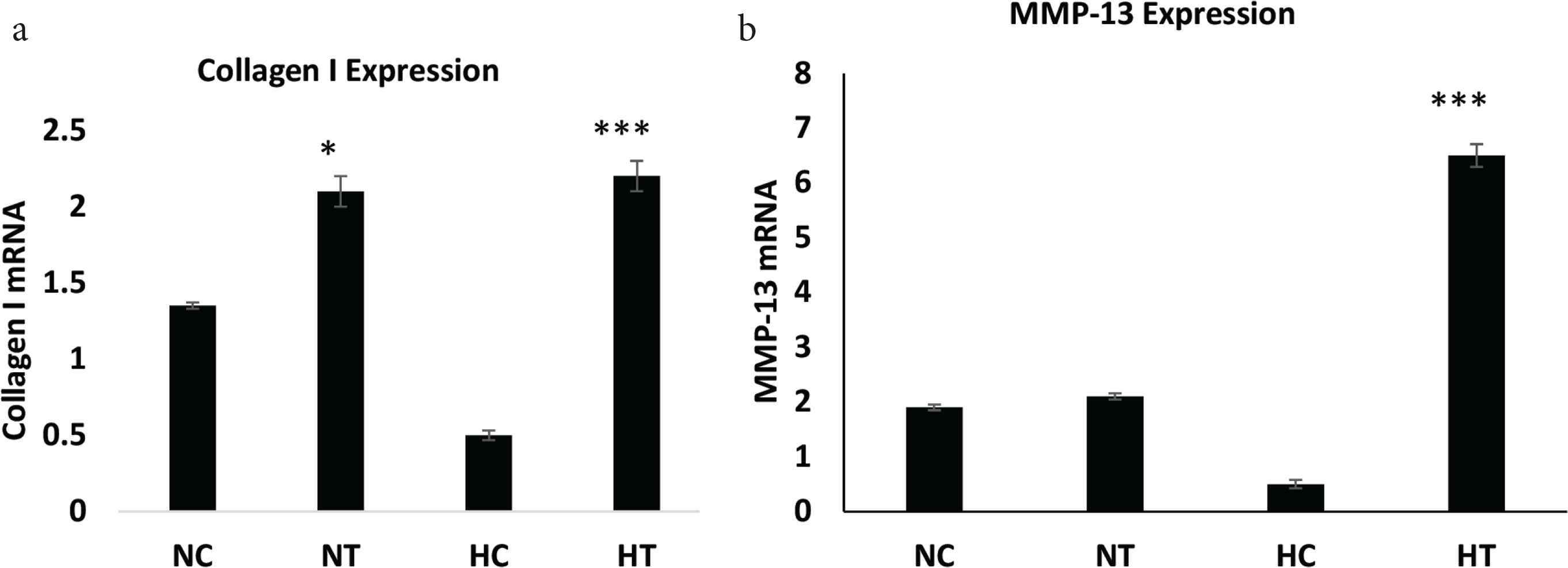
RT-PCR analysis of collagen I and MMP-13 mRNA levels. (a) Collagen I, *p < 0.05 NT vs NC; ***p < 0.001 HT vs HC and (b) MMP-13 in anastomotic tissue as measured using RT-PCR normalized to β-actin (data: mean ± SD; n = 8), ***p < 0.001 HT vs HC. Student’s t-test was used for significance. HC, hypoxic control; HT, hypoxic treated with IGF-1; MMP-13, matrix metalloproteinase-13; NC, normoxic control; NT, normoxic treated with IGF-1; RT-PCR, reverse transcription-polymerase chain reaction.
3.3. Effect of IGF-1 Treatment on Proinflammatory Cytokines
No significance alterations were noted on the levels of proinflammatory cytokines IL-4, IL-1β, IL-12, and IFN-γ between the two groups (Figure 3a–3d). However, a significant decrease in TNF-α and IL-10 levels were found when IGF-1 was given to hypoxic animals (p < 0.05 and <0.05, respectively) (Table 2 and Figure 3e and 3f).
