Primary African American Endothelial Cells Exhibit Endothelial Dysfunction with an Exacerbated Inflammatory Profile and Blunted MMP-2 Activity
 , Chenyi Ling2,
, Chenyi Ling2,  , Heather Grimm3, Adelola Adeyemo6,
, Heather Grimm3, Adelola Adeyemo6,  , Maitha Aldokhayyil4,
, Maitha Aldokhayyil4,  , Kevin Heffernan6, Bo Fernhall7, Michael Brown4, 7
, Kevin Heffernan6, Bo Fernhall7, Michael Brown4, 7- DOI
- 10.2991/artres.k.201102.005How to use a DOI?
- Keywords
- Endothelial dysfunction; hypertension; inflammation; matrix metalloproteinase-2; racial difference
- Abstract
Endothelial dysfunction is associated with the racial health disparity in vascular dysfunction in African Americans (AAs). Matrix Metalloproteinase (MMP)-2 is constitutively expressed in endothelial cells (EC) and is a biomarker that has been associated with hypertension, as its properties are involved in pathologic oxidative stress and pro-inflammation that may affect vascular homeostasis. Herein, we report significant inverse relationships between MMP-2, stroke volume, carotid and aortic systolic pressures in a small cohort of young AA men. In the current study, we postulated that basal activation in AA Endothelial Cells (EC) may include different responses in MMP-2 activity, compared to Caucasian (CA). We evaluated gene and protein expression and activity of MMP-2, and related peptides, in multiple different primary Human Umbilical Vein Endothelial Cells (HUVEC) isolated from four different AA and CA donors. Compared to CA, AA HUVEC exhibited greater basal MMP-2, MMP-14, Tissue inhibitor of metalloproteinase-2, Vascular cell adhesion molecule-1, Intracellular adhesion molecule-1, and Interleukin (IL)-1β gene expression and greater endothelin-1 secretion (p < 0.05). Interestingly, basal MMP-2 protein expression was greater while relative secreted MMP-2 activity was lower (p = 0.041). Inflammatory stimuli (tumor necrosis factor-alpha) exacerbated relative MMP-2 activity in AA HUVEC (p = 0.007). These in vitro data offer insights into a potential mechanism involving primary endothelial cell inflammatory mediated MMP-2 activities that may contribute to poorer vascular outcomes. Further studies are necessary to investigate endothelial intracellular transcriptional, translational, and activity regulation of MMP-2.
- Copyright
- © 2020 Association for Research into Arterial Structure and Physiology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
African American (AA) race is an independent risk factor for vascular disease (e.g., hypertension) [1,2]. Hypertension disproportionately affects AAs, regardless of admixture [3], coupled with increased severity of hypertension and nearly a twofold increase in mortality associated with cardiovascular disease [4], thus revealing the epidemiological racial disparity and public health impact regarding hypertension [5,6]. Therefore, investigations are necessary to understand potential mechanism(s) that ultimately lead to premature and exacerbated hypertension-related cardiovascular diseases (e.g., stroke, kidney disease, hypertensive neuropathy, heart dysfunction) and end-organ failure in AA.
Endothelial Dysfunction (EnDy), which can precede hypertension, has been shown to be greater in AA [7,8]. EnDy is characterized by a chronic low-grade inflammatory state of the endothelium and is marked by an exacerbated immune and oxidative stress response to inflammation. In vitro, Kalinowski et al. [1] showed that AA Human Umbilical Vein Endothelial Cells (HUVEC) exhibit EnDy marked by increased oxidative stress (e.g., superoxide and peroxynitrite production; Reactive Oxygen Species (ROS) and consequent reduced nitric oxide (NOx) bioavailability). Studies have previously shown that AA had significantly greater ROS in vitro and in vivo [9,10], and had greater circulating inflammatory endothelial microparticles (a novel biomarker of EnDy) [11,12]. One mechanistic explanation of these in vitro observations was that AA Endothelial Cells (EC) exhibited greater expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, increased basal inflammation, and lower antioxidant capacity [9] compared to Caucasian (CA) HUVEC that significantly improved in response to in vitro exercise mimetic (i.e., laminar shear stress) [13,14]. Heightened ROS is a potent secondary messenger that initiates a cascade of dysfunctional activities of other proteins. Therefore, this profile in AA HUVEC provoked our examination of Matrix Metalloproteinase (MMP)-2 expression and activity, which may be directly affected by ROS.
Matrix metalloproteinase-2, a biomarker associated with hypertension, is an enzyme that is constitutively expressed in EC and involved in chronic vascular disease processes [15,16]. Coutinho et al. [17] reported that MMP-2 may be a predictor of cardiovascular events in AA. MMP-2 activities can propagate EnDy by activating vasoactive pro-proteins, such as the potent vasoconstrictor pro-Endothelin (ET)-1 [18] and pro-Interleukin (IL)-1β [19]. Due to greater reported ROS in AA [9,10], MMP-2 may have a role in the disproportionate inflammation witnessed in AA by acting on these cytosolic pro-proteins mentioned above. We previously reported that circulating levels of MMP-2 were slightly lower and resistant to change following resistance exercise training in young AA men [10]. With these differential responses, we hypothesized that MMP-2 might be associated with vascular measures in this group. Following a secondary analyses of data reported in Cook et al. [10], we observed moderate relationships between plasma MMP-2 and stroke volume, carotid and aortic Systolic Blood Pressure (SBP) in young AA men but not CA (reported herein). As EC are a source of MMP-2, we initiated an in vitro investigation in multiple individual primary AA and CA HUVEC to examine the extent of EnDy, MMP-2 expression and activity. Given previous reports of racial differences in EC, we hypothesized that MMP-2 expression and activity would be greater in AA HUVEC.
2. MATERIALS AND METHODS
2.1. In vivo: MMP-2 and Blood Pressure
2.1.1. Subject characteristics
Healthy young self-reported AA and Caucasian CA men, between 18 and 30 years old, were recruited at the University of Illinois at Urbana [10].
2.1.2. Blood measures
Matrix metalloproteinase-2 was measured in serum via Elisa (R&D Systems, Minneapolis, MN, USA) as previously shown [10]. Brachial BP (mmHg) was measured by an automated oscillometric cuff (HEM-907 XL; Omron, Shimane, Japan). Carotid and aortic pressures (mmHg) were calculated via applanation tonometry (Millar Instruments; Houston, TX, USA) and stroke volume (ml/min) via ultrasonography (SSD5500; Aloka, Tokyo, Japan) as previously reported by Heffernan et al. [20]. The study was approved by the University of Illinois at Urbana Institutional Review Board.
