Inflammation and large arteries: Potential mechanisms for inflammation-induced arterial stiffness
- DOI
- 10.1016/j.artres.2012.03.002How to use a DOI?
- Keywords
- Aortic stiffness; Inflammation; Rheumatoid arthritis
- Abstract
Systemic inflammatory conditions are associated with an increased risk of cardiovascular disease (CVD). How exactly inflammation leads to this is not fully understood, but it has been suggested that arterial stiffening, could provide potential mechanisms to explain it. Chronic, systemic inflammatory conditions, as well as acute-models of inflammation are associated with arterial stiffening. Moreover, aortic stiffness can be reversed with successful immunomodulatory therapy. Although it seems evident that inflammation is involved in the process of aortic stiffening, the precise mechanism responsible for this remains unclear.
There are number of possible mechanisms by which inflammation could lead to arterial stiffening. (1) Inflammation is associated with endothelial dysfunction and this can regulate arterial stiffness via changes in smooth muscle tone. (2) Inflammation leads to increased synthesis of matrix metalloproteinases, which can degrade elastin, resulting in stiffening. (3) Several mediators of inflammation may directly stimulate vascular calcification, whereas endogenous inhibitors of vascular calcification are downregulated during inflammation, both of which can lead to stiffening. (4) During inflammation arterial glycosaminoglycan (GAG) synthesis is upregulated. In animal models, an overproduction of certain GAGs in the aorta results in stiffening of the arterial wall by thinning of elastic lamellae. (5) Finally, direct vascular inflammation could lead to arterial stiffening by changing the composition of extracellular matrix. This review aims to discuss potential mechanisms by which inflammation could lead to aortic stiffening.
- Copyright
- © 2012 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Cardiovascular disease is widely recognised as an inflammatory condition, where inflammation plays a pivotal role in the initiation, progression and propagation of the disease.1 Levels of the acute phase reactant C-reactive protein (CRP) predict the risk of future cardiovascular events both in subjects with known atherosclerosis,2 and in apparently healthy individuals.3 Inflammation can lead to an increase of cardiovascular risk via different mechanisms: directly, by accelerating the atherosclerosis process or destabilizing plaques,1 or indirectly, via endothelial dysfunction,4 and a premature stiffening of the large arteries.5
In recent years, great emphasis has been placed on the role of arterial stiffness in the development of CVD. There is a wealth of information about the association between the established CV risk factors and aortic stiffness6 and that by treating these risk factors either with life-style modifications or pharmacological interventions, arterial stiffness can be reversed.7 Most importantly, the results from recent longitudinal studies have demonstrated that aortic stiffness is an important independent predictor of fatal and non-fatal CV events. Aortic pulse wave velocity (aPWV), a measure of aortic stiffness, predicts CV outcome in patients with end-stage renal disease,8–10 hypertension,7,11–14 diabetes,15 in elderly subjects16 and in the general population.17–19 Recent large scale meta-analysis of 17 studies (n=15,877) concluded that aPWV is a strong independent predictor of CV events and all-cause mortality. An increase of aPWV by 1 standard deviation, corresponded to an increase of a risk-factor-adjusted risk of CVD by 42%.20
The incidence of cardiovascular disease (CVD) is increased in patients with rheumatoid arthritis (RA),21–24 and the inflammatory process of RA resembles that seen in atherosclerosis. Indeed, both diseases involve activation of macrophages, T-cells, especially CD4+CD28− and B-cells as well as increased expression of adhesion molecules and increased circulating levels of TNF-α and CRP.25 These similarities make RA a useful “model” in which to investigate the relationship between systemic inflammation and CVD.
RA26,27 and other inflammatory conditions28 as well as acute-models of inflammation29 are associated with arterial stiffening. We have also shown that aortic stiffness is greatest in patients with active RA compared to those with quiescent disease, and that CRP independently predicts aortic stiffness.26 Furthermore, immunomodulatory therapy with the anti-TNF-α therapy leads to a reduction in inflammation, improvement in endothelial function, and fall in aortic stiffness in RA.26,30 We have also shown that a modest reduction in inflammation following 6 weeks of cholesterol reduction therapy with simvastatin and ezetimibe reduces aortic stiffness and improves endothelial function.31 These findings suggest, firstly, that arterial stiffness correlates with the degree of active inflammation and secondly, that vessel stiffness can be reversed with successful immunomodulatory therapy.
