Pharmacological Management of Hypertension Guided by Central or Peripheral Blood Pressure Measurement: Comparison of Two Strategies on the Incidence of Intermediate Outcome
- DOI
- 10.2991/artres.k.200104.001How to use a DOI?
- Keywords
- Central blood pressure; hypertension; risk factors; evidence-based practice
- Abstract
Background: Central blood pressure values and arterial stiffness have demonstrated to be a useful tool to stratify cardiovascular risk and also as a biomarker. A question that is still unanswered is if hypertension treatment guided by central blood pressure parameters will be even better to promote cardiovascular protection than peripheral one.
Methods: With this proposition we have designed an open prospective multicentric randomized protocol to compare central (G1) and peripheral (G2) blood pressure targets during 1 year follow-up concerning target organ damage (carotid intima media thickness, left ventricular hypertrophy, microalbuminuria and pulse wave velocity). OMRON 1100 will be used to access peripheral and Mobil O’Graph to access central blood pressure and pulse wave velocity, TOSHIBA Xsario with longitudinal linear transdutor 7.5 MHz bidimensional mode B to access carotid and left ventricular parameters.
Expected Results: This paper aims to describe methodological aspects concerning this research and we expect to find results to answer some open questions about hypertension treatment and cardiovascular protection.
- Copyright
- © 2020 Association for Research into Arterial Structure and Physiology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Hypertension [1] is one crucial modifiable cause of cardiovascular morbidity and mortality in the adult world population, and is an independent Risk Factor (RF) for Cardiovascular Disease (CVD) [2]. CVDs constitute the leading cause of death in both developed and developing countries, with hypertension and atherosclerosis as their main risk factors [3]. Methods that allow early identification of cardiovascular structural and functional modifications may improve the treatment and control strategy [4].
Consequently, it is necessary to identify patients who are exposed to the highest cardiovascular risk early in order to implement lifestyle changes and treatments that can prevent complications and progression of CVD [2]. Besides, an accurate estimation of vascular damage and aging in individuals with hypertension, risk prediction can be better identified [4]. Major changes in the cardiovascular system are known to withstand oxidative stress, free radical production, neuroendocrine changes and genetic predisposition [5]. The sum of these factors will act mainly on the myocytes and as a consequence will contribute to the increase of ventricular and vascular stiffness, consequently related to the cardiovascular aging process [5].
Arterial stiffness increases the risk of cardiovascular morbidity and mortality, and an issue is the possibility of treatment with pharmacological intervention [6]. Therefore, treatment strategies should be well established, as the choice of antihypertensive drug is recommended considering its effect on lowering blood pressure and also reducing arterial stiffness [7]. The alterations that a drug produces in arterial stiffness may be independent of pressure, directly affecting the arterial wall through elastic and collagen fiber remodeling or pressure-dependent remodeling, occurring indirectly by reducing blood pressure [8].
Pharmacological therapy aims to manage Blood Pressure (BP) control mechanisms in order to reduce BP and the risks associated with its elevation, promoting reduction of cardiovascular outcomes, without negatively affecting the quality of life [9]. Antihypertensive pharmacological treatment should be individualized and the initial choice of drug used for patients with stage 1 hypertension and low to moderate cardiovascular risk is monotherapy [2]. Monotherapy contemplate the ability to reduce cardiovascular morbidity and mortality, drug safety profile, predominant pathophysiological mechanism in the patient being treated, and associated diseases [10]. On the other hand, studies show that about two-third of the cases, monotherapy was not sufficient to achieve pressure reductions; requiring antihypertensive associations [10].
It is also significant that the residual risk in hypertensive patients, even when properly treated and controlled, may be related to the delay in diagnosis and initiation of treatment, but may also be associated with the limitations of peripheral BP measurement as a determinant method of drug strategy [10]. Few studies directly compare the effect of antihypertensive drugs on brachial and central pressure. It is plausible that an inadequate reduction in central pressure may be associated with an adverse outcome. So, a central rather than brachial pressure treatment decision is likely to have important implications for hypertension prognosis and control [11,12].