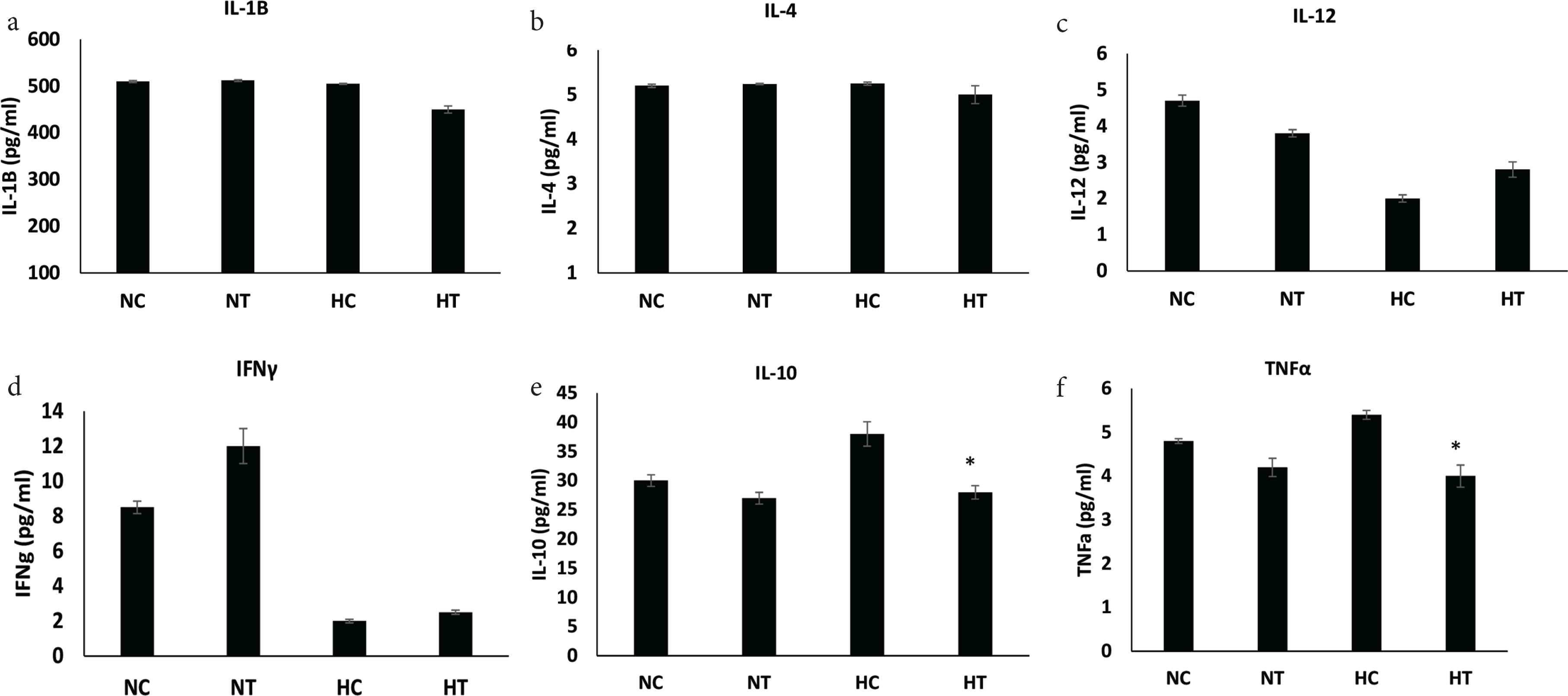
Levels of proinflammatory and anti-inflammatory cytokines. (a–f) All indicated cytokines measured by ELISA in anastomosed tissue from normoxic and hypoxic animals treated with IGF-1 (data: mean ± SD; n = 8). Student’s t-test was used for significance. *p < 0.05 HT vs HC. HC, hypoxic control; HT, hypoxic treated with IGF-1; NC, normoxic control; NT, normoxic treated with IGF-1; SD, standard deviation.