2.2. Endothelial Cell Culture and Experimental Conditions
Primary HUVEC lines isolated from self-reported AA (n = 4) and CA (n = 4), all different donors with both male and female gender evenly split within groups, were obtained from Lonza (Walkersville, MD, USA) and cultured in parallel in the same manner as experiments previously performed and described [9,14]. Specific clinical characteristics of the donors are protected and only information about self-identified race/ethnicity, gender of the fetus, general health, and smoking status (none) was collected by Lonza. Cell cultures were grown to ~95% confluence in 100-mm tissue culture dishes in full EGM-2 media (Lonza). Both basal (control) and stimulated conditions [Tumor Necrosis Factor-alpha (TNF-α); 10 ng/ml], at 4 and 24 h, were performed in serum free media to avoid the measurement of MMPs present in fetal bovine serum. Although fetal bovine serum was excluded from culture medium, all other growth factors, antioxidants, and antibiotics were added to media from the EGM-2 bullet media kit. Experiments were performed in duplicate and carried out in passage 6 for each HUVEC line.
2.2.1. Ethical guidelines
Ethical concerns pertaining to human cell culture work are important and not overlooked. Lonza Walkersville, Inc. accepts tissue only if consent for research has been obtained. Audits are frequently conducted to ensure appropriate operational procedures, and compliance with the Protection of Human Subjects regulations, of consent processes and receipt of any de-identified demographic or medical history information from donors. The in vitro studies were deemed to be exempt by the University of Illinois at Chicago Institutional Review Board.
2.3. Zymography
In each experimental condition, zymography (secreted activity) was assessed following the protocol outlined by Ben-Yosef et al. [21]. Briefly, the supernatant of cultured cells was harvested and centrifuged at 12,000 RPM to remove any cellular debris. After, supernatant (500 µl) was concentrated (20×) by centrifugation (Millipore – Amicon ultra 0.5 filter devices) and loaded onto Tris-Gly gelatin-containing gels. A 10 ng pro-MMP-2 (70–72 kDa; Abcam; Cambridge, MA, USA) and fully activated MMP-2 (58–62 kDa) standard was loaded on each gel for the evaluation of MMP-2 activity. Densitometric analyses of zymography was completed using the ImageJ software (NIH) to quantify MMP-2 activity in digested zones. Data are expressed as relative band intensity of active MMP-2-to-Pro-MMP-2 (mean ± SEM). Representative zymograms blots are shown (n = 4, performed in duplicate).
2.4. Western Blotting
Culture dishes were harvested for protein analysis as previously described [9]. Protein from cell lysates were separated by NuPAGE (Bis-Tris) gels and transferred to PVDF membranes (Invitrogen), blocked with 5% nonfat dry milk dissolved in Tris-Buffered saline, and incubated overnight with primary antibodies at 4°C. Immuno-reactive proteins were detected by chemiluminescence with Thermo Scientific SuperSignal (Pierce Biotechnology; IL, USA). Primary antibodies included MMP-2 (D8N9Y) and housekeeping genes anti-β-actin, and anti-α-tubulin (Cell Signaling Technologies; Danvers, MA, USA). Densitometric analyses of protein was completed using the ImageJ software (NIH). Band intensity of proteins were divided by the band intensity of their internal control for normalization. Data are expressed as relative protein expression (fold change Δ; mean ± SEM) and AA HUVEC data are compared to basal expression of CA HUVEC (referent control).
2.5. Reverse Transcriptase Qualitative Polymerase Chain Reaction
Reverse Transcriptase Qualitative Polymerase Chain Reaction (RT-qPCR) was performed to quantify the EC inflammatory activation profile of HUVEC under basal and TNF-α stimulated conditions. Total RNA was harvested from HUVECs using RNeasy Mini extraction kit (Qiagen; MD, USA), converted to cDNA using the Affinity Script cDNA synthesis kit (Agilent Technologies; Wilmington, DE), and RT-qPCR was performed for gene expression of MMP-2, MMP-14, Tissue Inhibitor of Metalloproteinase (TIMP)-2, Endothelial Nitric Oxide Synthase (eNOS), IL-1β, Vascular Cell Adhesion Molecule (VCAM)-1, and Intracellular Adhesion Molecule (ICAM)-1. Rt-qPCR was performed using an Agilent Stratagene MX3005P machine and all PCR primers were purchased from Life Technologies utilizing Taqman® assays (Grand Island, NY, USA). Gene expression data are expressed as relative mRNA fold expression (ΔΔCT method) by normalizing values to housekeeping gene expression of GAPDH and basal expression in CA (referent group). ΔΔCT method is explained by Livak and Schmittgen [22].
2.6. Enzyme Linked Immunosorbent Assay
Enzyme linked immunosorbent assay colorimetric assay kits were used to measure ET-1 (Enzo Life Sciences; Farmingdale, NY, USA) and NOx (Abcam) in cell culture media of basal conditions (unstimulated for 24 h; n = 4). The NOx colorimetric kit measurement utilized nitrate/nitrite quantification via Griess Reagent. This method has been validated to reflect NOx in biological samples [23]. Assays were performed per manufacturer’s instructions. Data are expressed for ET-1 in pmol/L and NOx in µmol/L as mean ± SEM with ET-1 intra/inter-assay CV of 8.9% and 5.9%, respectively, and <5% for NOx in the observed ranges.
2.7. Data Analysis
Statistical analysis includes descriptive statistics for in vivo and in vitro studies. In vivo, baseline measure analysis was performed using two-tailed t-tests between groups. One-way ANOVA analysis (within race and within experimental conditions), with Bonferroni pairwise comparisons, was performed to examine race and TNF-α interaction effects concerning gene and protein expression and MMP-2 activity. Within and between-HUVEC racial group analysis was performed using t-tests. Non-parametric statistical analysis (with Welch’s correction or Kruskal–Wallis with Dunn’s comparisons) was used when data were not normally distributed. Correlation analyses for in vivo data were performed using Pearson r and Spearman when not normally distributed (MMP-2/Aortic SBP AA data only). All statistical analyses were performed using SPSS version 21.0 (SPSS Inc.; Chicago, IL, USA) and GraphPad Prism 8.0 (La Jolla, CA, USA). Data are expressed as mean ± SEM with a level of significance set at p < 0.05.