However, the mechanisms behind inflammation-induced aortic stiffening remain unclear. A greater understanding of this mechanism would provide valuable information about aortic stiffness not only in patients with RA, but also in healthy individuals with modest elevations in inflammatory markers. It would also lead to a better understanding of how to treat inflammation in high risk individuals in order to minimise their CVD risk. This review aims to discuss potential mechanisms by which inflammation could lead to aortic stiffening (Fig. 1).
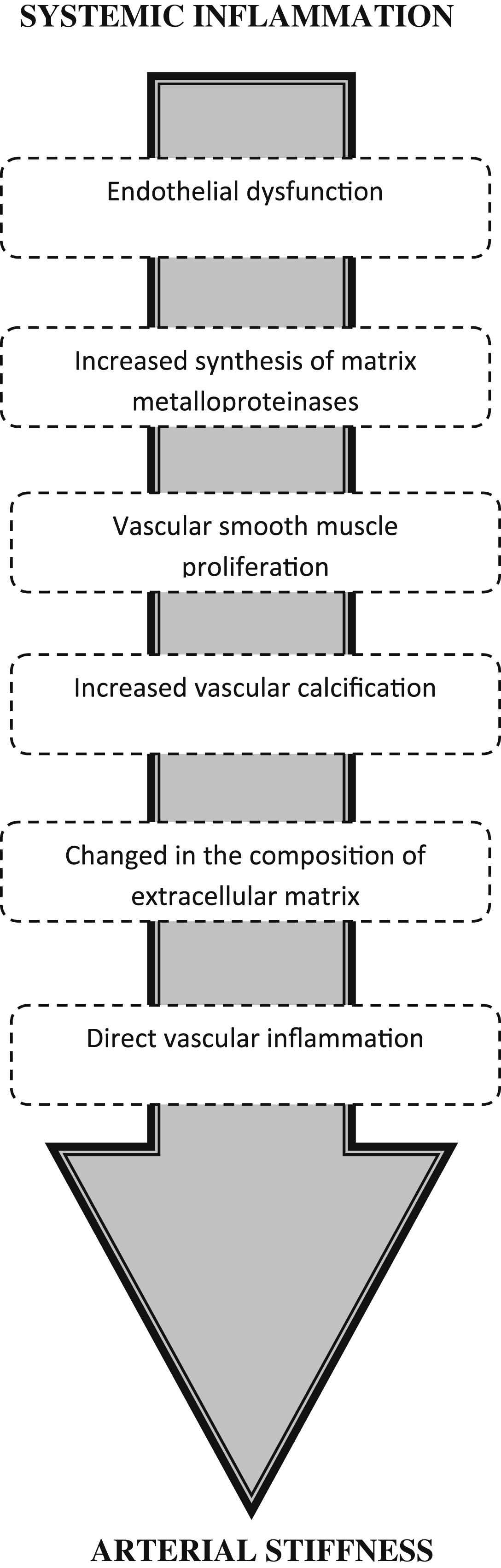
Potential mechanisms for inflammation-induced arterial stiffening. Systemic inflammation leads to numerous changes within the arterial wall, which in turn can lead to stiffening.
How does inflammation lead to arterial stiffening potential mechanisms?