| Cytokines | n | Mean ± SEM (HC) | Mean ± SEM (HT) | Difference between means | 95% CI | p* |
|---|---|---|---|---|---|---|
| IL-4 | 8 | 5.376 ± 0.3263 | 4.890 ± 0.2951 | 0.4857 ± 0.4400 | –0.4580 to 1.429 | 0.2882 |
| IL-10 | 8 | 36.43 ± 2.506 | 26.86 ± 2.102 | 9.573 ± 3.271 | 2.557 to 16.59 | 0.0110 |
| IL-12 | 8 | 2.136 ± 0.4325 | 2.647 ± 0.3079 | –0.5112 ± 0.5309 | –1.650 to 0.6275 | 0.3519 |
| IL-1β | 8 | 534.9 ± 58.27, n = 8 | 406.7 ± 32.63, n = 8 | 128.2 ± 66.78 | –15.05 to 271.4 | 0.0755 |
| IFN-γ | 8 | 2.305 ± 0.6123 | 2.941 ± 0.6634 | –0.6354 ± 0.9028 | –2.572 to 1.301 | 0.4931 |
| TNF-α | 8 | 5.263 ± 0.4827 | 3.939 ± 0.2464 | 1.324 ± 0.5420 | 0.1618 to 2.487 | 0.0284 |
Student’s t-test.
HC, hypoxic control group; HT, hypoxic IGF-1 treated group; IFN, interferon; n, number; SEM, standard error of the mean.
Proinflammatory cytokines
3.4. IGF-1 Treatment Increases Prohealing Peptides
The IGF-1 and VEGF levels were found to increase significantly in the hypoxic IGF-1 treated group compared to the hypoxic control group (p = 0.0001). The TGF-β mRNA level also increased significantly in the hypoxic IGF-1 treated group compared to the hypoxic control group (p = 0.0110) (Table 3 and Figure 4a–4c).
| Prohealing peptides | n | Mean ± SEM (HC) | Mean ± SEM (HT) | Difference between means | 95% CI | p* |
|---|---|---|---|---|---|---|
| TGF-β | 8 | 0.2574 ± 0.02941, n = 8 | 0.4000 ± 0.03874, n = 8 | –0.1426 ± 0.04864 | –0.2469 to –0.03822 | <0.0110 |
| IGF-1 | 8 | 2.078 ± 0.4756, n = 8 | 11.37 ± 1.143, n = 8 | –9.288 ± 1.238 | –11.94 to –6.632 | <0.0001 |
| VEGF | 8 | 2.508 ± 0.1792, n = 8 | 6.673 ± 0.7534, n = 8 | –4.164 ± 0.7745 | –5.825 to –2.503 | <0.0001 |
Student’s t-test.
HC, hypoxic control group; HT, hypoxic IGF-1 treated group; n, number; SEM, standard error of the mean.