3. RESULTS
3.1. In vivo: Relationship between MMP-2 and Vascular Measures
Baseline subject characteristics for AA (n = 13) and CA (n = 11) men (age, body mass index, MMP-2 (ng/ml), stroke volume, carotid and aortic systolic BP) are reported in Table 1. A secondary analysis of blood and vascular measures revealed moderate but significant inverse correlations between circulating MMP-2 and stroke volume, carotid SBP, and aortic SBP in AA with no relationship in CA (Table 1). We questioned that naïve EC (i.e., HUVEC) may provide insight into differential MMP-2 expression between race and, therefore, initiated an in vitro investigation of EnDy and MMP-2 activity in primary AA and CA HUVEC.
| African American | Caucasian | p | |||
|---|---|---|---|---|---|
| n = 13 | n = 11 | ||||
| Age (years) | 22.1 ± 0.512** | 27.0 ± 1.07 | 0.0002 | ||
| Body Mass Index (BMI) | 29.3 ± 1.20 | 29.5 ± 1.18 | 0.913 | ||
| MMP-2 (ng/ml) | 231.8 ± 10.68 | 229.4 ± 23.89 | 0.921 | ||
| Stroke volume (ml/beat) | 111.5 ± 4.99 | 112.6 ± 5.98 | 0.891 | ||
| Carotid SBP (mmHg) | 128.7 ± 2.89 | 122.2 ± 3.67 | 0.166 | ||
| Aortic SBP (mmHg) | 113.1 ± 2.41 | 109 ± 2.58 | 0.288 | ||
| MMP-2 (ng/ml) | r | p | r | p | |
| Stroke volume (ml/beat) | –0.558 | 0.047* | –0.2172 | 0.521 | |
| Carotid SBP (mmHg) | –0.555 | 0.050* | 0.07738 | 0.821 | |
| Aortic SBP (mmHg) | –0.601 | 0.023* | 0.4097 | 0.210 | |
Data are reported as average ± SEM. Pearson r-values with corresponding p-values in AA and CA. Spearman r is reported for MMP-2 and Aortic SBP in AA only.
p < 0.05,
p < 0.001.
Relationship between circulating MMP-2, stroke volume, and vascular pressures
3.2. Endothelial Dysfunction in AA EC
AA HUVEC (n = 4 different cell lines per group) exhibited EnDy observed by lower basal NOx production (CA: 11.09 ± 1.002; AA: 8.25 ± 0.53 µmol/L, p = 0.046) (Figure 1A) and higher ET-1 production (CA: 4.01 ± 0.4; AA: 5.14 ± 0.36 pmol/L, p = 0.001) (Figure 1B) compared to CA (n = 4 different cell lines per race). Further, AA HUVEC exhibited an inflammatory activated gene expression profile. eNOS gene expression was not significantly different under basal (p = 0.065) or TNF-α (p = 0.060) treated conditions, while EC inflammatory activation markers (VCAM-1: basal, p = 0.019; TNF-α, p ≤ 0.0001; and AA - TNF-α, p = 0.030, n = 4; and ICAM-1: basal, p = 0.013 and TNF-α, p ≤ 0.0001, n = 4) were significantly different between race. IL-1β gene expression was greater in AA HUVEC under basal (p = 0.002), TNF-α (p < 0.0001), and AA - TNF-α, p = 0.003 conditions (Figure 2A–2D).
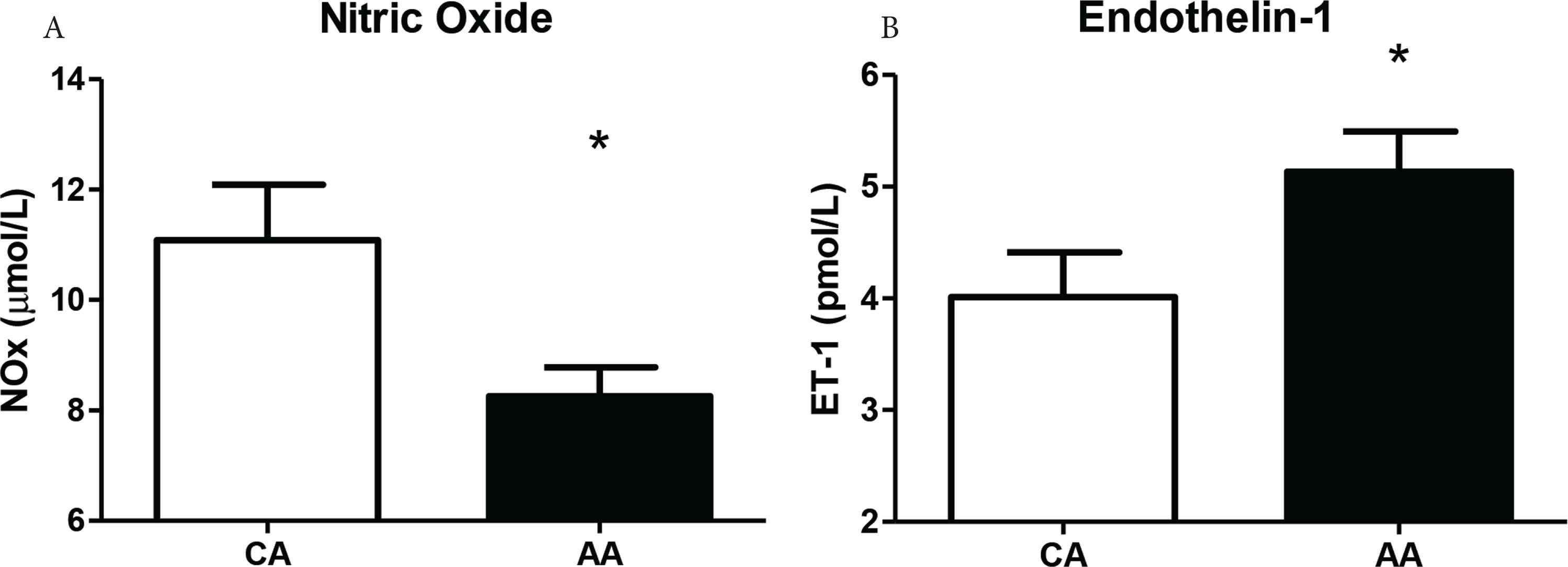
The secretion of NOx and ET-1 in the basal (control) condition in AA and CA HUVEC. (A) NOx (µmol/L) in the cell culture media of AA HUVEC is lower compared to CA after 24 h. (B) ET-1 (pmol/L) secretion is greater in AA HUVEC, compared to CA, after 24 h. Data are reported as mean ± SEM. * (A) p < 0.05, (B) p = 0.001; (n = 4).
