Emicizumab Associated Rhabdomyolysis in Hemophilia A

- DOI
- 10.2991/chi.k.200924.001How to use a DOI?
- Keywords
- Emicizumab; rhabdomyolysis; hemophilia A
- Abstract
Emicizumab is increasingly the front-line treatment for patients with Hemophilia A with or without inhibitors. Rhabdomyolysis is a syndrome of muscle necrosis and release of intracellular muscle constituents into the circulation. Creatine kinase (CK) levels are typically markedly elevated, and muscle pain and myoglobinuria may be present. The severity of illness ranges from asymptomatic elevations in serum muscle enzymes to life-threatening disease associated with extreme enzyme elevations, electrolyte imbalances, acute kidney injury and disseminated intravascular coagulation. We present a case of an African American male with severe hemophilia A and history of factor VIII inhibitor, maintained on emicizumab prophylaxis, who developed rhabdomyolysis with a symptomatic hyperCKemia. To date, there is no known link between rhabdomyolysis to emicizumab. This report brings to light the possibility of symptomatic rhabdomyolysis as a potential side effect of emicizumab after moderate exertional activity.
- Copyright
- © 2020 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Rhabdomyolysis is a syndrome characterized by muscle necrosis resulting in the leakage of the intracellular muscle constituents into the circulation and extracellular fluid. Rhabdomyolysis ranges from an asymptomatic illness with elevation in the creatine kinase (CK) level to a life-threatening condition associated with extreme elevations in CK, electrolyte imbalances, acute renal failure (ARF) and disseminated intravascular coagulation [1]. Although the cause of rhabdomyolysis is usually identifiable, in some instances the etiology is elusive. Musculoskeletal trauma, particularly crush syndrome, accounts for a large proportion of the cases of rhabdomyolysis. Less common causes include muscle enzyme deficiencies, electrolyte abnormalities, infectious episodes, drugs, toxins and endocrinopathies. Rhabdomyolysis is commonly associated with myoglobinuria, and if this is sufficiently severe, it can result in ARF. Weakness, myalgia and tea-colored urine are the main clinical manifestations [2]. Drug-induced rhabdomyolysis can be induced by a large group of substances which can affect muscles by different mechanisms. Any drug that directly or indirectly impairs the production or use of ATP by skeletal muscle, or increases energy requirements that exceed the rate of ATP production, can cause rhabdomyolysis. The potential mechanism of drug-induced sarcolemmal injury is presumably due to changes in the viscosity of the sarcolemma, caused by activation of phospholipase A. These changes result in increased permeability of the sarcolemma, permitting leakage of intracellular contents, as well as an increase in the entry of sodium ions into the cell [3]. The increased intracellular sodium ion concentration activates Na+/K+-ATPase, a process that requires energy. This exhausts the supplies of ATP and impairs cellular transport proteins. The increase in cellular sodium ion concentration leads to the accumulation of intracellular calcium, which activates neutral proteases causing further cellular injury [3].
Emicizumab is a bispecific monoclonal antibody, which restores the function of FVIII by bridging the enzyme (FIXa) and substrate (FX) together and facilitating FIXa-mediated FX activation. The net effect is the formation of the prothrombinase complex and downstream clot formation. Emicizumab is associated with two known cases of asymptomatic rhabdomyolysis in adult patients in the context of strenuous activity [4]. We present a case of an African American male with severe hemophilia A and history of factor VIII inhibitor, maintained on emicizumab prophylaxis, who developed rhabdomyolysis with a symptomatic hyperCKemia.