Prohealing peptides
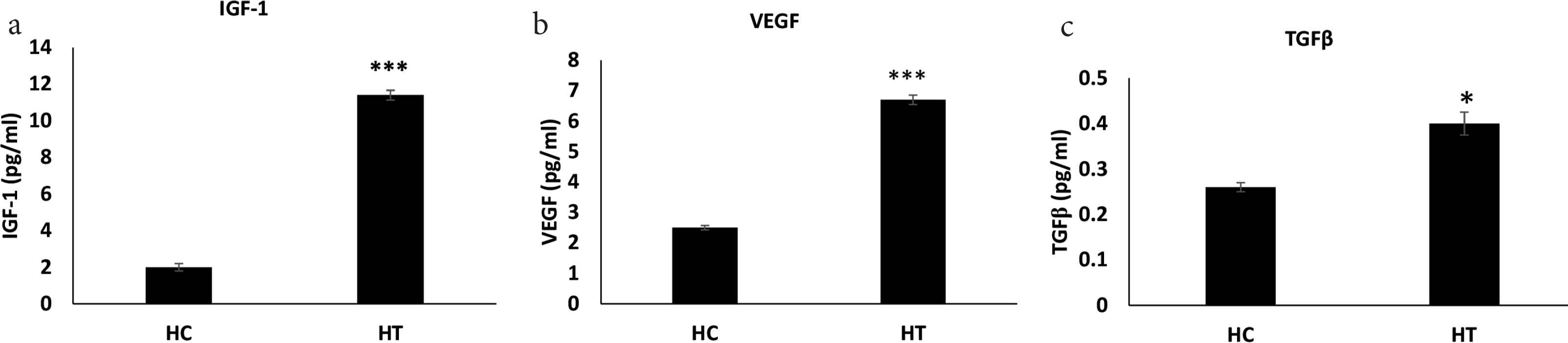
Prohealing cytokines levels. (a–c) The indicated cytokines measured by ELISA in anastomosed tissue from normoxic and hypoxic animals treated with IGF-1 (data: mean ± SD; n = 8). Student’s t-test was used for significance. ***p < 0.001 HT vs HC; *p < 0.05 HT vs HC. HC, hypoxic control; HT, hypoxic treated with IGF-1; NC, normoxic control; NT, normoxic treated with IGF-1; SD, standard deviation.
4. DISCUSSION
Anastomotic leak remains the cause of morbidity and mortality after colorectal surgery to surgical patients. The reported incidence of anastomotic failures has been shown to fall in the range between 1% and 26% [1–3]. Adequate oxygenation is necessary to the process of wound healing in all stages, during the inflammatory phase; oxygen is required by polymorphonuclear cells to produce superoxide free radicals to kill microorganisms and prevent infections [25]. Jonsson et al. [26] evaluated collagen deposition in wounds of surgical patients and found that the quantity of collagen deposited was directly proportional to the measured tissue oxygen tension. Neoangiogenesis, an important step during the proliferative phase of wound healing, is highly dependent on oxygen as well. Although hypoxia may induce angiogenesis, it cannot sustain the process [10]; therefore, it has been found that oxygen treatment induce VEGF mRNA levels in endothelial cells and macrophages [27–29]. During the last stage of wound healing process, oxygen is an important cofactor for collagen cross-linking responsible for tensile strength of the wound [30]; oxygen may also trigger fibroblasts differentiation to myofibroblasts, which is required for wound contraction [31].
In our study, we encountered no leaks in both groups while we kept the animals under hypoxia. Previous studies have shown that this level of systemic hypoxic condition directly translated into local tissue hypoxic environment, which affected the healing of anastomotic tissue of the rat colon [32]. Cytokines and growth factors supplementation were effective in producing wound-healing enhancement in a large number of experimental studies [3], but the translation of this basic science research to the clinical environment has been slow. We intended in our experiment to study the effect of a growth factor to the process of wound healing that in the future might help to us further understand the detailed mechanism of wound healing and overcome the factors that impair it by means of manipulating these factors.
In our study, IGF-1 induced weight gain in animals as compared to control animals but it also decreased weight loss in stressed (hypoxic) animals. Our results regarding the trophic effects of IGF-1 on the bowel are pretty much in agreement with most of the studies conducted previously [14,15,23]. The proposed mechanism of such effect is thought to be secondary to its known role in the metabolism of protein and fat, increase cell proliferation, and increase the absorptive capacity of the intestine to glucose, water, and minerals [33]. Peterson et al. [14] showed that IGF-1 administration increases median body weight by 5%. By contrast, in a similar study, Steeb et al. [33] showed no significant increase in mean body weight in IGF-1 infused rats. Other studies also have shown that IGF-1 induced weight gain in animals that had undergone small bowel transplant [34]. In our study, with respect to controlled food intake among all groups, the effect of hypoxia on rat’s weight during postoperative period was significant as the hypoxic controls lost about 28% of their weight whereas the hypoxic IGF-1 group lost only 16%.
