Histopathology and histomorphometry of umbilical cord blood vessels. Findings in normal and high risk pregnancies☆
Parts of this study were presented at the World Congress of Cardiology. 18–21 May 2008, Buenos Aires, Argentina. Circulation May 13, 2008;117(19):22 [abstr 0104].
- DOI
- 10.1016/j.artres.2011.02.001How to use a DOI?
- Keywords
- Umbilical cord; Preeclampsia; Hypertension; Diabetes; Histomorphometry; PAI-1; TGF-β1
- Abstract
Objective: A systematic histological, morphometric and immunohistochemical (PAI-1 and TGF-β1) study of umbilical vessels in normal and pathological conditions was undertaken in order to describe and compare the lesions found.
Methods: Segments of umbilical cords were obtained from 92 pregnancies/107 newborns from normal gestations (n = 20) or from gestational diabetes mellitus (n = 13), chronic hypertension (n = 14), preeclampsia (n = 9), intrahepatic cholestasis (n = 13), antiphospholipid syndrome (n = 11), fetal growth restriction (n = 9), oligohydramnios (n = 6), premature rupture of membranes (n = 12), antiphospholipid antibodies (n = 11) and fetal distress (n = 23). Thirty-four of these patients presented combined pathologies.
Results: “Pathological” umbilical cords presented perivascular/intraparietal hemorrhages with wall dissections, parietal recent thrombosis and focal moderate or extensive Wharton’s jelly hemorrhages. Pathological pregnancies presented more microscopic lesions (35/73; 48%) than normal pregnancies (4/20; 20%; p = 0.039). The wall:lumen ratio of arteries was significantly higher in all pathologies (32.6 ± 16) as compared to 3.1 ± 0.6 in the control group (p < 0.0001), also due to the significantly higher values belonging to outer plus inner layer areas in opposition to much less increases in luminal areas (p = 0.03). Concerning veins, wall:lumen ratio was also higher in the pathological groups (p = 0.0086) due to a 2-fold increase in wall areas.
Conclusion: Quantitative histomorphometry of the pathological alterations and pathophysiologic disorders of the umbilical cord has the potential to enhance investigation and treatment of maternal and fetal diseases.
- Copyright
- © 2011 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
The umbilical cord is the cable linking the fetus to the placenta. It usually has two arteries and a vein, suspended in a hydrated extracellular matrix known as Wharton’s jelly. Originally two veins are present, but the second one normally atrophies during the pregnancy.1 The arteries return poorly oxygenated blood to the placenta while the vein carries oxygenated blood from this tissue to the fetus.1–3
Human umbilical vessels differ from the major vessels of the same caliber in the body for many reasons. Transudation of fluid occurs in these vessels and contributes to the formation of the amniotic fluid.2
In spite of complete and accurate descriptions of the histology of umbilical cord vessels,1–3 the histopathology and histomorphometry of these structures have been insufficiently studied, despite the wide broad spectrum of pathologies affecting the mother and the fetus.3
It has been suggested that transforming growth factor-β1 (TGF-β1) plasma levels in the human fetal circulation could potentially clarify the role of this growth factor in the fetal endothelial cell function in health and disease.4 Plasminogen activator inhibitor-1 (PAI-1), an inhibitor of tPA and uPA/urokinase and a biochemical marker of impaired fibrinolysis, is strongly expressed in endothelial cells (where it is mainly produced) and in the clefts of Wharton’s jelly.5 Pandolfi et al.6 have pointed out in diabetic patients the presence of high amounts of PAI-1, at the site at which thrombosis occurs, in particular, in endothelial and smooth muscle cells. Additionally, Sartori et al.7 concluded that PAI-1 tests and inhibin-A monitoring may help in the early diagnosis of pregnancy-related hypertensive disorders.
The objective of this paper was to describe and compare the microscopic lesions observed in non-pathological conditions and in an extensive number of different clinical diseases and intrinsic fetal sickness affecting pregnancy, namely: gestational diabetes,8 chronic hypertension,9 preeclampsia,9 intrahepatic cholestasis,10 antiphospholipid syndrome,11 fetal growth restriction,12 oligohydramnios13 and fetal distress.14
To resolve this, a systematic histological, morphometric and immunohistochemical study of umbilical arteries and veins in normal and pathological conditions was undertaken. TGF-β1 and PAI-1 were selected as the main biological markers involved in the regulation of extracellular matrix throughout the process of synthesis and degradation of collagen, respectively.15,16
Methods
Segments of umbilical cords 2 cm away from the placental were obtained from 92 pregnancies/107 newborns, gestational age varying from 29 to 41 weeks. Mothers were negative for American trypanosomiasis (Chagas’ disease) and human immunodeficiency virus (HIV). From the clinical point of view, gestations were classified as being normal, n = 20; gestational diabetes mellitus, n = 13; chronic hypertension, n = 14; preeclampsia, n = 9; intrahepatic cholestasis of pregnancy, n = 13; antiphospholipid syndrome, n = 11; fetal growth restriction, n = 9; oligohydramnios, n = 6; premature rupture of membranes, n = 12; antiphospholipid antibodies (lupus anticoagulant or anticardiolipin antibodies), n = 11 and fetal distress, n = 23. Thirty-four of these patients presented two or more (combined) pathologies.
This study was approved by the Ethics Committee for Scientific Research of the ININCA (UBA-CONICET).