2. CASE
A 19-year-old African American male with severe hemophilia A and history of persistent factor VIII inhibitor, despite multiple attempts at immune tolerance induction, and maintained on weekly emicizumab prophylaxis presented with severe muscle cramps in his anterior pectoral regions without a clear source, highlighted by imaging completed on admission (Figure 1). The patient had required change in prophylactic medication from factor eight inhibitor bypassing activity to emicizumab a year before, as he continued to have many hospitalizations for hemarthrosis, resulting in contractures and discordant musculoskeletal exam. On-demand factor replacements did not occur since the initiation of emicizumab. The patient had a job at a restaurant that involved moderate exertion. However, he had been working at the restaurant for a year without any symptoms until the hospitalization. On examination, he had diffuse muscle tenderness of his anterior pectoral regions. Laboratory studies showed hyperCKemia (Table 1). Emicizumab was considered as a likely candidate causing symptomatic rhabdomyolysis. Laboratory testing did not demonstrate endocrine pathology, the complete blood counts and metabolic panel were normal, the history was not concordant with toxin-based insult, and the rheumatology workup was unremarkable. An extensive infectious disease workup done including Hepatitis panel, human immunodeficiency virus, Epstein-Barr virus, and cytomegalovirus studies, all of which were normal. CRP was not elevated; urinalysis did not demonstrate myoglobinuria. Coagulation studies showed no abnormalities, consistent with emicizumab in a hemophilia patient. He was managed with intravenous fluids and oral opioids around the clock for 24–48 h, and then changed to ‘as needed’ medication. He required two doses of oral opioids after this transition in care plan, due to retention of anterior pectoralis muscle tenderness during the remainder of his 5-day hospital course.
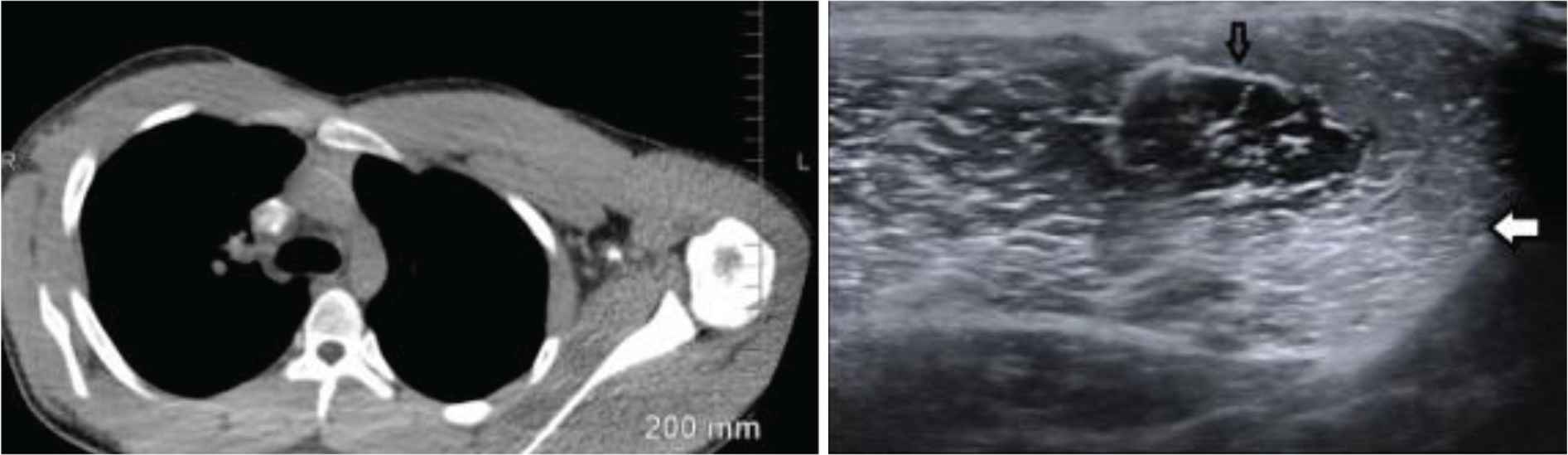
Two panels showing different imaging modalities that highlight the asymmetry between the patient’s inflamed left and minimally affected right pectoralis muscle. The left panel is a CT of the chest showing drastic difference between the left and right musculature. The right panel is an ultrasound that demonstrates the normal muscle with a black arrow and hyperechoic, edematous muscle with the white arrow.
| Hospital day | CK value |
|---|---|
| Day of admission | 28,423 |
| Day 2 | 25,131 |
| Day 3 | 23,442 |
| Day 4 | 9008 |
| Day 5 | 2689 |
CK, creatine kinase.