Bursting pressure measurement has proved effective in determination of wound strength especially during the first week of the wound healing process [35]. We found that hypoxia has a negative effect on anastomotic bursting pressure, collagen I, and MMP-13 expressions as compared to nonhypoxic animals; however, when we administered IGF-1 to the animals we noticed a statistically significant change in these parameters. This is in keeping with previous reports [13–15] showing that IGF-1 increases anastomotic collagen content early after surgery. It was also shown that IGF-1 increases collagen staining on histology of the anastomotic segment [13]. The authors proposed that IGF-1 promotes healing of colonic anastomosis by inducing matrix synthesis and collagen deposition.
Proinflammatory cytokines seemed to have no role in improved anastomotic healing under hypoxic conditions when IGF-1 is used. The amount of IL-4, IL-12, IL-1β, IFN-γ , IL-10, and TNF-α did not differ significantly when we use IGF-1. This indicates that the increase in anastomotic strength is not attributable to a change in inflammation or increased anti-inflammatory cytokines. It has been shown that proinflammatory cytokines impair signals from growth factor receptors; in addition, they suppress proliferation and induce growth factor functional resistance in many tissues [36]. O’Connor et al. [37] showed that IGF-1 would act directly to suppress the activity of proinflammatory cytokines and will induce decreased sensitivity to TNF-α in the brain. Hypoxia may lead to increased expression of inhibitory cytokines TNF-α and IL-10; however, there is an obvious indication that IGF-1 may act to decrease levels of these inhibitory mediators (Figure 5).
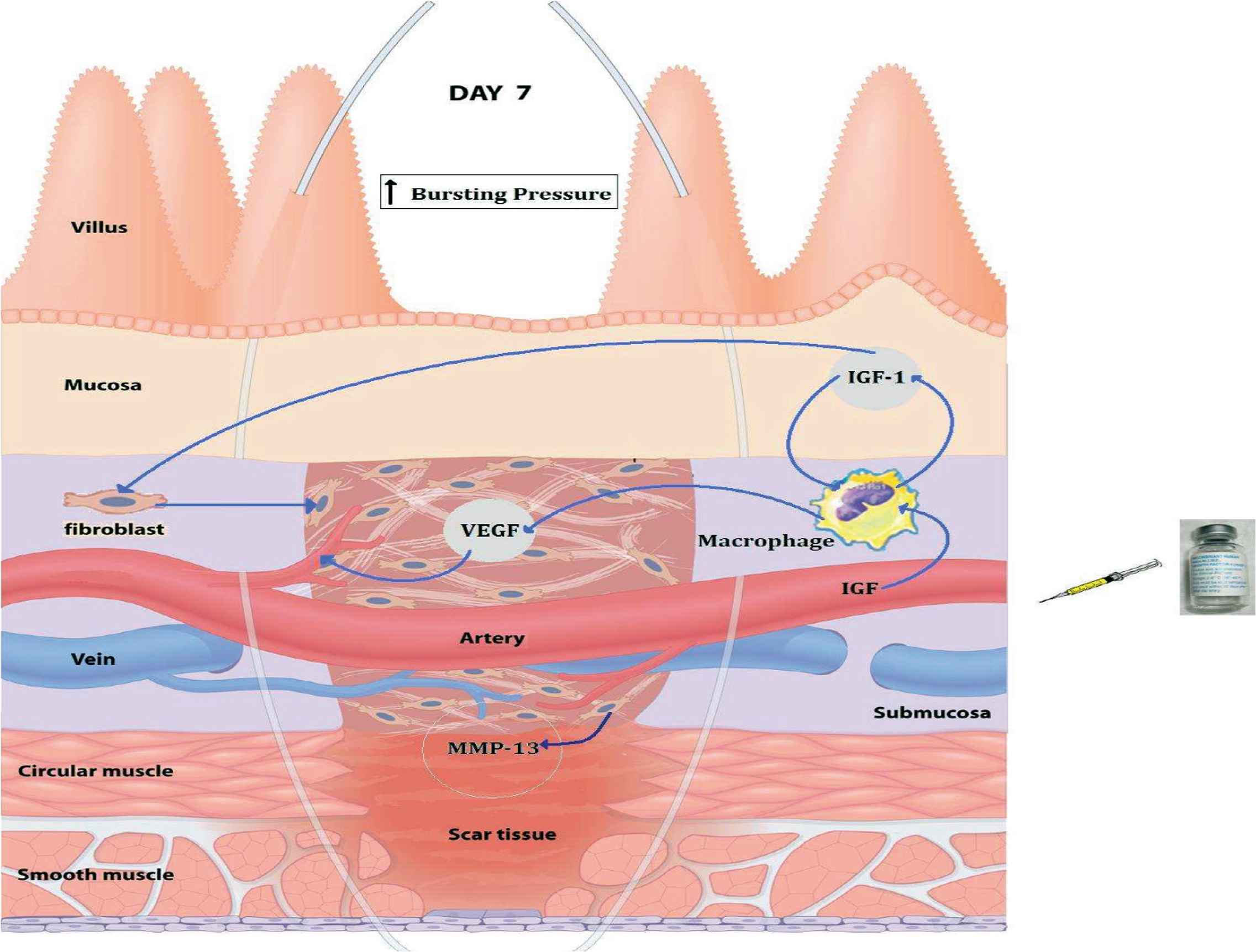
Mechanism of IGF-1 mediated intestinal wound healing.
5. CONCLUSION