Thus, this study aims to test the hypothesis that central BP will be more efficient in defining the dosage adjustment of antihypertensive drugs compared with the current methodology by measuring peripheral BP in correlation to prevention or even regression of target organ damage (intermediate outcome).
2. MAIN ISSUE
All hypertension treatments are guided by peripheral blood pressure measurements. After identifying these peripherals parameters, the diagnosis is done, then the establishment stratification risk and institution of treatment goals. Based on the current knowledge of the significance as for central BP measurements in the prognosis of cardiovascular disease, is the treatment of arterial hypertension guided by the central measurement parameters more efficient compared with the treatment guided by the peripheral measurement parameters?
3. OBJECTIVES
3.1. Main Objective
To compare the incidence of target-organ damage between patients who had the therapeutic strategy guided by central BP values (G1) and patients by peripheral BP values (G2).
3.2. Secondary Objectives
- •
To assess the left ventricular mass index performance during the treatment of hypertension in both groups.
- •
To assess the response of intima media thickness during the treatment of hypertension in both groups.
- •
To assess the response of microalbuminuria and glomerular filtration rate during the treatment of hypertension in both groups.
- •
To assess Pulse Wave Velocity (PWV) response during treatment of hypertension in both groups.
- •
Compare treatment adherence in both groups.
- •
Assess the incidence of adverse events in both groups.
3.3. Methods
3.3.1. Study design and population
This will be a prospective, open, randomized, multicenter study. Outpatients with arterial hypertension will be selected and attended at the Hypertension League (LHA/UFG) and Campus Rio Verde, Federal University of Goiás, Brazil.
Both units attend approximately 26 patients per day with an average of 130 patients per week. Patients with hypertension will be selected and invited to participate in the study, with or without the use of antihypertensive drugs, who meet the inclusion and exclusion criteria [2].
3.3.2. Inclusion criteria
- •
Patients older than 18 years.
- •
Hypertension with indication for pharmacological treatment, assessed through casual blood pressure measure.
3.3.3. Exclusion criteria
- •
Participation in other research protocols for less than one year as per ANVISA – Brazil regulations.
- •
Chronic diseases in terminal stages.
- •
Prior cardiovascular disease including coronary artery disease (AMI, angina, previous revascularization surgery or angioplasty) or stroke (ischemic stroke or TIA) for <6 months. Exclusion criteria for prior CVD will be defined with information obtained from patients by direct interview or evidence through complementary exams.
3.3.4. Criteria for interruption of the study
Individuals who wish to discontinue the participation in the study or who during follow-up present one or more exclusion criteria.
3.3.5. Recruitment
Sample calculation of minimal 84 patients selected from consultations scheduled in the institutions will be randomized to two groups: central (G1) and peripheral BP measurement guided drug treatment group (G2).
3.3.6. Randomization procedures
Randomization will be done at www.randomizer.org proportion 1:1.
3.3.7. Study visits
There will be a total of five visits: selection, randomization visit, and medication supply (V1); three follow-up visits (V2–V4) in the 3rd, 6th and 9th months respectively and one final visit (V5) in the 12th month (Figure 1).
