The Association between Homocysteine, Arterial Stiffness and Executive Function Middle-age and Older Women

Present address:
Department of Physical Therapy, Faculty of Medical and Health Sciences, Tsukuba International University, 6-8-33, Manabe, Tsuchiura, Ibaraki, Japan
Department of Sports Research, Japan Institute of Sport Sciences, 3-15-1 Nishigaoka, Kitaku, Tokyo, Japan
- DOI
- 10.2991/artres.k.201102.003How to use a DOI?
- Keywords
- Plasma homocysteine; arterial stiffness; executive function
- Abstract
Age-related decreases in executive function and an increase in arterial stiffness and plasma homocysteine levels are related to the risk of dementia. However, the association between executive function, arterial stiffness, and homocysteine levels remains unclear. This study aimed to investigate the relationship between executive function, arterial stiffness, and plasma homocysteine in 82 middle-aged and older women. The Stroop interference time, Brachial-ankle Pulse Wave Velocity (baPWV), and plasma homocysteine concentration were collected. The correlation analyses revealed that the Stroop interference time was significantly correlated with plasma homocysteine (r = 0.40, p < 0.001) and baPWV (r = 0.38, p = 0.001). In addition, plasma homocysteine levels were significantly correlated with baPWV (r = 0.48, p < 0.001). In the mediated analyses, the plasma homocysteine level directly (β = 0.24; p = 0.037) and indirectly (β = 0.12, 95% confidence interval [0.007, 0.238]) affected the Stroop interference time. These results suggest that higher plasma homocysteine levels are associated with a decline in executive function mediated by higher artery stiffness in middle-aged and older women.
- Copyright
- © 2020 Association for Research into Arterial Structure and Physiology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
The prevalence of cognitive impairment and dementia is increasing in Japan owing to the aging population. It is important to examine the age-related decline of cognitive function and prevent the onset of dementia [1,2]. Cognitive function, especially executive function, is required for functional mobility and is required for making decisions and solving problems. Furthermore, executive function helps the elderly maintain their independence. Executive function plays a role in controlling attention and judgment about one’s surroundings and maintaining daily quality of life [3]. Cognitive dysfunction is associated with cerebral hypoperfusion and vascular dysfunction [4,5]. Increases in central arterial stiffness are correlated with cerebral arterial lesions and decreased cognitive function, including memory and executive processes in older individuals [6,7]. Recently, we reported that arterial stiffness is associated with executive function in middle-aged and older adults [8]. Moreover, postmenopausal depletion of sex hormones was found to contribute to a higher risk of dementia and accelerate the increase in arterial stiffness [9]. Therefore, it is important to further investigate executive function and arterial stiffness in postmenopausal women.
Blood plasma homocysteine is an amino acid that is associated with the methyl donor, methionine, and helps to regulate intracellular metabolism [10]. Homocysteine stimulates oxidative stress and increases matrix metalloproteinase activity, which contributes to vascular damage and endothelial dysfunction [11]. It has been reported that homocysteine levels increase after menopause, and hyperhomocysteine is a risk factor for arteriosclerosis [12], myocardial infarction [13,14], and ischemic cerebrovascular disorder [15]. A meta-analysis revealed that a high blood concentration of homocysteine might be the cause of cerebrovascular disease [16]. It has been reported that hyperhomocysteine is associated with dementia in Alzheimer’s disease [17]. In addition, it has been shown that blood homocysteine levels are associated with cognitive decline and dementia [18–20]. It is plausible that these detrimental effects of homocysteine on the vasculature could affect executive function. Mooijaart et al. [21] reported that elevated homocysteine levels were associated with cognitive impairment in individuals over 85 years. Although both plasma homocysteine and arterial stiffness may affect executive function, the causal relationships are not well understood. This study aimed to investigate the association between executive function, arterial stiffness, and plasma homocysteine in postmenopausal women using a cross-sectional design.