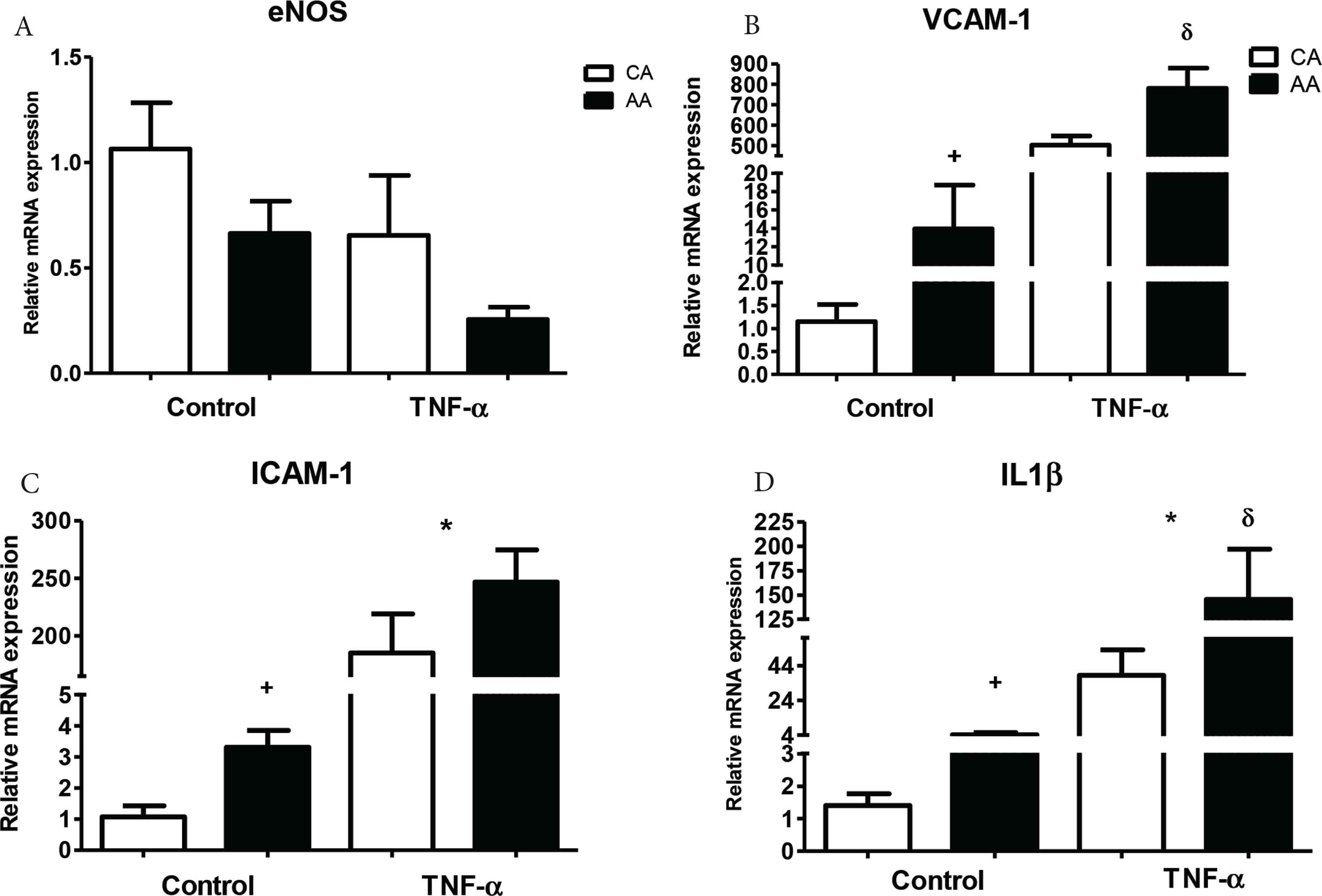
Relative gene expression of eNOS, VCAM-1, ICAM-1, and IL-1β under basal and TNF-α stimulation (10 ng/ml). After a 4 h incubation, eNOS was lower under both basal and TNF-α stimulation in AA (A). VCAM-1 was significantly higher under basal and TNF-α stimulated conditions in AA (B). ICAM-1 was significantly higher under basal and TNF-α conditions in AA (C). IL-1β was significantly higher under basal and TNF-α stimulated conditions in AA (D). Data are reported as mean ± SEM relative fold expression compared to control CA (white bar) referent group. + denotes significant basal racial difference, * denotes significant TNF-α effect, δ denotes significant race by TNF-α effect; duplicated, p < 0.05 (n = 4).
3.3. MMP-2 Expression and Activity
In our assessment of MMP-2 activity, gene and protein expression, MMP-2 gene expression was significantly greater in AA HUVEC (p < 0.05) under basal conditions and TNF-α significantly decreased expression in AA and CA HUVEC (F3,25 = 8.32; p = 0.0007) (Figure 3A). We also measured the gene expression of peptides related to MMP-2 activation. Under basal conditions, MMP-14 (p < 0.01) and TIMP-2 (p < 0.05) expression were also significantly greater in AA HUVEC (Figure 3B and 3C). Basal and TNF-α stimulated MMP-2 protein expression was slightly greater in AA, with a twofold difference (basal; CA-C: 1.83 ± 0.58 vs. AA-C: 3.68 ± 0.61) and that was sustained after TNF-α stimulation (CA-T or AA-T; F3,15 = 3.256; p = 0.0596) (Figure 4). Representative blots show MMP-2 band for each race and condition with corres-ponding α-tubulin bands.