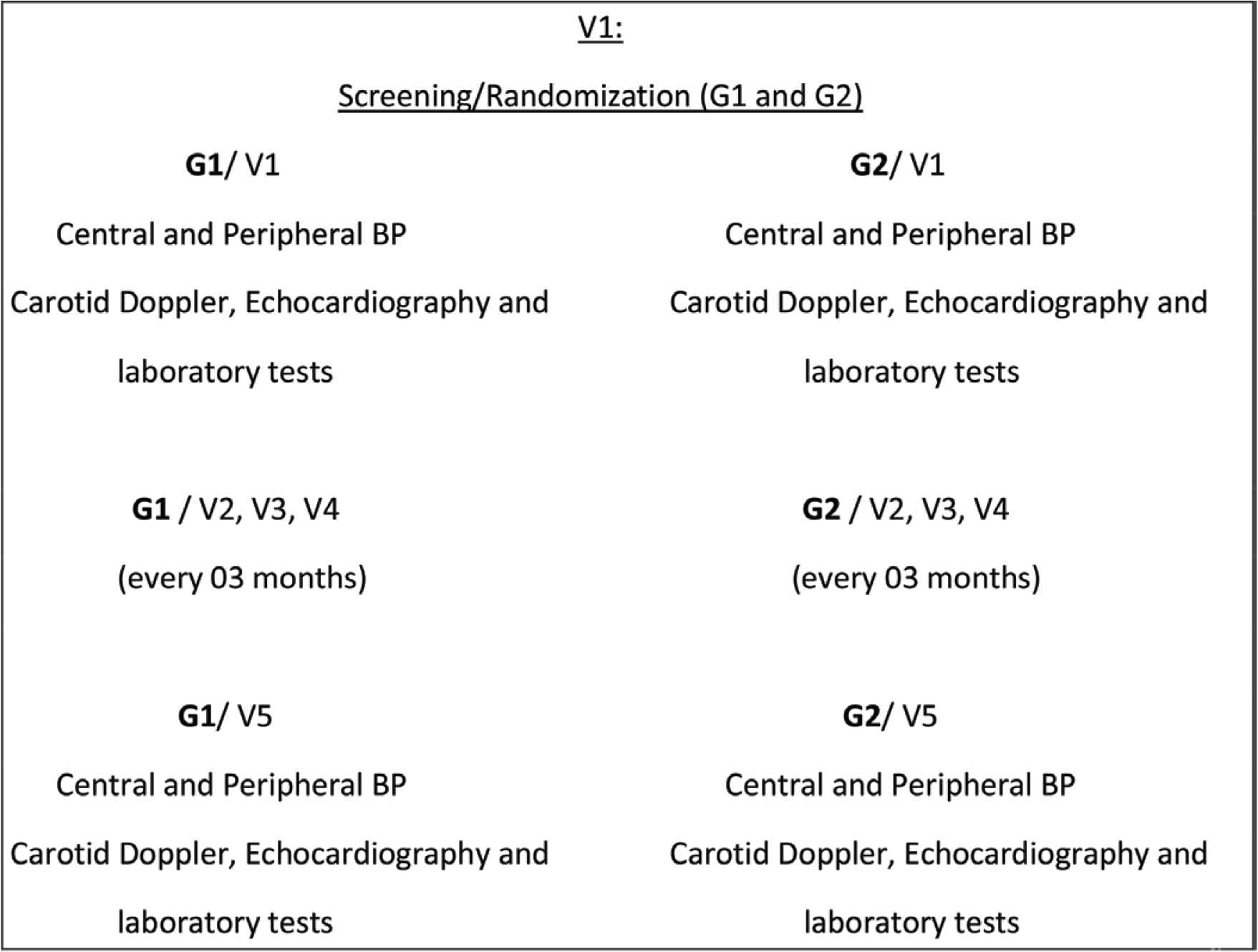
Study flowchart
At visit 1 (day 0) and 5 (12 months ± 1 week) the following procedures will be performed: blood pressure measurement (central and peripheral); carotid Doppler, Doppler echocardiography and laboratory tests.
In visits 2–4, there will be a measurement of central and peripheral BP measurement and dose adjustment according to randomization.
3.3.8. Study procedures
Casual peripheral BP measurement will be performed by automatic OMRON 1100 (HEM 1100, Omron Healthcare, Kyoto, Japan) according with 2018 Brazilian Guidelines of Hypertension procedures [2]. Average of 02 BP measurement, 2 min interval will be considered for the study.
Central BP measurement will be assessed non-invasively using a blood pressure cuff that measures peripheral blood pressure from which and through the algorithm. ARCSolver estimates the central arterial blood pressure values oscillometric Mobil-O-Graph® (IEM, Stolber, Germany). Procedures will be performed by the same person and device, using C2 PWV validated protocol [13,14].