Clinical definitions
Gestational diabetes
Diabetes with initial onset or recognition or glucose intolerance that is first detected during pregnancy.8
Chronic arterial hypertension
Blood pressure higher than140 mm Hg systolic, and or 90 mm Hg diastolic, known or detected prior to conception diagnosed before the 20th gestational week, or diagnosed during pregnancy that failed to resolve by 12 weeks postpartum.9
Preeclampsia
An increase in blood pressure higher than 140/90 mm Hg after the 20th week of gestation, combined with proteinuria (protein excretion, higher than 0.3 g per 24 h).9
Intrahepatic cholestasis
Pruritus associated with elevated levels of serum bile acids and/or aminotransferases, and the absence of diseases that may produce similar symptoms.10
Antiphospholipidic syndrome
(a) One or more unexplained deaths of a morphologically normal fetus at or beyond the 10th week of gestation, with normal fetal morphology documented by ultrasound or by direct examination of the fetus, or (b) one or more premature births of a morphologically normal neonate at or before the 34th week of gestation because of severe preeclampsia or eclampsia, or severe placental insufficiency, or (c) three or more unexplained consecutive spontaneous abortions before the 10th week of gestation, with maternal anatomic or hormonal abnormalities and paternal and maternal chromosomal causes excluded, plus 1. Anticardiolipin antibody of IgG and/or IgM isotype in blood, present in medium or high titer, on 2 or more occasions, at least 6 weeks apart, and or 2. Lupus anticoagulant present in plasma, on 2 or more occasions at least 6 weeks apart, detected according to the guidelines of the International Society on Thrombosis and Hemostasis.11
Fetal growth restriction
A birth weight below the 10th percentile for gestational age.8 Low birth weight neonates are subgrouped according to the degree of smallness at the first weight determination after birth: low, less than 2500 g; very low, less than 1500 g and extremely low birth weight less than 1000 g.12
Oligohydramnios refers to amniotic fluid volume that is less than expected for gestational age.13
Morphologic study
Transversal sections of the umbilical cords 5 mm in thickness were fixed in buffered formaldehyde, pH 7.0, embedded in paraffin, serially sectioned at 4–6 μm and stained with hematoxylin and eosin, Masson Trichome, acetic orceine for elastic fibers, PAS, Alcian Blue pH 2.5 and Mallory phosphotungstic acid hematoxylin.
Microscopic whole sections of the arteries and the vein were digitalized and used for histomorphometry. In this way it measurements (in pixels) were obtained in veins of (a) the surface area of the light and (b) the surface area of the muscular layer, and in arteries of (a) the surface area of the light, (b) the surface area of the internal muscular layer and (c) the surface area of the external muscular layer. Measurements were obtained using a microscope WPI Professional H602 (World Precision Instruments Inc.) and the software Image J 1.37 v Wayne Rasband, National Institutes of Health, USA (Java 1.5.0_09 Sun Microsystems). All the parameters were normalized for bodyweight of the newborn.
Immunocytochemical staining
All tissue sections were incubated with primary antibodies for anti human PAI-1 (mouse monoclonal antibody to PAI-1-(ab82218) Abcam Lab, Cambridge, MA, USA 1: 100) and TGF-β1 (mouse monoclonal antibody [TGFB17] to TGF-β1-(ab49574) Abcam Lab, Cambridge, MA, USA 1:40) overnight at 4 °C. After several washes in PBS, the sections were incubated with the proper secondary antibody (biotinylated anti-mouse IgG, Vector Laboratories, Burlingame, CA, USA, 1:500) for 1 h at room temperature and followed by incubation with Streptavidin Biotin Peroxidase System (BioGenex Inc). Development of peroxidase reactions was carried out with 3,3′-diaminobenzidine. Negative controls were run simultaneously with irrelevant antibodies of the same isotype.
Statistical analysis
Values were expressed as mean ± SD except for gestational age or gestational period which was expressed as median (interquartile range). All statistical analyses were processed through GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA). The significance of differences between group parameters was evaluated by Fisher’s exact test or Student t unpaired test as appropriate. The selected level of significance was p < 0.05 (two-tailed). The significance of differences between groups in relation to histomorphometric parameters was determined by an analysis of variance (ANOVA), followed by a post hoc Tukey’s test when the analysis of variance suggested a significant difference between groups.
Results
Maternal and fetal demographic data are shown in Table 1. Forty-five newborns were males and 62 females. Birth weight was 2928 ± 613 g for control group vs. 2232 ± 652 g of all pathological groups (p < 0.01; ANOVA), and vs. 2166 ± 624 g of subjects with combined pathologies (p < 0.001; ANOVA). Also, a significant diminution was observed in birth weight in newborns belonging to preeclampsia (p < 0.01), fetal growth restriction and premature rupture of membrane (p < 0.05) groups (Table 1).
| Birth weigth (g) | Male | Female | Gestation period (weeks) | Maternal age (years) | |
|---|---|---|---|---|---|
| Chyp | 2779 ± 750 | 5 | 10 | 36.5 (2.75) | 34 (13.75) |
| FD | 2386 ± 807 | 12 | 11 | 36 (4.25) | 31 (8.75) |
| AphS | 2685 ± 442 | 5 | 6 | 36 (1.5) | 34 (4) |
| AphAb | 2264 ± 498 | 6 | 5 | 37 (2) | 36 (7.5) |
| Ich | 2407 ± 484 | 8 | 6 | 36 (2) | 31 (11.5) |
| GDBT | 2489 ± 478 | 10 | 6 | 37 (2) | 34.5 (6.25) |
| O | 2361 ± 745 | 3 | 3 | 36.5 (2.5 | 31.5 (8) |
| Pe | 1749 ± 656** | 5 | 4 | 35 (4) | 28 (16) |
| FGR | 1969 ± 465* | 3 | 6 | 36 (3) | 28 (3) |
| PRM | 2087 ± 579* | 8 | 6 | 34 (2.25) | 27 (14.25) |
| NORMAL | 2928 ± 613 | 11 | 11 | 39 (1) | 31 (16.5) |
| COMBINED | 2166 ± 624*** | 20 | 18 | 36 (3) | 31 (8) |
p < 0.05,
p < 0.01,
p < 0.001 vs. normal.