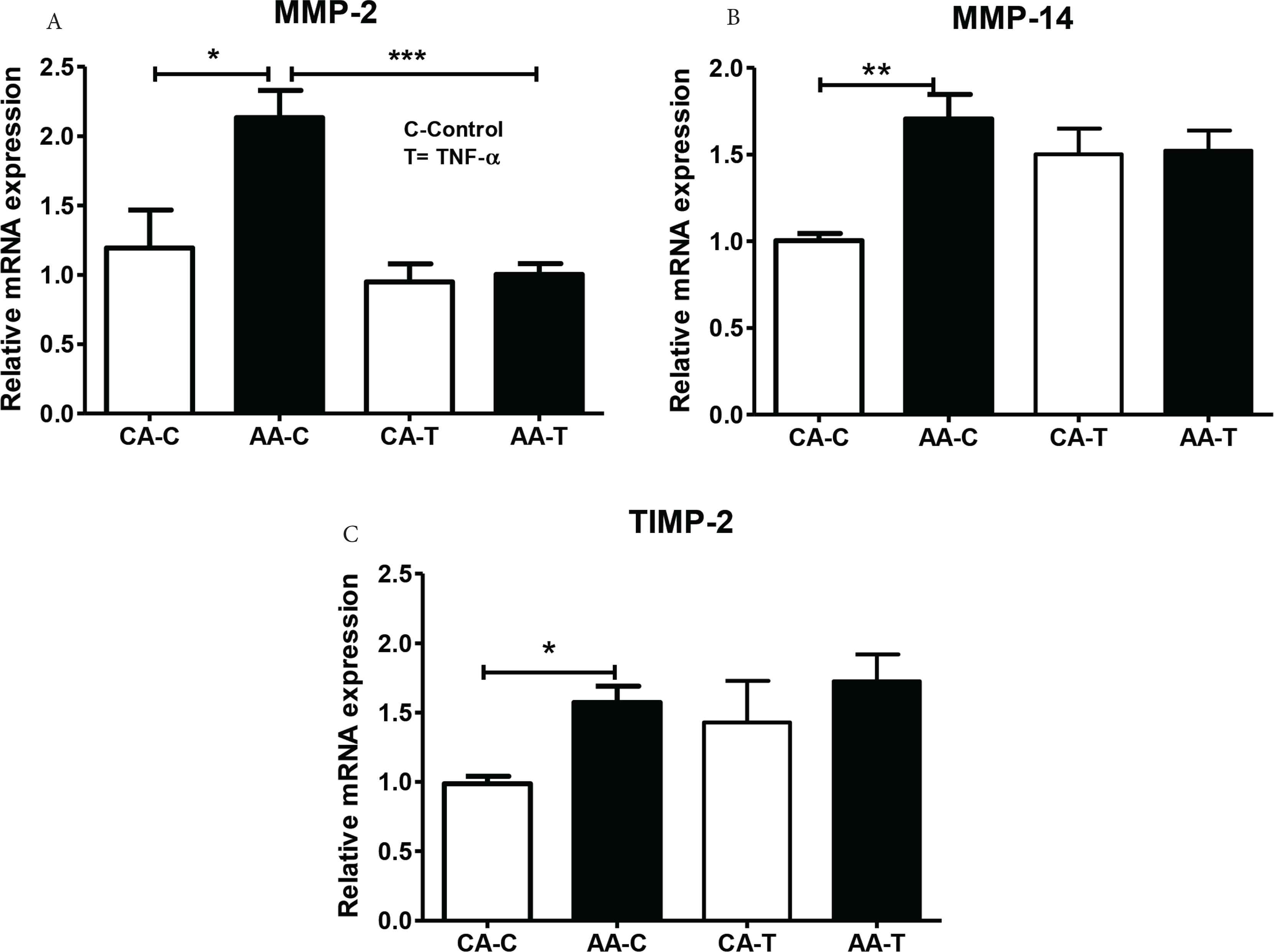
Relative gene expression of (A) MMP-2, (B) MMP-14, and (C) TIMP-2 under control (-C) and TNF-α (-T) conditions. AA EC had significantly greater basal MMP-2, MMP-14, and TIMP-2 gene expression relative to CA. TNF-α reduced MMP-2 (A). Data are reported as mean ± SEM relative fold expression compared to CA referent group. 4 h; *p < 0.05, **p < 0.01, ***p < 0.001 (n = 4, duplicated).
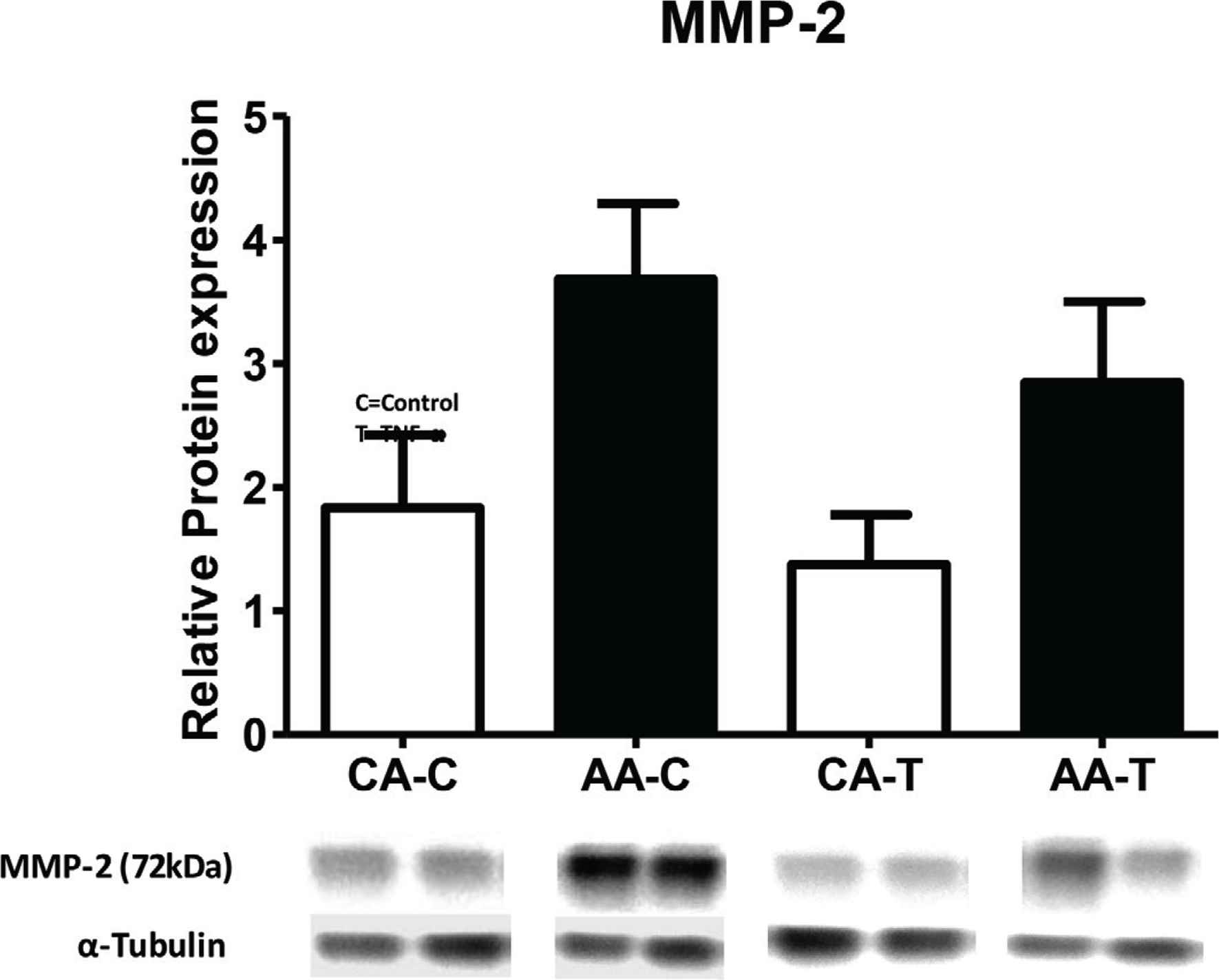
Relative protein expression of MMP-2 under control (-C) and TNF-α (-T). We observed slightly elevated MMP-2 protein expression in AA, compared to CA EC (p = 0.07). Data are reported as mean ± SEM. Representative blots below show two adjacent lanes per group with representative α-tubulin bands; (n = 4).
In lieu of elevated MMP-2 gene and protein expression, we obse-rved significantly lower basal activated MMP-2 (64 kDa) to pro-MMP-2 (72 kDa) ratio (marked as relative activity) in AA HUVEC after 4-h incubation (CA: 1.050 ± 0.2961; AA: 0.3008 ± 0.1559 relative units, t2.23, 