The evaluation of carotid Doppler and Echocardiogram will follow the American [15] and European [16,17] consensus guidelines. Both of them will be assessed by two-dimensional transthoracic echocardiography using a TOSHIBA Xsario and longitudinal linear transdutor 7,5 MHz, bidimensional mode B.
Fasting laboratory tests: blood count, glycated hemoglobin, insulin, lipid profile, creatinine, potassium and microalbuminuria.
3.3.9. Pharmacological treatment
Both G1 and G2 will receive same non and pharmacological treatment. G1 BP target will be defined as reference values at Table 1 [18]. G2 BP target will follow 2018 Brazilian Hypertension Guidelines (<140 × 90 and 130 × 80 mmHg in patients with low/intermediate and high risk respectively) [2].
| Age (years) | Women (mmHg) | Men (mmHg) |
|---|---|---|
| <20 | 99 | 109 |
| 20–29 | 101 | 110 |
| 30–39 | 111 | 114 |
| 40–49 | 116 | 118 |
| 50–59 | 120 | 123 |
| 60–69 | 128 | 128 |
| 70+ | 138 | 135 |
Systolic and diastolic central blood pressure reference values (mmHg) according with age and gender [18]
Pharmacological treatment will follow whenever possible steps proposed in Table 2.
| Level | Medication |
|---|---|
| Level 1 | Losartan 50 mg/day |
| Level 2 | Losartan 50 mg 12/12 h |
| Level 3 | Losartan 50 mg 12/12 h + Amlodipine 5 mg/day |
| Level 4 | Losartan 50 mg 12/12 h + Amlodipine 10 mg/day |
| Level 5 | Losartan 50 mg 12/12 h + Amlodipine 10 mg/day + HCTZ 12.5 mg/day |
| Level 7 | Losartan 50 mg 12/12 h + Amlodipine 10 mg/day + HCTZ 25 mg/day + Espironolactone 25 mg/day |
HCTZ: hydrochlorothiazide.
Pharmacological treatment strategy to reach blood pressure target
3.3.10. Target organ damage analyses
- •
Carotid intima media thickness and atheromatous plaques.
- •
Left ventricular mass and septal/posterior wall thickness.
- •
Microalbuminuria.
- •
PWV.
3.4. Ethical Aspects
The research will follow the standard of resolution number 466/12. It was submitted and approved by the Ethics Committee of the Hospital das Clínicas da Universidade Federal de Goiás.
4. CONCLUSION
With this study we intend to contribute with a knowledge concerning central blood pressure as a useful parameter to guide hypertension treatment and if, compared with peripheral values, it may improve cardiovascular protection.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
WKSB contributed in study conceptualization and writing (review & editing) the manuscript. WKSB, SI, GCG, PVV and ALLS contributed in data curation, formal analysis and writing (original draft) the manuscript. WKSB, SI and GCG contributed in funding acquisition. WKSB, SI, GCG, RPP, VAM, LFO, ES, GRM and DSCV contributed in project administration. PCBVJ and AC contributed in supervising the project.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Weimar Kunz Sebba Barroso AU - Sayuri Inuzuka AU - Gilberto Campos Guimarães AU - Robson Pierre Pacífico AU - Victoria Alves Melo AU - Luiz Fernando Oliveira AU - Eduarda Silva AU - Gustavo Ribeiro Mesquita AU - Deborah Silva Cintra Valle AU - Priscila Valverde Vitorino AU - Ana Luiza Lima Sousa AU - Paulo César Brandão Veiga Jardim AU - Antonio Coca PY - 2020 DA - 2020/01/23 TI - Pharmacological Management of Hypertension Guided by Central or Peripheral Blood Pressure Measurement: Comparison of Two Strategies on the Incidence of Intermediate Outcome JO - Artery Research SP - 1 EP - 4 VL - 26 IS - 1 SN - 1876-4401 UR - https://doi.org/10.2991/artres.k.200104.001 DO - 10.2991/artres.k.200104.001 ID - Barroso2020 ER -