Endothelial dysfunction
Association between acute and chronic inflammation and endothelial dysfunction has been demonstrated in numerous studies.4,32–34 However, the mechanisms behind this are incompletely understood. One possibility is that certain cytokines induce expression of inducible nitric oxide synthase (iNOS) leading to a production reactive oxygen species (ROS) and subsequent uncoupling of endothelial NOS (eNOS) and reduction in nitric oxide (NO) production.35 Moreover, acute phase protein CRP may also decrease eNOS expression and thus reduce NO bioavailability directly.36,37 Production of myeloperoxidase (MPO) is another potential key mediator in inflammation-induced endothelial dysfunction. MPO is released for activated neutrophils during inflammation. MPO can catalytically consume NO, thus reducing NO bioavailability. Furthermore, MPO has the unique ability to produce hypochlorous acid and subsequently lead to uncoupling of eNOS,38,39 oxidation tetrabiopterin (BH4)40,41 and again further superoxide (O2−) production. Tetrahydrobiopterin (BH4), a naturally occurring essential co-factor for endothelial nitric oxide synthase (eNOS)42 is thought to play an important role. Recent, in vitro studies, suggest that activation of inducible NOS (iNOS) may lead to endothelial dysfunction by depleting the bioavailability of BH4 from eNOS and subsequently uncouple eNOS, resulting to production of superoxide (O2−) rather than NO.35,43,44 When O2− reacts with NO in vivo, peroxynitrite is formed, leading to oxidation of BH4 and a reduction in the allosteric stability of eNOS, further uncoupling of eNOS. Furthermore, increased levels of adhesion molecules may damage the endothelial cells and lead to altered endothelial function,45 activation of neutrophils by anti-neutrophil cytoplasm antibodies within the vascular lumen may contribute to endothelial cell injury46 or oxidation of LDL promoted by inflammation may lead to direct endothelial cell toxicity47 and disturbed eNOS function.
Endothelial function can regulate arterial stiffness via changes in smooth muscle tone and indeed, both basal and stimulated NO production can regulate muscular artery distensibility. Indeed, there is an inverse correlation between endothelial function, as measured by flow mediated dilatation response, and aortic PWV, which remained significant after correcting for potential confounding factors, including age and MAP.48 Furthermore, local arterial distensibility is reduced by blockade of endogenous NO synthesis with the NO synthase inhibitor L-NMMA in the ovine common iliac artery49 and also in human iliac artery.50 However, the role of nitric oxide in regulating stiffness of more elastic aorta, remains controversial, and may not be as important as regulation of muscular artery stiffness.
Increased synthesis of matrix metalloproteinases
Another mechanism, which could be responsible arterial stiffening during inflammation, is an accelerated elastin breakdown by matrix metalloproteinases (MMP). MMP synthesis is induced by CRP51 and the release of MMPs from the leukocytes can degrade elastin within the media.52 Yasmin et al. demonstrated in 677 subjects, that MMP-9 levels are independently associated with aortic stiffness.52 Results from a smaller study (n=213) contradict these findings by reporting that there is a negative association between MMP-2 and MMP-9 and aortic stiffness.53 Nevertheless, Yasmin et al. demonstrated for that aortic stiffness and elastase activity are influenced by MMP-9 gene polymorphisms, suggesting that the genetic variation in this protein may have a causal role in the process of large artery stiffening.54 Although, elastin degradation may play important role in arterial stiffness over long periods of time, it is unlike to explain the more acute changes seen and also to explain how anti-inflammatory therapies are able to reduce stiffness. After all very little, if any, elastin is synthesised beyond the first year of life.55
Calcification
Calcification is another potential mechanism behind inflammation-induced arterial stiffness. Several mediators of inflammation such as oxidation, carbonyl stress, C-reactive protein, and cytokines may directly stimulate vascular calcification.56 This can lead to a phenotypic transformation of vascular smooth muscle cells, which increases bioapatite formation and therefore calcification57 as well as a transformation of vascular smooth muscle cells to osteoblast-like cells. Also, fetuin-A, an endogenous inhibitor of vascular calcification, is downregulated during inflammation and recently it has been demonstrated that fetuin-A is an independent risk factor for progressive arterial stiffness58 in patients with chronic kidney disease. A study in children on dialysis, demonstrated that fetuin-A, and another physiological inhibitor of calcification osteoprotegerin are associated with increased aortic stiffness and calcification.59
Smooth muscle proliferation
Inflammatory response initiates an accumulation of leukocytes into the vascular endothelium. This can lead to a complex cascade of vascular smooth muscle cell (VSMC) activation, migration and proliferation. Activated VSMC can synthesise and secrete biologically active mediators such as endothelin, angiotensin II, cytokines, proteases, collagen and proteoglycans that regulate contraction, relaxation, inflammation, proliferation, apoptosis and matrix alterations60 and can therefore subsequently lead to arterial stiffening.