14: p = 0.041; n = 4) which was still blunted after 24-h (CA: 1.101 ± 0.1913; AA: 0.6965 ± 0.1487; n = 4). However, MMP-2 relative activity 24-h post TNF-α stimulation was exacerbated in AA HUVEC (CA: 3.29 ± 0.23; AA: 6.15 ± 0.05 relative units, p = 0.007, n = 4) (Figure 5A and 5B).
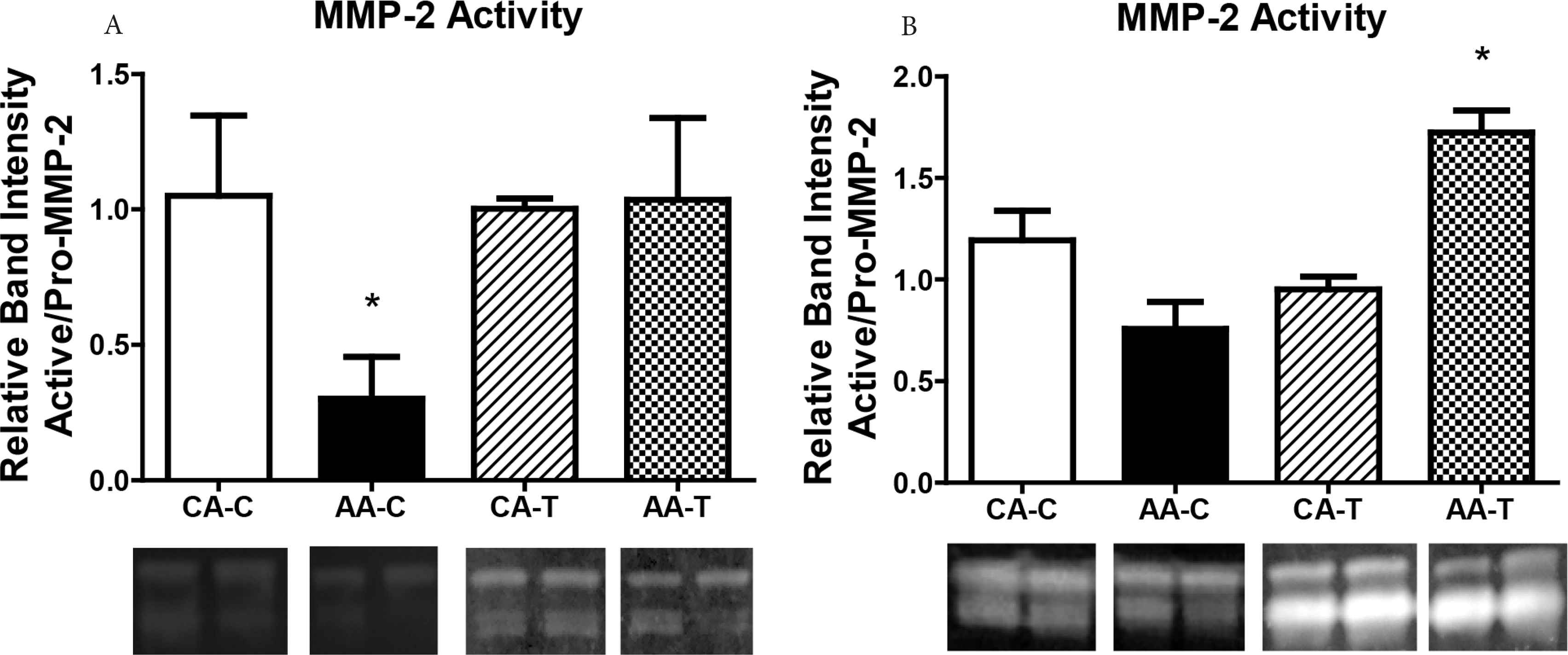
Relative activity of MMP-2 via zymography in AA and CA EC. (A) representation shows secreted MMP-2 activity after 4 h basal (unstimulated) and TNF-α conditions while (B) shows secreted MMP-2 activity after 24 h. Representative graphs show (A) AA HUVEC have significantly less relative MMP-2 activity after 4 h in the basal condition (n = 4). Relative MMP-2 activity after 24 h (B) was not different under basal condition between race, however, TNF-α stimulation exacerbated secreted MMP-2 activity in AA EC (n = 4). Data are reported as mean ± SEM relative fold expression compared to control expression of CA referent group. Representative zymogram blots with two adjacent bands per group are shown; *p < 0.05.