2. MATERIALS AND METHODS
2.1. Participants
Eighty-two healthy middle-age and older postmenopausal women (49–78 years, mean: 61.8) were recruited by local advertisements. The participant characteristics are presented in Table 1. The inclusion criteria were (1) nonsmoker, (2) not taking any medication, and (3) receiving hormone replacement therapy. The exclusion criteria were (1) history of neurological disorder, (2) cerebrovascular disease, (3) receiving treatment for hypertension, (4) dyslipidemia, (5) diabetes, (6) history of gastroenterological surgery, and (7) use of dietary supplements that influence blood pressure. All participants provided their informed consent to participate in the study. The study protocol was reviewed and approved by the ethics committee of the University of Tsukuba.
| Variables | Values |
|---|---|
| n | 82 |
| Age, years | 61.8 ± 6.2 |
| Height, cm | 155.8 ± 5.0 |
| Weight, kg | 53.5 ± 7.7 |
| BMI, kg/m2 | 22.0 ± 2.8 |
| TC, mg/dL | 231.5 ± 43.4 |
| HDL, mg/dL | 66.5 [54.8–80.3] |
| LDL, mg/dL | 140.9 ± 34.9 |
| TG, mg/dL | 83.5 [60.8–122.55] |
| SBP, mmHg | 123.6 ± 14.4 |
| DBP, mmHg | 74.9 ± 9.2 |
| HR, bpm | 62.4 ± 7.4 |
| baPWV, cm/s | 1387.0 [1276.5–1514.5] |
| Homocysteine, nmol/mL | 9.1 [7.8–10.2] |
| Stroop interference time, s | 0.38 [0.28–0.47] |
Data are shown as the mean ± SD, median [interquartile range], as appropriate.
n, number of subjects; BMI, body mass index; TC, Total cholesterol; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; TG, Triglycerides; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; baPWV, brachial pulse wave velocity.
Characteristics of the participants
2.2. Measurements
2.2.1. Executive function
The participants performed the Stroop task [22] using the Multi-PAS System (DKH, Tokyo, Japan). Based on a previous study [23], the color word Stroop task was used in this study, which involved two experimental conditions, namely the non-executive naming condition (EASY) and the executive condition (HARD). In the EASY condition, the color visual mark stimulus (XXXXX, one each of red, blue, green, and yellow) was presented at the top of the screen. The participants were asked to identify the ink color for the presented color word by selecting the corresponding word presented in black ink at the bottom right or left of the screen. In the HARD condition, participants responded to color words displayed in incongruent colors (e.g., the word RED was presented in blue); participants were asked to identify the corresponding ink color for the right or left words, which were also displayed in incongruent colors at the bottom of the screen. Each condition consisted of two sets of 15 trials, each of 60 s. We evaluated the Stroop interference time, which was estimated as the reaction time difference between the EASY and HARD conditions and is an index of executive function.
2.2.2. Arterial stiffness
Brachial-ankle Pulse Wave Velocity (baPWV) was measured using a pulse wave analyzer (PWV/ABI, Komaki, Japan). The cuffs were wrapped around the upper arm and ankles, and the ankles were connected to a plethysmograph sensor. Pressure waveforms were recorded simultaneously in the brachial and tibial arteries to estimate the time interval between the first rise of the brachial and tibial waveforms. The distances from the aortic valve opening to the upper arm to the ankle were determined using an estimation formula obtained from the patient’s height. The baPWV is obtained by dividing La − Lb by the latency difference of the pulse wave rise of the upper arm and ankle [24].
2.2.3. Blood chemistries
A sample of blood was collected using an antecubital vein after fasting overnight. Plasma samples were centrifuged at 3000 rpm at 4°C for 15 min and stored frozen at −80°C until use. Plasma homocysteine levels were measured using liquid chromatography with tandem mass spectrometry by a commercial laboratory (LSI Medience Co. LTD., Tokyo, Japan).
