Arterial calcification and its physiological consequences: Ideas from animal models
- DOI
- 10.1016/j.artres.2009.10.183How to use a DOI?
- Keywords
- Artery; Calcification; Animal model; Wall stiffening; Ageing; Cardiac function; Human
- Copyright
- © 2009 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
In man age-linked aortic wall calcification occurs at two main levels: intimal plaques and the medial elastic fibre network (medial elastocalcinosis 37,48). Several recent reviews deal with the subjects of arterial ageing and elastocalcinosis.1,3,10,11,19,23,25,35,36,45
Although vascular calcification was described at the beginning of the 20th century37 it was not until the 1940s that research on this subject intensified. After finding that vascular wall calcification was less marked in syphilitic aortitis in which the elastic elements of the media are destroyed, Blumenthal et al.5 suggested that medial calcification is primarily associated with elastic fibres. In the age range of 81–103 years, the aortic wall elastin content fell and the calcium content rose concomitantly.27 Furthermore, in coronary arteries, calcification was accompanied by elastic fibre fragmentation.27 All of these observations suggest that medial calcification involves destruction of elastic fibres.4
As elastin plays a major role in aortic wall elasticity, it is possible that the development of elastocalcinosis with age is involved in age-linked stiffening of the aortic wall as described by Wilens in 193749. However, although elastocalcinosis and wall stiffening were described over 60–70 years ago, evidence of the way in which the two are linked is still lacking. This short review is an attempt to provide evidence from animal models of these possible links (Fig. 1).
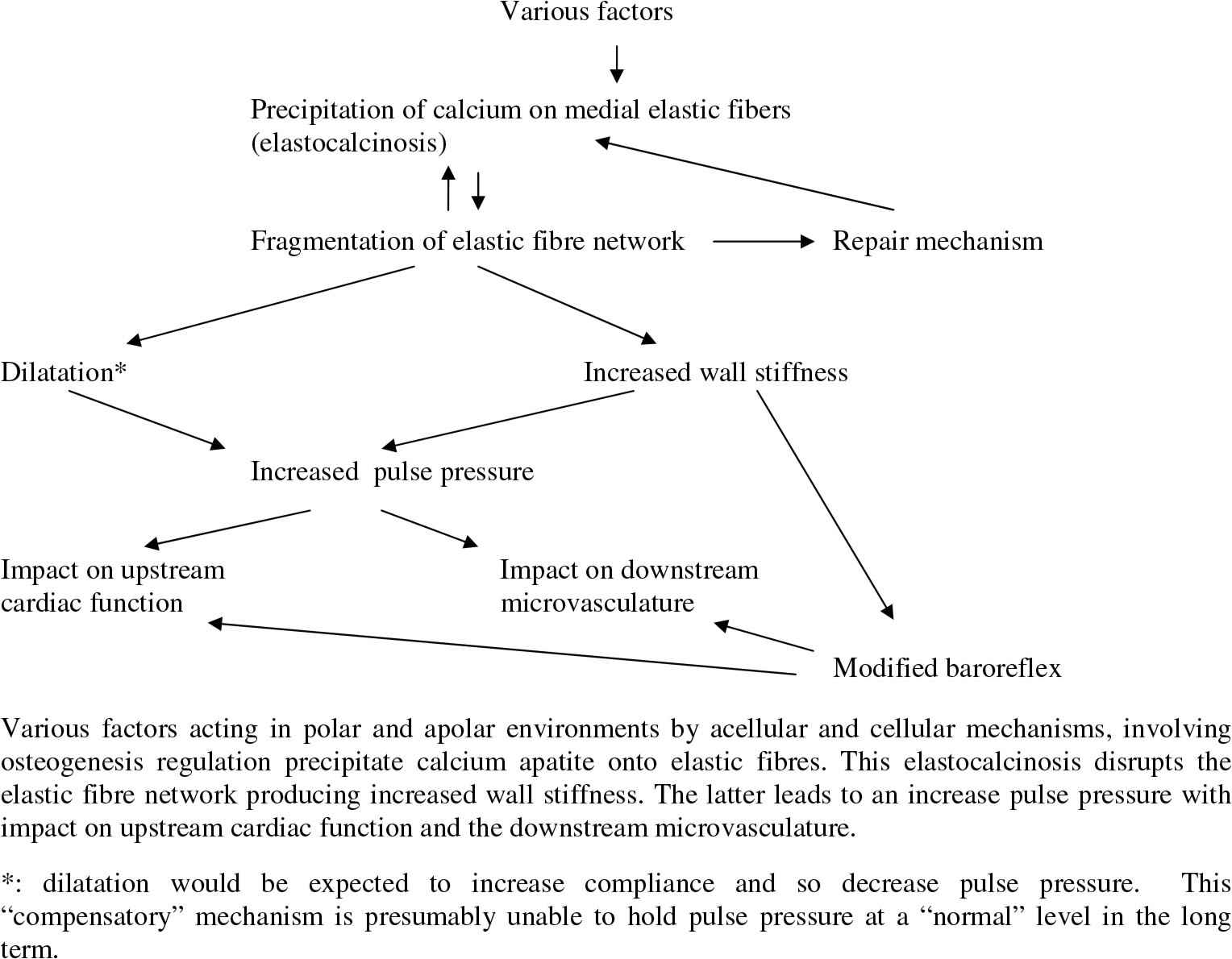
Mechanisms and consequences of arterial medial elastocalcinosis.