Birth weight is expressed as mean ± SD, while gestational period and maternal age are expressed as median (interquartile range). Normal, n = 20; gestational diabetes mellitus (GDBT) n = 13; antiphospholipid syndrome (AphS) n = 11, chronic hypertension (Chyp) n = 14, preeclampsia (Pe) n = 9, intrahepatic cholestasis of pregnancy (Ich) n = 13, fetal growth restriction (FGR) n = 9, premature rupture of membranes (PRM) n = 12, antiphospholipid antibodies (lupus anticoagulant or anticardiolipin antibodies) (AphAb) n = 11, oligohydramnios (O) n = 6, fetal distress (FD) n = 23, combined n = 34.
Maternal and fetal demographic data.
A significant shortening of the gestation period (p < 0.0001) was noted in the pathological groups vs. control group: 36 ± 2.5 (median 36) vs. 39 ± 1.2 (median 39).
From the histological point of view, arterial and venous findings allowed to classify umbilical cords as: “normal” or “pathological”. As it will be demonstrated along the description, human umbilical vessels differ in many ways from the major vessels of the body.
Normal umbilical cords
“Normal” cords presented 2 arteries with a helical course around a unique vein, giving the cord a twisted appearance. No adventitia, external elastic lamina or vasa vasorum was present. Lumens were lined by endothelium (Fig. 1A).
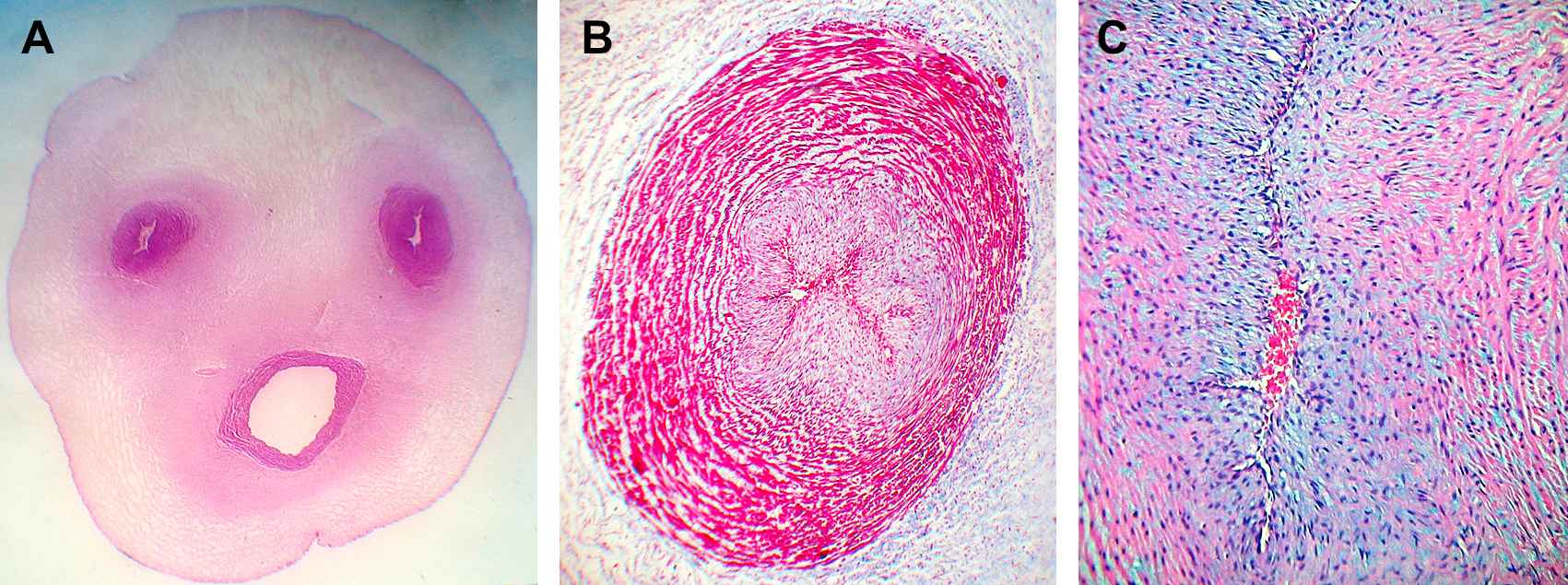
A. Umbilical cord (39 weeks) showing two constricted arteries with irregularly branched lumen and a helical course around a unique vein which presents a clearly patent lumen. Wharton’s jelly contains much fibrous tissue surrounding the vessels in contrast to the peripherical areas. H&E ×4. B. Umbilical artery of 39 weeks. The lumen is constricted with a typical irregular branched shape, with little staining in the pale intima in the center. The media is particularly thick showing an inner layer of longitudinal smooth muscle cells (SMCs), poorly differentiated and an outer coat consisting of a system of crossing spiraled SMCs with heavily stained myofibrils and with remnants of the internal elastic lamina. Masson’s trichrome. ×40. C. The constricted irregularly branched lumen of a fetal umbilical artery of 39 weeks is observed. A well developed intimal layer is shown with variable thickenings and partly folded into longitudinal pleats presenting thin, elongated or wavy SMCs cut in transverse section, with fewer myofibrils than in the media and with pale staining of cytoplasms. The media is particularly thick showing an inner layer of longitudinal smooth muscle cells (SMCs), poorly differentiated. Interlaminar and intercellular spaces consisting of proteoglycan deposits are observed in the media and intimal layers. Masson’s trichrome. ×100.