2.3. Statistical Analysis
The Shapiro–Wilk test was used to evaluate normality. Normally distributed data are presented as the mean ± standard deviation, while non-normally distributed data are presented as the median and interquartile range. Relationships were evaluated using Spearman’s rank correlation coefficients and partial correlation analyses adjusted for age. Mediated analyses were performed using the plasma homocysteine as the independent variable, baPWV as the mediated variable, and Stroop interference time as the dependent variable to investigate the association between the Stroop interference times, plasma homocysteine, and baPWV. Mediated analyses were performed using IBM SPSS macro functions (INDIRECT macro for SPSS [25]). The mediation effect was examined using non-parametric bootstrapping tests. SPSS statistical package version 24 (SPSS, IBM, Chicago, IL, USA) was used for the statistical analyses.
3. RESULTS
Table 1 displays the characteristics of the participants. The Stroop interference time was significantly correlated with the plasma homocysteine levels (Figure 1, r = 0.40, p < 0.001). The Stroop interference time was also significantly correlated with baPWV (Figure 2, r = 0.38, p = 0.001). Furthermore, plasma homocysteine levels were significantly correlated with baPWV (Figure 3, r = 0.48, p < 0.001). After adjusting for age, significant correlations were observed between the Stroop interference time and plasma homocysteine (partial r = 0.37, p = 0.001), the Stroop interference time and baPWV (partial r = 0.24, p = 0.035), and plasma homocysteine and baPWV (partial r = 0.47, p < 0.001). Figure 4 shows the mediation analysis. Plasma homocysteine significantly explained the Stroop interference time (β = 0.36; p = 0.001) and baPWV (β = 0.44; p < 0.001). Furthermore, baPWV significantly explained the Stroop interference time (β = 0.27; p = 0.020). Moreover, when baPWV was used as the mediating variable, plasma homocysteine directly (β = 0.24; p = 0.037) and indirectly (β = 0.12; 95% confidence interval [0.007, 0.238]) affected the Stroop interference time, respectively. This result indicates that baPWV partially