4. DISCUSSION
Racial disparity studies are carried out solely in vivo making it difficult to understand the underlying mechanisms of EnDy that precede vascular disease(s). To the best of our knowledge, this is the first in vivo study to report associations between MMP-2 and vascular measures in AA and first in vitro study to investigate the contributions of EnDy concerning MMP-2. The main findings in our studies were, in vivo, MMP-2 was significantly and inversely related to stroke volume, carotid, and aortic BP in young AA men with no significant relationship in CA. In vitro, primary AA HUVEC exhibited basal inflammatory EnDy and differential MMP-2 expression and secreted activity as indicated by: (1) blunted basal NOx and elevated ET-1 vasoconstrictor secretion, (2) greater pro-inflammatory and MMP-2 related gene expression, and (3) greater MMP-2 protein expression with lower basal relative MMP-2 activity, compared to CA EC. These data reveal significant differential activity and responses in basal and TNF-α stimulated MMP-2 expression and activity between AA and CA HUVEC. MMP-2 is gaining recognition as therapeutic targets of endothelial and vascular dysfunction [24].
The in vitro investigation was performed to provide insight into the contribution of MMP-2 in EC dysfunction. Utilizing multiple individual primary HUVEC, a naïve EC line, lessens the impact of pre-existing factors from humans and permits identification of EC profiles related to racial/ethnic groups. This outcome in AA HUVEC is novel considering that reduced NOx bioavailability and ET-1 have been implicated in AA hypertension [25] and the effect that heightened inflammation has on EC function [26]. Further, MMP-2 activity is a key component of vascular Extracellular Matrix (ECM) maintenance, atherosclerosis progression, and hypertension which emphasizes its role in maintaining vascular structure and homeostasis [27,28]. Thus, differential activity of this key enzyme in AA EC underscores endothelial participation in MMP-2 activity related to vascular diseases as in vivo data implies [15–17,29]. These data are consistent with Kalinowski et al. [1] demonstrating that primary HUVEC from AA exhibit an EnDy profile associated with vascular dysfunction.
4.1. EnDy, Vascular Dysfunction, and Regulation of MMP-2
The association of abnormal MMP-2 activity has been more often studied in the context of vascular complications associated with cardiovascular disease, in vivo [30], and has been positively correlated with increased aortic intimal thickness without the presence of inflammatory cells [31]. Functionally, reduced MMP-2 secreted activity from EC may perhaps limit vascular maintenance and promote vascular dysfunction, as MMP-2 is important for ECM turnover, vascular remodeling, angiogenesis, and participates in vascular inflammatory responses [32]. Of note, aging has been associated with increased MMP-2 activity in the human aorta [33] and we observed a significant inverse relationship between MMP-2 and aortic systolic BP. In this study, the CA group is significantly older but there was no difference in MMP-2 (Table 1).
In vitro, increased gene expression of cell adhesion molecules and IL-1β is a reflection of the heightened inflammatory state of AA EC in the basal and stimulated (TNF-α) conditions. There is evidence for the role of IL-1β in EnDy, hypertension, and related cardiovascular diseases [34,35] as inflammation can precede the progression of hypertension and promotes MMP expression and activation in vascular tissue [36]. Further, there may be intracellular interactions between pro-IL-1β and MMP-2 proteins [19] that precede exaggerated IL-1β activity. Clinically, anti-hypertensive agents have proven to be effective, in part, by decreasing inflammation as evidenced by significant systemic reductions of IL-1β in essential hypertensive patients [37]. We chose not to stimulate or co-stimulate HUVEC with IL-1β due to its capacity to directly interact with MMP-2, as noted above.
In the context of EnDy, it is equally important to outline the basal racial differences and identify responses to additional relevant inflammatory mediators. Our choice of TNF-α as an inflammatory stimulus was based on previous reports from our group showing differential and dysfunctional responses to TNF-α between AA and CA HUVEC [12]. In vivo, TNF-α was reported to be greater in individuals with resistant hypertension and correlated with greater pulse wave velocity (vascular dysfunction) in those patients [38]. Treating HUVEC with serum from patients with resistant hypertension while inhibiting TNF-α significantly reduced endothelial dysfunction and indirectly triggered pro-MMP-2 [39].
Inflammation and ROS have been shown to have a substantial impact on endothelial function [1], vascular health [2], and MMP-2 function regarding vascular disease [40,41]. ROS, which is nearly ubiquitous in AA EnDy, has been demonstrated to activate MMP-2 [42,43] and concomitantly inactivate TIMPs (an inhibitor of MMP-2 activation) [44] indicating a potentially damaging cascade that disrupts EC homeostasis and promotes vascular dysfunction. We did not directly measure ROS in this particular in vitro study, but we have previously reported higher ROS production in AA HUVEC that is characterized by significantly greater NADPH oxidase subunit expression in these cells [9]. Yoo et al. [45] has also shown that NADPH oxidase subunit Nox4-derived ROS modulates MMP-2 in oral fibroblast cells. Although these current data do not distinguish a ratio of normal to ROS-mediated MMP-2 activity, future studies are necessary to investigate this occurrence.