Arteries
The lumens were constricted with a branched shape and lined by endothelial cells with prominent nuclei. A well developed intimal layer with variable thickenings showed thin, elongated or wavy smooth muscle cells (SMCs) cut in transverse section (Fig. 1B). The media was thick showing an inner layer of longitudinal SMCs, poorly differentiated and an outer coat consisting of a system of crossing spiraled SMCs (Fig. 1C) and with remnants of the internal elastic lamina (IEL) at the intima-medial junction and in the intima. The media was irregularly arranged with widened intermuscular spaces occupied by mucopolysaccharides.
Vein
The lumen presented circular or triangular shapes. The intima layer, the inner intima and the muscular coats were thinner than in arteries. At the intima-medial junction segments of the IEL with waving, duplication and folding of elastic fibers were found. (Fig. 2A). The medial SMCs were arranged in circumferential branched laminae of 2 or 3 cells separated by blebs positive for Alcian blue (Fig. 2B). Scarce groups of longitudinally or oblique SMCs could also be observed.
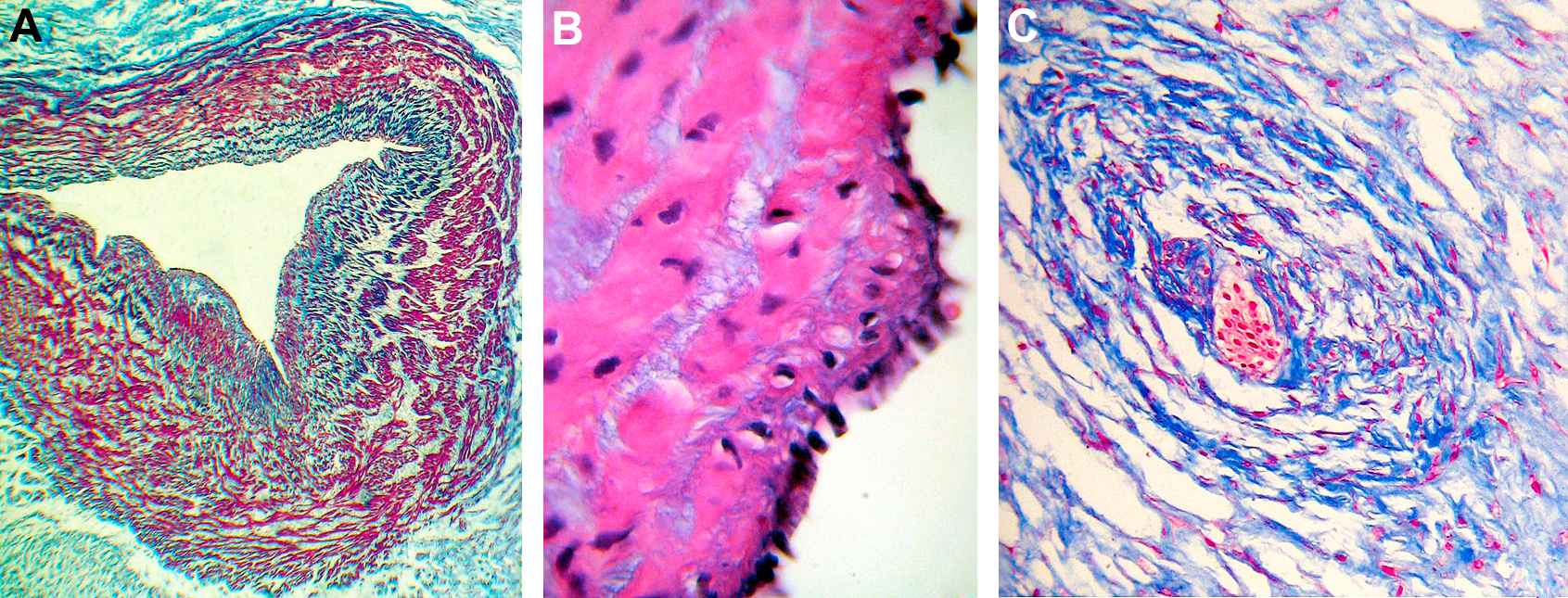
A. The lumen of the umbilical vein of 39 weeks shows preservation of the triangular shape and is lined by a row of prominent endothelial cells. The intima and the inner intima are thinner than in arteries. At the intimo-medial junction discontinuous segments of the internal elastic lamina with waving, duplication and folding of elastic fibers are shown. The venous muscular coats are thinner than those of the arteries and composed of more separate layers of fibers. Masson’s trichrome. ×40. B. Umbilical vein of 39 weeks. The intima and the inner intima are thin (much more than in arteries). The endothelium is evenly tall and nuclei prominent. The medial SMCs are arranged in circumferential branched laminae consisting of 2 or 3 cells with scarce myofibrils and separated by blebs positive for Alcian blue. Masson’s trichrome. ×400. C. A scarce developed vessel is shown in Wharton’s jelly, distant from the main vessels, where they were frequently found. The lumen has plenty of blood cells, some could be erythroblasts. Note the widespread distribution of stromal clefts in the adjacent jelly. Masson’s trichrome. ×100.