mediates the relationship between plasma homocysteine and the Stroop interference time, to a significant level (Table 2).
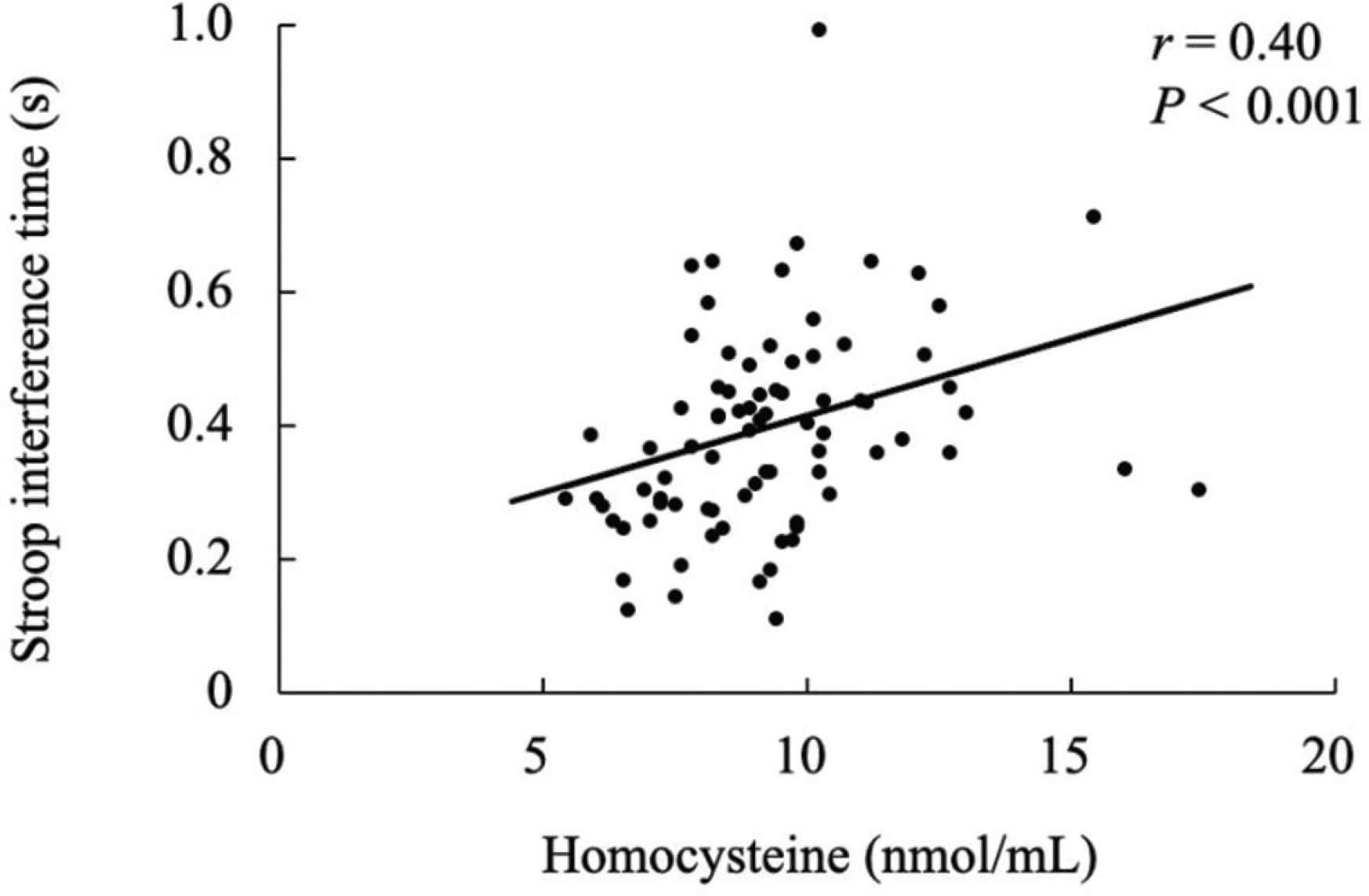
Relationship between homocysteine and Stroop interference time.
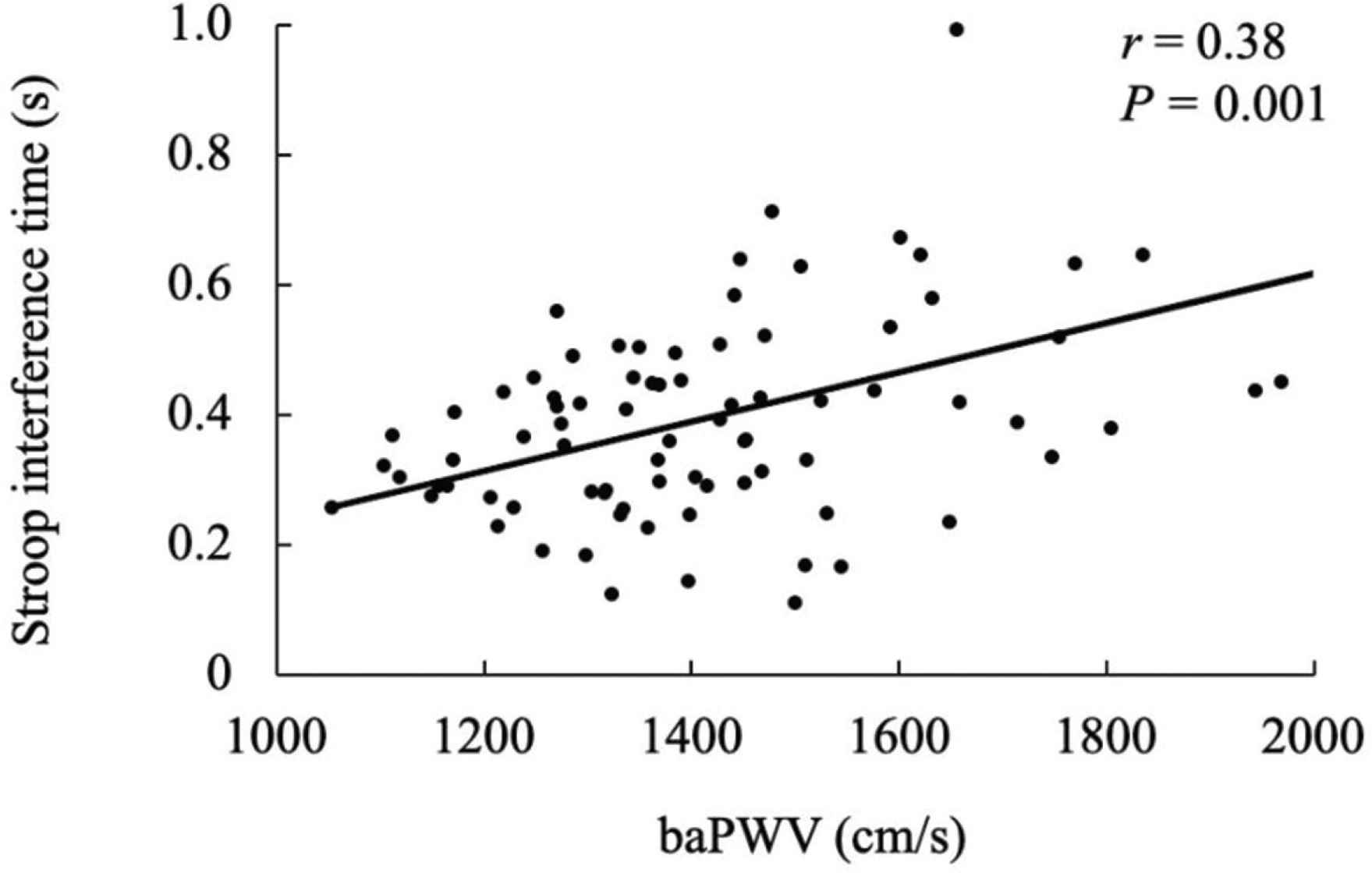
Relationship between baPWV and Stroop interference time. baPWV, brachial pulse wave velocity.
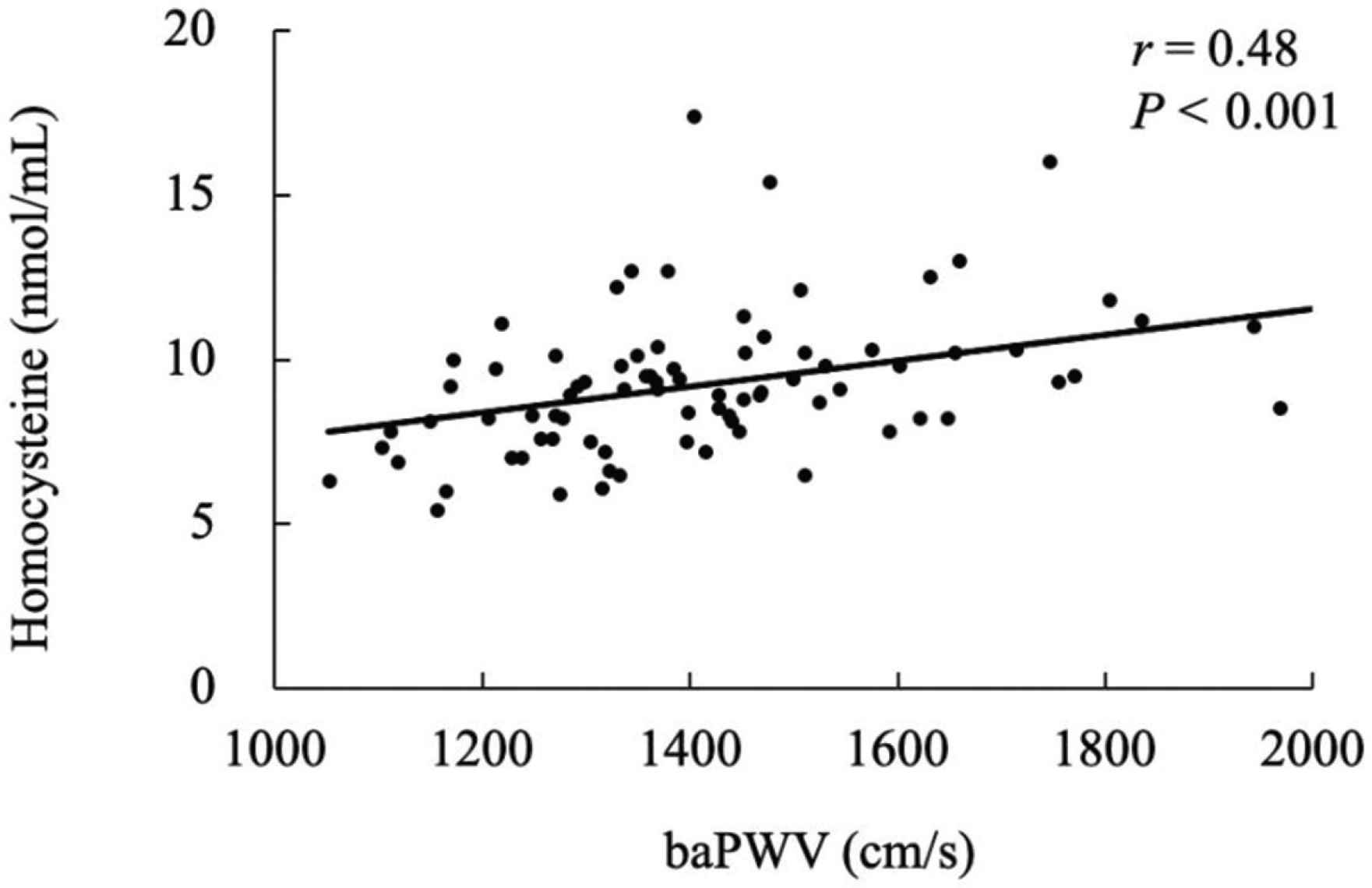
Relationship between baPWV and homocysteine. baPWV, brachial pulse wave velocity.
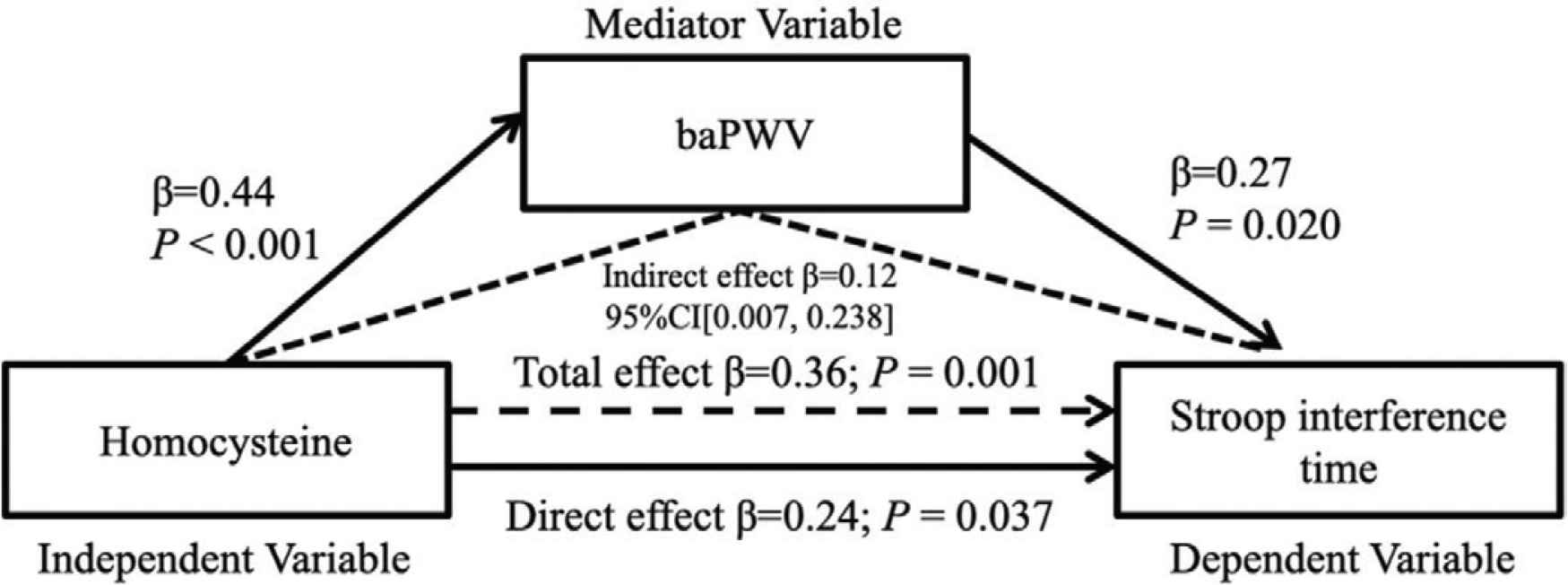
Mediation analysis. The effect of homocysteine on Stroop interference time mediated by baPWV. baPWV, brachial pulse wave velocity; β, standardized regression coefficient; CI, 95% confidence interval.