Changes in the composition of extracellular matrix
Normal blood vessel walls are composed of endothelial cells, smooth muscles cells and ECM. Healthy ECM is a complex collection of fibrous proteins and glycoproteins, which are embedded in a hydrated ground substance of proteoglycans (PG),proteins with glycosaminoglycan (GAG) chain attached to them.61 PGs have numerous specific roles within the vascular extracellular matrix, such as hydration, filtration, and regulation of various cellular activities as well as inflammatory processes.62 During atherosclerosis and inflammation arterial GAGs within the intima accumulate, where inflammatory cytokines, TNF-a and TGF-B have capacity to alter GAG in both quantitatively and qualitatively manner.63 In intermediate and advanced lesions, GAGs decorin, versican, biglycan and hyaluronan (HA) are upregulated and alterations in HA metabolism, distribution, function has been documented in many diseases, e.g. RA & other inflammatory conditions and vascular disease.64 Tissue enriched with hyaluronan tends to trap water and swell, forming a viscous hydrate gel which allows ECM to resist compression forces,64 i.e. making the wall stiffer. Also, overproduction of HA in the aorta results in stiffening of the arterial wall by thinning of elastic lamellae in animal models.65 In vitro findings indicate that upregulation of hydrating GAGs lead to an increased water content of the vessel wall. This has been confirmed in a recent in vivo experiment using MRI with a gadolinium-based contrast, which indicated that in patients with CVD, inflammation causes increase in water content in the arterial wall, which correlated with histological markers of inflammation.66,67 Although changes in the amount of hydrating GAGs in ECM can alter stiffness in vitro, to date there are no published in vivo data available in humans.
Direct vascular inflammation
One very plausible candidate mechanism for aortic stiffening seen in RA is direct aortic inflammation. Large vessel vasculitis is associated with an increase in aortic stiffness.68–71 This is often reversible, although in Kawasaki disease, it appears to be party irreversible,70 possibly because this disease directly involves large vessels. Clinically manifest vasculitis in RA is thought to be rare, being present in only 1–3% of RA patients.72,73 However, the lack of large scale studies addressing this issue makes it very difficult to know the true prevalence of vasculitis in RA. An autopsy series in 188 RA patients indentified 10 patients with aortitis, of which 7 died as a result, of note was that, a diagnosis of vasculitis was not made before death in these patients.74 The possibility that many RA patients have a sub-clinical vasculitis is high. Indeed, a study in patients undergoing bypass graft surgery that those patients with inflammatory rheumatic disease (IRD) (n=65) had a greater occurrence of mononuclear cell infiltrates within their aortic media or adventitia than those patients without IRD (n=51); odds ratio (OR=3.6, 95% CI: 1.6–8.5; P=0.002),75 indicating vasculitis. Although, to date, there are no studies demonstrating the link between aortic inflammation and stiffness, theoretically, inflammatory cell infiltration within the aortic wall would lead to stiffening by accelerating all of the mechanism discussed in this review.
Conclusion
A strong body of evidence demonstrates that inflammation plays an important role in arterial stiffening. There are number of potential mechanisms by which inflammation could lead to arterial stiffening such as endothelial dysfunction and subsequent change in smooth muscle tone, smooth muscle proliferation and activation, changes in composition of extracellular matrix and direct vascular inflammation. However, studies addressing these possible mechanisms need to be done to ascertain the role of these processes in inflammation-induced arterial stiffening.
References
Cite this article
TY - JOUR AU - Kaisa M. Mäki-Petäjä AU - Ian B. Wilkinson PY - 2012 DA - 2012/04/15 TI - Inflammation and large arteries: Potential mechanisms for inflammation-induced arterial stiffness JO - Artery Research SP - 59 EP - 64 VL - 6 IS - 2 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2012.03.002 DO - 10.1016/j.artres.2012.03.002 ID - Mäki-Petäjä2012 ER -