One or two scarce developed vessels were frequently found in Wharton’s jelly, distant from the main vessels (Fig. 2C).
Wharton’s jelly consisted of a mucoid structure consisting a ground substance of mucopolysaccharides, accumulated around cleft-like territories (“stromal clefts”),2 occupied by homogeneous extracellular matrix, devoid of collagen and basal lamina. It contained evenly distributed spindle-shaped fibroblasts with long extensions and different degrees of differentiation from mesenchymal cells to myofibroblasts.
“Pathological” umbilical cords
“Pathological” umbilical cords presented mainly several types of lesions namely: perivascular and/or intraparietal hemorrhages with wall dissections (Fig. 3A), parietal recent thrombosis (Fig. 3B) and focal moderate or extensive Wharton’s jelly hemorrhages (Table 2).
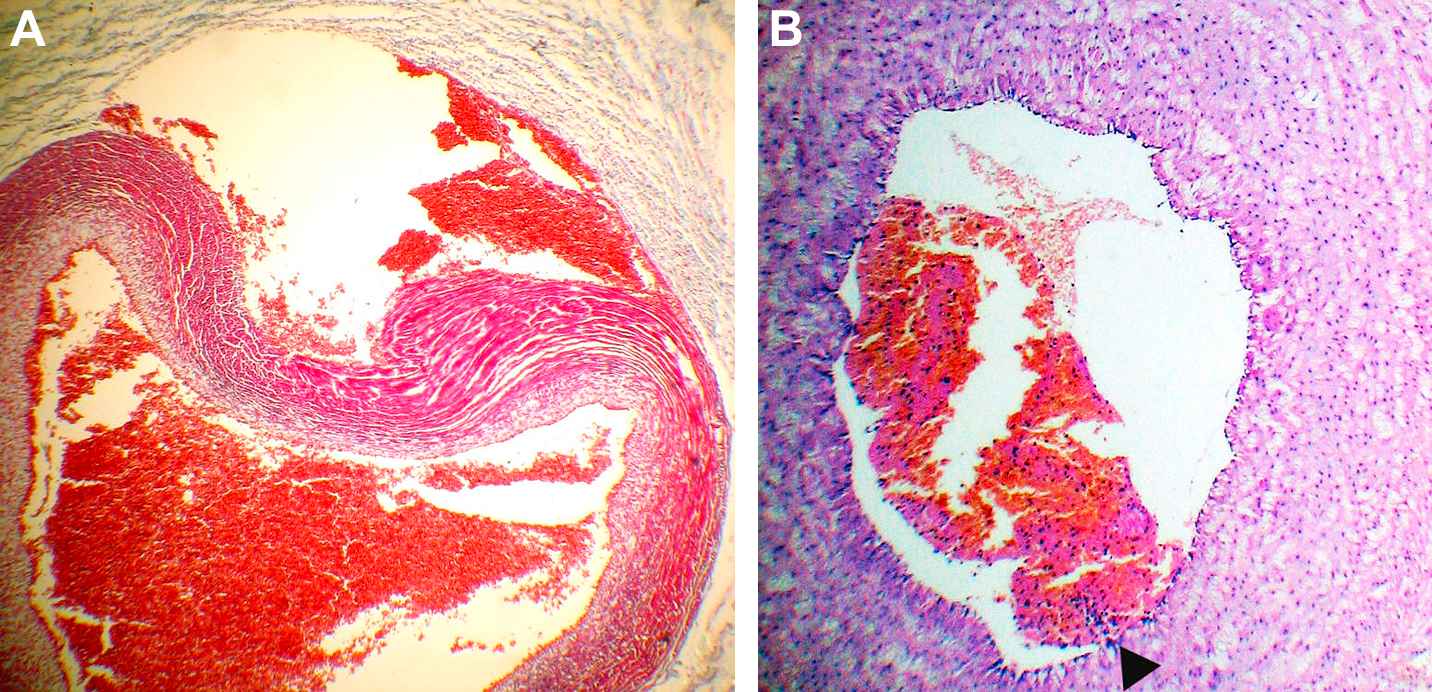
A. Extense hemorrhage of Wharton’s jelly adjacent to a vein, with severe distortion of its wall, dissection of the external layers and diminution of the lumen. Masson’s trichrome. × 40. B. Non-occlusive recent venous thrombus is observed. The area of attachment is pointed out by an arrow. H&E ×40.