| Dependent variable | Stroop interference time (s): ST | |||
| Independent variable | Homocysteine (nmol/mL): HCY | |||
| Mediator variable | brachial Pulse Wave Velocity (cm/s): baPWV | |||
| Sample size | 82 | |||
| Model 1: HCY to baPWV | ||||
| R2 = 0.19 | p < 0.001 | β | p | CI |
| HCY | 0.44 | <0.001 | [0.24, 0.64] | |
| Model 2: HCY to ST | ||||
| R2 = 0.13 | p = 0.001 | β | p | CI |
| HCY | 0.36 | 0.001 | [0.15, 0.57] | |
| Model 3: The effect of HCY and baPWV on ST | ||||
| R2 = 0.19 | p < 0.001 | β | p | CI |
| HCY | 0.24 | 0.037 | [0.02, 0.46] | |
| baPWV | 0.27 | 0.020 | [0.04, 0.49] | |
| Indirect effect of HCY on ST | β | Boot LLCI | Boot ULCI | |
| baPWV | 0.12 | 0.007 | 0.238 | |
β, standardized regression coefficient; CI, confidence interval; Boot, bootstrap.
Statistics of mediation analysis
4. DISCUSSION
In this study, we investigated the association between executive function, arterial stiffness, and plasma homocysteine in middle-aged and older women. We found that the Stroop interference time was significantly correlated with plasma homocysteine and baPWV. In addition, plasma homocysteine levels were significantly correlated with baPWV. Furthermore, the mediation analyses showed that baPWV partially mediated the relationship between plasma homocysteine and the Stroop interference time. These results suggest that higher plasma homocysteine levels are associated with a decline in executive function partially mediated by higher artery stiffness in postmenopausal women.
Emerging evidence has shown that a higher homocysteine level is a risk factor for stroke and dementia [26]. Homocysteine has a neurotoxic action that damages neurons and further promotes apoptosis [20]. Homocysteine overstimulates the N-methyl-d-aspartate receptor, which leads to neurotoxicity [27] and smooth muscle cell proliferation, as well as increased platelet aggregation, strokes, and white matter lesions [20]. It has been shown that blood homocysteine concentration is associated with cognitive decline and dementia [28,29]. Prins et al. [28] reported a relationship between an increase in homocysteine levels and a decrease in cognitive function, particularly psychomotor speed, in 1077 older adults [28]. Lewerin et al. [29] reported that high homocysteine adversely affects movement and cognitive performance, especially in digit symbol and block design tests [29]. Consistent with previous studies, we demonstrated that plasma homocysteine levels were significantly correlated with the Stroop interference time. These results suggest that blood homocysteine levels are associated with executive function in postmenopausal women.
Homocysteine is also known to reduce nitric oxide bioavailability by stimulating reactive oxygen species. Endothelial function and compliance of the arterial wall are lost due to vascular damage [11]. It has been reported that higher levels of homocysteine are associated with the prevalence of arteriosclerosis [30]. Chen et al. [31] recently demonstrated that higher homocysteine levels were associated with an increased prevalence of arterial stiffness. In the present study, we showed a relationship between executive function and homocysteine, and between executive function and arterial stiffness. Increased arterial stiffness by higher homocysteine may partly contribute to age-induced cognitive decline.