This study demonstrates that IP injection of IGF-1 stimulates the healing of colonic anastomosis under hypoxia. IGF-1 treatment results in the increased expression of collagen I and MMP-13. IL-10 and TNF-α decreased however, VEGF, TGF-β, and IGF-1 levels increased after IGF-1 treatment under hypoxia. These factors promote neoangiogenesis, collagen deposition and the paracrine expression of IGF-1 leading to intestinal wound healing. Further studies are warranted to determine if these findings can be replicated in human patients.
5.1. Focus
After gastrointestinal surgery, anastomotic leak is associated with high morbidity and mortality. There are some inevitable factors impairing wound healing that might not be prevented because of hypoxia. Recently it has been shown that growth factors such as IGF-1 may accelerate and improve anastomotic healing in an animal model. Understanding the effects and mechanism of these factors in anastomotic healing in humans is of considerable clinical value, and it may reduce the mortality and morbidity associated with leaks. It may also eliminate the traditional use of diverting stomas and two-step surgery in high-risk patients.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
KA and ASA conceptualized the study and performed experiments. KA, MA and RA did data analysis, interpretation, and writing of the manuscript.
ACKNOWLEDGMENTS
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs.
ABBREVIATIONS
- IGF-1,
insulin-like growth factor;
- ELISA,
enzyme-linked immunosorbent assay;
- MMP-13,
matrix metalloproteinase-13;
- IL-1β,
interleukin 1 beta;
- IL-4,
interleukin 4;
- IL-12,
interleukin 12;
- IL-10,
interleukin 10;
- IFN-γ,
interferon gamma;
- TNF-α,
tumor necrosis factor alpha;
- VEGF,
vascular endothelial growth factor;
- TGF-β,
transforming growth factor beta.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Khayal Al-Khayal AU - Alanoud Saad AlWesaimer AU - Maha Abdulla AU - Rehan Ahmad PY - 2020 DA - 2020/06/03 TI - Insulin-like Growth Factor Administration Stimulates Wound Healing on Colonic Anastomosis in Hypoxic Rat Model JO - Dr. Sulaiman Al Habib Medical Journal SP - 43 EP - 50 VL - 2 IS - 2 SN - 2590-3349 UR - https://doi.org/10.2991/dsahmj.k.200529.001 DO - 10.2991/dsahmj.k.200529.001 ID - Al-Khayal2020 ER -