| With alterations | Perivasc./intraparietal hemorrhage | Arterial/venous thrombus | Wharton’s jelly edema | Venous dilation | |
|---|---|---|---|---|---|
| Preeclampsia | 4/9 | 2/9 | 0/9 | 1/9 | 1/9 |
| Chronic hypertension | 11/14 | 2/14 | 5/14 | 4/14 | 1/14 |
| Antiphospholipid syndrome | 3/11 | 2/11 | 0/11 | 1/11 | 0/11 |
| Gestational diabetes mellitus | 3/13 | 1/13 | 1/13 | 1/13 | 0/11 |
| Intrahepatic cholestasis | 4/13 | 2/13 | 1/13 | 1/13 | 0/11 |
| Antiphospholipid antibodies | 5/11 | 2/11 | 4/11 | 1/11 | 2/11 |
| Fetal growth restriction | 5/9 | 0/11 | 4/9 | 0/11 | 2/9 |
| Oligohydramnios | 6/6 | 0/11 | 2/6 | 3/6 | 2/6 |
| Premature rupture of membranes | 6/12 | 2/12 | 4/12 | 1/12 | 0/11 |
| Fetal distress | 8/23 | 6/23 | 3/23 | 2/23 | 1/23 |
| Combined pathologies | 18/34 | 6/34 | 11/34 | 3/34 | 1/34 |
| Pathologic groups | 35/73 (48%)* | 14/73 | 12/73 | 8/73 | 9/73 |
| Normal pregnancies | 4/20 (20%) | 1/20 | 1/20 | 1/20 | 1/20 |
Two-sided p = 0.039 (Fisher’s exact test) vs. normal.
Type and frequency of lesions according to maternal or fetal disease.
Perivascular hemorrhages consisted of extended accumulation of fresh erythrocytes surrounded by marked edema of Warthon’s jelly and severe distortion of the walls of either arteries and/or veins. Conversely, intraparietal hemorrhages with wall dissections showed leaks of the external layers of arteries or veins, plenty of erythrocytes causing mild to moderate diminution of the corresponding lumens and moderate distortion of its shapes, generally accompanied by hemorrhages and edema of the adjacent Warthon’s jelly.
Isolated non-occlusive recent venous thrombi with a clearly delineated area of attachment were observed in umbilical cords belonging to pathological pregnancies presented significantly more microscopic lesions (35/73; 48%), than normal pregnancies (4/20; 20%; p = 0.039).
Histomorphometry
The histomorphometry of umbilical arteries and veins of all pathologic groups vs. control group showed that wall:lumen ratio of arteries was significantly higher in all pathologies (32.6 ± 16) as compared to 3.1 ± 0.6 in the control group (p < 0.0001). In this regard, the outer (142.8 ± 67 vs. 56.6 ± 25; p < 0.0001) and the inner layer areas (57.8 ± 23 vs. 28.6 ± 8; p = 0.0002) were higher in opposition to much less increases in luminal areas (5.6 ± 3 vs. 3.4 ± 2; p = 0.03) of all pathologies vs. control group, respectively.
Concerning veins, wall:lumen ratio was also higher in the pathological groups (5.3 ± 3 vs. 2.9 ± 1; p = 0.0086) due to a 2-fold increase in wall areas (225.5 ± 118 vs. 109.8 ± 35; p = 0.003).
The histomorphometry of each pathologic group vs. control group is displayed in Table 3.
| Arteries | Veins | ||||||
|---|---|---|---|---|---|---|---|
| Outer layer area | Inner layer area | Lumen area | Wall:lumen ratio | Wall area | Lumen area | Wall:lumen ratio | |
| Chyp | 131.7 ± 69 | 53.6 ± 13 | 5.9 ± 2 | 31.2 ± 21** | 211.9 ± 101 | 50.4 ± 18 | 6.2 ± 3** |
| FD | 132.2 ± 54* | 54.1 ± 20 | 4.5 ± 2 | 38.5 ± 12*** | 192.4 ± 92 | 44 ± 19 | 5.4 ± 4 |
| AphS | 66.9 ± 13 | 28 ± 4 | 4.7 ± 3 | 34.4 ± 13*** | 90.2 ± 9 | 23.9 ± 8 | 4.6 ± 3 |
| AphAb | 148.3 ± 84 | 99 ± 12*** | 7.3 ± 5 | 30.3 ± 17** | 230.6 ± 53 | 56 ± 10 | 4 ± 2 |
| Ich | 178.9 ± 60* | 57.5 ± 17 | 6.6 ± 3 | 29.5 ± 13** | 343.4 ± 96*** | 31.6 ± 19 | 5.6 ± 5 |
| GDBT | 134.1 ± 67 | 54.8 ± 22 | 3.9 ± 3 | 26.7 ± 9* | 197 ± 76 | 46.4 ± 16 | 5.8 ± 4 |
| O | 142.2 ± 47 | 51.5 ± 26 | 8.4 ± 1 | 23.4 ± 18 | 324 ± 62*** | 74.3 ± 57 | 35.2 ± 22*** |
| Pe | 116.4 ± 55 | 63.1 ± 16* | 8.4 ± 1 | 20.3 ± 9** | 155.9 ± 86 | 35.9 ± 29 | 4.8 ± 2 |
| FGR | 148.7 ± 17* | 54.6 ± 13 | 5.2 ± 3 | 29 ± 13* | 222.4 ± 39 | 37.3 ± 12 | 6 ± 3 |
| PRM | 217.2 ± 144*** | 67.3 ± 17** | 4.6 ± 3 | 51.6 ± 22*** | 308.7 ± 64*** | 65.6 ± 14 | 4.4 ± 2 |
| Normal | 56.5 ± 25 | 28.6 ± 8 | 3.4 ± 2 | 3.1 ± 0.6 | 109.8 ± 35 | 36.6 ± 18 | 2.9 ± 1 |
p < 0.05,
p < 0.01,
p < 0.001 vs. normal (ANOVA).