We found that baPWV was significantly related to executive function, which was evaluated using the Stroop interference time. Concerning vascular function and cognitive function, some studies have focused on vascular function as a predictor of cognitive decline [32,33]. Previous studies have reported that higher baPWV and blood pressure fluctuations increase the risk of a decline in cognitive function in older adults [34]. Furthermore, pulse pressure and PWV have also been associated with cognitive decline [32]. Recently, we found that carotid arterial stiffness was correlated with executive function, which was evaluated using the Stroop interference time, similar to the present study [8]. Taniguchi et al. [33] found that higher baPWV was related to cognitive decline in patients with hypertension using the mini-mental state examination. Central artery stiffness and ability to buffer pulsatile strain may be attributed to cerebral hypoperfusion and microvascular damage, which may deteriorate cognitive function [35,36]. Consistent with previous studies, our results also showed a relationship between baPWV and executive function. Specifically, we found that individuals with lower baPWV had shorter Stroop interference times. It has been reported that the increase in arterial stiffness in patients with end-stage renal disease was independently correlated with plasma homocysteine concentration [37]. In addition, some evidence shows that an increase in homocysteine may play a role in vascular damage and arterial stiffness [38,39]. In this study, a significant relationship between homocysteine and executive dysfunction mediated by arterial stiffness was evidenced. However, the mediation analysis did not exhibit a significant relationship. Therefore, increased arterial stiffness may lead to a decline in cognitive performance, especially in executive function.
There are several limitations to this study. First, the present study had a relatively small sample size, and we focused only on healthy postmenopausal females. The mediation analysis adjusted for age and blood pressure did not reach statistical significance between baPWV and the Stroop interference time. On the other hand, each relationship between homocysteine, baPWV, and the Stroop interference time was significant after adjusting for age and blood pressure in this study. Although homocysteine, baPWV, and the Stroop interference time seem to be associated with each other, the hypothesis that arterial stiffness mediates the impact of homocysteine on executive function may not be affected by age and blood pressure. In the future, a mediation analysis with a larger sample size, including young adults, patients with hypertension, and males, should be conducted. Second, we only used the Stroop task to measure executive function, but further studies are warranted to investigate other functions such as working memory, verbal learning, processing speed, and category fluency. Third, homocysteine is a metabolite of the amino acid, methionine, which is primarily metabolized in the kidney, and chronic renal dysfunction increases blood homocysteine levels. It has been reported that a higher blood homocysteine concentration was found in patients with renal disease [40,41]. However, we did not measure renal function, such as the estimated glomerular filtration rate or albuminuria, as there were no subjects with renal disease in this study.
5. CONCLUSION
The present study demonstrated that plasma homocysteine was significantly associated with arterial stiffness and executive function, and arterial stiffness was also associated with executive function in postmenopausal women. Furthermore, the mediated analysis revealed that the association between plasma homocysteine and executive function was partially mediated by arterial stiffness. These results suggest that the homocysteine-induced increase in arterial stiffness involves decreased executive function.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
AH, NA and SM contributed to conceptualization and funding acquisition. AH, NA and RM contributed to data curation and formal analysis. AH, NA and SM contributed in writing (reviewed and edited).
FUNDING
This study was supported by
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Ai Shindo-Hamasaki AU - Nobuhiko Akazawa AU - Reiko Momma AU - Seiji Maeda PY - 2020 DA - 2020/11/13 TI - The Association between Homocysteine, Arterial Stiffness and Executive Function Middle-age and Older Women JO - Artery Research SP - 32 EP - 37 VL - 27 IS - 1 SN - 1876-4401 UR - https://doi.org/10.2991/artres.k.201102.003 DO - 10.2991/artres.k.201102.003 ID - Shindo-Hamasaki2020 ER -