Trajectory of CK values throughout 5-day hospital admission
A metabolic myopathy and rhabdomyolysis 60 gene panel next generation sequencing was performed through a commercial laboratory. The panel comprised genes associated with mitochondrial fatty acid oxidation defects, glycogen storage diseases, mitochondrial myopathy genes, channelopathies, etc. The patient was found to have two heterozygous variants of unknown significance in the ETFDH and the RYR1 genes, respectively. Pathogenic variants in the ETFDH cause autosomal recessive glutaric aciduria type 2C. The ETFDH variant seen in this patient is in the heterozygous form and two pathogenic variants are necessary to cause disease. His variant, denoted as c.1049G>A (p.Arg350Gln) is present in the African population at a frequency of 0.2% and in the total population at 0.02%. This means this variant is ten times more common in the African population and may be a likely benign variant.
3. DISCUSSION
Emicizumab has remarkedly improved the treatment plan in patients with Hemophilia A whose immune systems produce antibodies against Factor VIII. Some adverse side effects have been linked to emicizumab, including injection site reactions, thrombotic microangiopathy and thrombosis in the absence of anti-drug antibodies [5]. There are two known reported cases of adult patients on emicizumab developing rhabdomyolysis. In both cases, there were no musculoskeletal or renal symptoms in the presence of the increased creatinine kinase levels [4]. Pathogenic variants in the RYR1 gene cause autosomal dominant malignant hyperthermia type 1, autosomal recessive conditions such as minicore myopathy, centronuclear myopathy and central core disease, some of which have both autosomal dominant and autosomal recessive inheritance. Rhabdomyolysis and hyperCKemia occurs in heterozygous pathogenic variants in the RYR1 gene. The heterozygous RYR1 variant denoted as c.1955C>T (p.Ala652Val) which was seen in this patient has reportedly not been observed in individuals with disease, and prediction algorithms (PolyPhen-2, SIFT, MutationTaster) were inconsistent. This variant has been seen in nine alleles in the general population suggesting that it may be rare. It is possible that this variant may have contributed, in part, to his presentation of rhabdomyolysis and hyperCKemia. More evidence is needed to determine the pathogenicity of this variant and whether other pathogenic gene variants which were not included in the panel test could have predisposed this patient to episodes of rhabdomyolysis and hypeCKemia.
To date, there is no known link between rhabdomyolysis and emicizumab in Hemophilia A. In the pediatric population, rhabdomyolysis can be caused by a myriad of factors with the most common often linked to infection and inherited disorders [6]. Rhabdomyolysis is also linked to overexertion. We ruled out exertional rhabdomyolysis as the sole potential cause for our patient’s symptoms, as he had been working at the same restaurant for over a year with no symptoms. In contrast to the two asymptomatic adult patients, our 19-year-old patient presented with rhabdomyolysis on emicizumab with musculoskeletal symptoms. This is an important finding for clinicians to be aware of when using emicizumab as a treatment.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
JAW wrote the largest share of the report and had direct patient contact. SH, AA, VRS and DCJ made significant contribution to interpretation of data of the work, and revised the work for important intellectual content. ABR made significant contribution to the conception and design of the work, analysis and interpretation of data.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Joseph A. Wilson AU - Stephanie Hayden AU - Alexander Asamoah AU - Vivek R. Sharma AU - David C. Jennings AU - Ashok B. Raj PY - 2020 DA - 2020/10/07 TI - Emicizumab Associated Rhabdomyolysis in Hemophilia A JO - Clinical Hematology International SP - 165 EP - 167 VL - 2 IS - 4 SN - 2590-0048 UR - https://doi.org/10.2991/chi.k.200924.001 DO - 10.2991/chi.k.200924.001 ID - Wilson2020 ER -