Normal, n = 20; gestational diabetes mellitus (GDBT) n = 13; antiphospholipid syndrome (AphS) n = 11, chronic hypertension (Chyp) n = 14, preeclampsia (Pe) n = 9, intrahepatic cholestasis of pregnancy (Ich) n = 13, fetal growth restriction (FGR) n = 9, premature rupture of membranes (PRM) n = 12, antiphospholipid antibodies (lupus anticoagulant or anticardiolipin antibodies) (AphAb) n = 11, oligohydramnios (O) n = 6, fetal distress (FD) n = 23. Area measurements were obtained in pixels and then they were normalized for bodyweight of the newborn.
Histomorphometry of pathologic groups vs. control group.
Immunohistochemistry (paraffin-embedded sections)
Positive immunostaining against TGF-β1 could be observed in the interstitial area of the muscular layer of the umbilical arteries and to a lesser extent in the umbilical veins. The endothelium and amnion were also positive (Fig. 4).
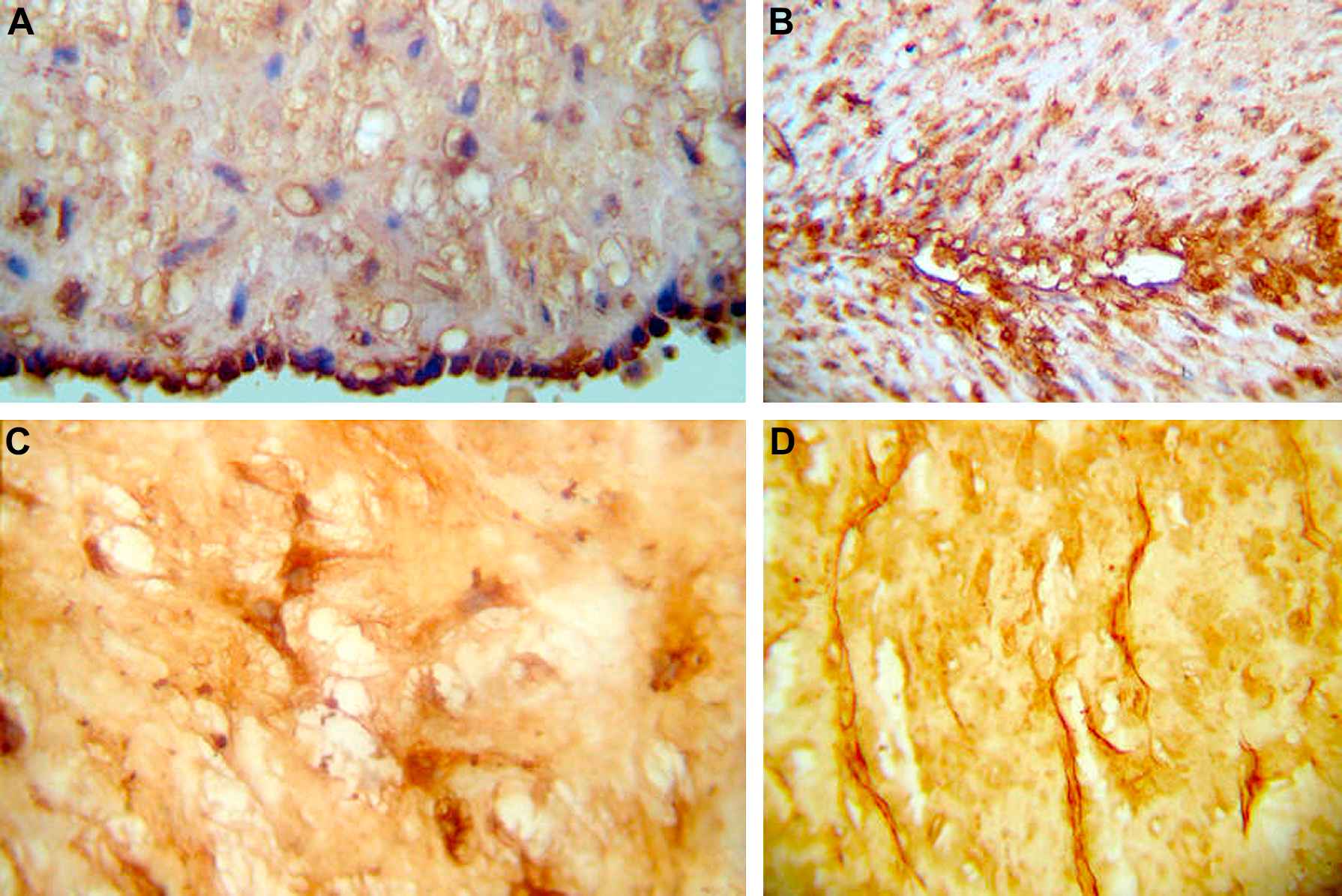
Immunohistochemistry. Deposits of TGF-β1 are observed in: A. Cytoplasms of endothelial cells. B. Spindle-shaped smooth muscle cells of the media and intima layers of arteries and veins. C. Cytoplasms and long extensions of the spindle-shaped mesenchymal cells of Wharton’s jelly. PAI-1 deposits are evenly found in the clefts of Wharton’s jelly, as shown in D.
Regarding PAI-1, it was expressed surrounding the muscle cells of the umbilical arteries, possibly indicating basal membrane positiveness. To a lesser extent the muscle cells of the umbilical veins were also positive. The endothelium was strongly positive, as well as the amnion and the stromal cells of the cord.