4.2. Regulation of MMP-2 Activity in EC
Activation of pro-MMP-2 requires TIMP-2 and MMP-14 [46]. Recently, MMP-2 and its inhibitors (e.g., TIMPs) have been significantly associated with vascular complications in left ventricular dysfunction and heart failure [47,48]. AA EC expressed significantly greater basal MMP-2, MMP-14, and TIMP-2 gene expression (Figure 3) with elevated MMP-2 protein expression (Figure 4). Although gene expression (which denotes the activation state of any cell) may not always coincide with translated protein levels, it is reasonable to expect greater MMP-14 and TIMP-2 gene expression related to the greater MMP-2 gene and consequent protein expression observed. In Figure 3, TNF-α stimulation resolved the racial difference of MMP-2 (reduction in both groups), MMP-14 (increase in CA) and TIMP 2 (slight increase in both groups). We speculate that the increase response in gene expression of MMP-14 to TNF-α is likely an inflammatory driven mechanism to control overactivity of MMP-2. Interestingly, MMP-2 protein expression was resistant to change in both groups following TNF-α stimulation but remained elevated in AA EC. The importance of MMP-2 on vascular homeostasis during inflammation appears to be an EC preserved response and the heightened basal inflammation observed in the AA EC may be culpable for racial differences reported in the gene expression.
Secreted MMP-2 relative activity [activated (64 kDa)/pro-MMP-2 (72 kDa)] was significantly lower in AA EC. This result suggests that there is either inefficient regulation of MMP-2 activation by the trimolecular activation complex that is MMP-2/TIMP-2/MMP-14 [46] or that post-translational modification is eliciting stricter control of MMP-2 protein in AA EC. We speculate this may be another compensatory mechanism to control overactivation of MMP-2 considering greater basal inflammation. With these data, it is evident that future studies are necessary to determine the extent of MMP-2 activity coupled with TIMP-2/MMP-14 activities to determine the mechanism(s) of abnormal regulation of MMP-2 in AA EC and should include multiple EC from different vascular depots of racially diverse groups (e.g., HUAEC). Under basal conditions, blunted MMP-2 secretion in AA EC implies there could be reduced ECM maintenance and reduction in ECM sequestered growth factors [49] that are important for vascular health. Conversely, TNF-α significantly increased relative MMP-2 activity in AA but not in CA EC. This implies that the exaggerated response evident in AA EC may exacerbate activity which could accelerate vascular damage during inflammation and suggests greater sensitivity to inflammatory stimuli (TNF-α).
4.3. Limitations
Although significant, our in vivo data can only provide associations between MMP-2 and vascular measures with no determination on cause or overall effect. These data did prompt us to examine characteristics in naïve EC isolated from racially different groups. In vitro studies are essential to understanding the pathophysiological consequences of dysfunction at the cellular and mechanistic levels. However, in vitro studies have their limitations as noted by Ghallab [50]. We recognize that these limitations include the inability to capture interactions between multiple cell types that are present in vascular tissues (which may mediate EC responses) and titrating direct stimulation of ECs with inflammatory stimuli that would vary in vivo. In attempt to understand the etiology of racial differences in EnDy, this background work is necessary in understanding how to make investigating racial differences at the cellular level more physiological. The novelty of this racial comparison study is outlining the consistent differences between cells that may shape a vascular phenotype that may predispose one of a racial/ethnic background to vascular dysfunction at an earlier age. In our recent publication, we acknowledge that societal determinants and maternal lifestyle are often overlooked and may affect cellular programming that impact EC pathophenotype and CVD risk [51]. Further, future studies must consider thorough characterization of EC donors and utilize epigenetic profiling (in vitro) and metabolomic approaches (in vivo) to aid in making any associations concrete.
Importantly, it has been shown that basal and inflammatory responses are comparable between cultured primary isolated HUVEC, Human Umbilical Arterial Endothelial Cells (HUAEC), and primary Human Coronary Artery Endothelial Cells (HCAEC) [52]. Unfortunately, primary HUAEC and HCAEC isolated from specific racial/ethnic groups are not currently available. Future studies must also include ECs from various vascular depots where cells have been least likely influenced by disease states, which is a fitting rationale for the use of isolated umbilical cord ECs.
5. CONCLUSION
The inverse relationships between MMP-2 and vascular measures prompted investigation into racial differences at the cellular level. The heightened activation of primary EC from AA and expression of MMP-2 was significantly greater in AA EC but did not correspond with secreted activity. Further, MMP-2 secreted activity in AA EC was lower under basal conditions (mirroring in vivo results from our previous study in young AA men) while exhibited an exacerbated response to TNF-α, compared to CA EC. These data add new insight into basal EC dysregulation of a protein related to overall vascular health. Further, these data underscore the differential activities of MMP-2 in AA EC that necessitate additional in vitro and in vivo studies to determine epigenetic and post-translational modification of MMP-2 to clearly define its role within the context of racial differences in EnDy (e.g., greater inflammation and oxidative stress), vascular maintenance, and BP regulation.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
MDC and MB contributed in conception and design. MDC, CL, HG and KH contributed in experimental execution (in vivo data). MDC, CL, AA, MA, MB and BF contributed in analysis and interpretation of data (in vivo data). MDC contributed in writing. All authors contributed in review and revision.
ABBREVIATIONS
- AA,
African American;
- CA,
Caucasian;
- EC,
endothelial cell;
- EnDy,
endothelial dysfunction;
- eNOS,
endothelial nitric oxide synthase;
- ET-1,
endothelin-1;
- ECM,
extracellular matrix;
- HUVEC,
human umbilical vein endothelial cells;
- ICAM-1,
intracellular adhesion molecule-1;
- IL-1β,
interleukin-1β;
- MMP-2,
matrix metalloproteinase 2;
- MMP-14,
matrix metalloproteinase 14;
- NADPH,
nicotinamide adenine dinucleotide phosphate;
- NOx,
nitric oxide;
- ROS,
reactive oxygen species;
- TIMP,
tissue inhibitor of metalloproteinase;
- TNF-α,
tumor necrosis factor-alpha;
- VCAM-1,
vascular cell adhesion molecule.
FUNDING
This work was partially funded by the
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Marc D. Cook AU - Chenyi Ling AU - Heather Grimm AU - Adelola Adeyemo AU - Maitha Aldokhayyil AU - Kevin Heffernan AU - Bo Fernhall AU - Michael Brown PY - 2020 DA - 2020/11/18 TI - Primary African American Endothelial Cells Exhibit Endothelial Dysfunction with an Exacerbated Inflammatory Profile and Blunted MMP-2 Activity JO - Artery Research SP - 38 EP - 46 VL - 27 IS - 1 SN - 1876-4401 UR - https://doi.org/10.2991/artres.k.201102.005 DO - 10.2991/artres.k.201102.005 ID - Cook2020 ER -