There was no correlation between the immunostaining semiquantitative score in the control group and pathological groups (data not shown).
Discussion
According to our results, pathological pregnancies present a significant diminution in birth weights, more arterial and venous lesions of umbilical cord vessels and significant increases of their wall areas with diminished wall:lumen ratios, suggesting that vascular alterations must be considered concurrent causes and/or consequences in the broad spectrum of high risk pregnancy.
Arterial and venous, perivascular or intraparietal hemorrhages with wall dissections, arterial and venous recent thrombosis, venous dilatation and Wharton’s jelly edema, were the main lesions found in the present study. Pathological pregnancies presented much more lesions: 35/73 (48%), than normal pregnancies: 4/20 (20%, p < 0.03), and among them, chronic hypertension, premature rupture of membranes, oligohydramnios, fetal growth restriction and combined pathologies were the clinical situations that presented more frequently (>50%) in those microscopic alterations. Of note, chronic arterial hypertension alone or associated with other pathologies contributed to the highest number of microscopic lesions (11/14; 79%).
Regarding birth weight, a significant diminution was observed in newborns belonging to pathological groups as compared to the normal group (p < 0.001). Preterm labor is often necessitated by maternal and fetal indications, and this could be the cause of low birth weight in conditions as preeclampsia, chronic hypertension, antiphospholipid syndrome, premature rupture of membranes and fetal distress. In fetal growth restriction the cause is inherent to the pregnancy itself and in diabetes is contradictory.
Concerning histomorphometry, significant differences were found. In arteries the areas of the outer layer, the inner layer, the lumen and the wall:lumen ratio were significantly higher in pathological groups compared to the normal group. In veins the lumen area was similar but the outer area and the wall/lumen ratio were higher.
To our knowledge a complete explanation for these findings is difficult to be given. However, some facts may be taken into consideration. Fetal responses to clinical diseases affecting pregnancy or intrinsic fetal sickness may include hormone production, tissue sensitivity to these hormones and alterations in metabolism that may affect organ development, leading to disturbances in anatomical, physiological and metabolic changes.
The changes in wall and luminal areas may be partially explained by the fact that throughout the last 2 weeks of pregnancy, the cord vessels show increasing responsiveness to mechanical irritation.14 Furthermore, these vessels are exquisitely sensitive to various endocrine mediators, such as serotonin, angiotensin, prostaglandins and oxytocin.15–17 Moreover, smooth muscle cells are influenced in paracrine loops by vasodilator substances produced within the neighboring endothelial cells, like prostaglandins and nitric oxide 17–19 and by the autocrine stimuli of atrial natriuretic peptide,20 and other substances.
Further studies will have to elucidate the complicated interactions of these substances, as they are very likely to be involved in abnormal conditions, such as intrauterine growth restriction and a high Doppler resistance index as well as in the thickening of the walls of the umbilical cord vessels, as it was found in the present paper.
The umbilical cord is an extension of the fetal weave and as so it allows assessing the impact of clinical diseases and/or intrinsic fetal sickness affecting pregnancy and its evaluation by different methods yields to a close relation to fetal evolution.21 Therefore the association between fetal growth restriction, low weight at birth and high risk pregnancies may be interpreted as long-term consequences of fetal adaptive responses.22,23
Perivascular or intraparietal hemorrhages and wall dissections, described in this paper could not be ascribed to short cords, trauma or entangling, neither to manipulation of the cord by the obstetrician during or after delivery, that may induce local hematoma formation, and that may be life threatening. Hematoma due to cord clamping is often obvious because the serrations of a clamp remain visible. Traumatic injury to cord vessels during amniocentesis has often been reported,24 but none of the patients included in this study required amniocentesis nor were diagnosed with vasa previa. Hemorrhages were found in 14/73 (19%) of pathological pregnancies and may be attributed to fetal and maternal diseases and more possible to a combination of both.
In the present study we found diffuse immunostaining for TGF-β1 and PAI-1 in umbilical blood vessels. The samples were positive only at high antibody concentration, indicating that the activation cascade of the mentioned immunomarkers is not a key event in the pathophysiology of umbilical cords. Furthermore, there was no correlation between the immunostaining score in the control group and the different pathological groups. We therefore suggest investigating further pathophysiological routes to explain the morphological differences found in our study.
Finally, we may stress that many lines of evidence, including epidemiologic, clinical and experimental studies, indicate that early life events that may be amplified by other risk factors,25 play a fundamental role in influencing later susceptibility to adult chronic diseases. An understanding of developmental plasticity provides the basis for these facts.26 Quantitative histomorphometry of the pathological alterations and pathophysiologic disorders of the umbilical cord has the potential to enhance investigation and treatment of maternal and fetal diseases.
Conflict of interest
None.
Acknowledgements
This study received financial support from CONICET and the University of Buenos Aires, Argentina (UBACYT M047 – 2008–2010).
References
Cite this article
TY - JOUR AU - Manuel Vázquez Blanco AU - Hilda Ruda Vega AU - Roberto A. Guerri-Guttenberg AU - Rodolfo Giuliano AU - Daniel R. Grana AU - Francisco Azzato AU - José Milei PY - 2011 DA - 2011/02/26 TI - Histopathology and histomorphometry of umbilical cord blood vessels. Findings in normal and high risk pregnancies☆ JO - Artery Research SP - 50 EP - 57 VL - 5 IS - 2 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2011.02.001 DO - 10.1016/j.artres.2011.02.001 ID - Blanco2011 ER -