Animal models of aortic elastocalcinosis: Spontaneous age-linked vascular calcification
Rat arteries contain up to 5 times more calcium than other soft tissues and calcify with age (2- to 3-fold) whereas other soft tissues do not.9,26 The old rat suffers from mild medial elastocalcinosis: calcium bound to vascular elastin increases with age in the rat.34,40 Studies on age-related changes in vascular mechanics show that although the elastic modulus of the aortic wall increases there is no rise in central aortic pulse pressure.9 Furthermore there is no correlation between elastic fibre calcification and increased arterial stiffness upon ageing in normotensive rats.34 Thus although elastocalcinosis occurs with ageing in the rat it is relatively mild and associated with but minor changes in wall mechanics.
Hypervitaminosis D plus nicotine (VDN model)
Possible correlations between elastocalcinosis and increased wall stiffness were evaluated in the hypervitaminosis D plus nicotine (VDN) model that suffers from a far more intense degree of medial elastocalcinosis (Table 1). Hass and co-workers20 and several laboratories used hypervitaminosis D, alone14,43 or in combination with nicotine2,13,50 or cholesterol44 to produce arterial calcification. Although arteries such as the aorta are most susceptible, hypervitaminosis D treatment also leads to calcification of the heart, kidneys and other organs21,22,26. Hypervitaminosis D with or without nicotine produces aortic elastocalcinosis with calcification localised on elastic fibres.12,38,39
| Control | VDN | P | |
|---|---|---|---|
| Aorta and aortic wall | |||
| Calcium content (micromol/g)a | 24±2 (14) | 318±85 (21) | <0.05 |
| Desmosines (microg/g)a | 1243±83 (14) | 555±36 (21) | <0.05 |
| Elastic fibre fragmentationb | 0.95±0.03 (10) | 0.61±0.04 (12) | <0.05 |
| Elastic modulus (106 dynes/cm2)a | 6.7±0.5 (14) | 13.2±1.4 (21) | <0.05 |
| Central pulse pressure (mmHg)a | 39±2 (14) | 66±6 (21) | <0.05 |
| Heart | |||
| Baseline stroke volume (microl)c | 223±18 (8) | 211±13 (10) | >0.05 |
| Left ventricle weight/body weight (g/kg)c | 1.54±0.07 (8) | 1.73±0.05 (10) | <0.05 |
| Collagen (microg/g)c | 31±4 (8) | 52±4 (10) | <0.05 |
| Beta myosin isoform (%)c | 18±2 (8) | 31±3 (10) | <0.05 |
Cardiovascular features of the hypervitaminosis D plus nicotine (VDN) model of aortic elastocalcinosis and increased wall stiffness.
We studied the links between elastocalcinosis, arterial stiffening and the consequences of the latter in the VDN rat (one day’s treatment with vitamin D and nicotine followed by several months of recovery). VDN treatment does not modify aortic wall thickness, wall thickness to lumen ratio, or wall stress; central aortic mean blood pressure does not increase.38,39 VDN rats show increased arterial wall rigidity (increased elastic modulus, increased isobaric elasticity, and decreased pulse amplification) with increased central aortic pulse pressure (with no statistically significant change in stroke volume) (Table 1). There is an increase in diameter.24 It should be remembered that induction of arterial calcification is also used in animal models of aneurysm.17 Albeit, in the VDN model, aortic impedance increases and systemic arterial compliance decreases.2,38,39,47
The VDN model is interesting in that a change in wall composition - elastocalcinosis - independent of any change in geometry determines the mechanical properties of the wall. VDN treatment produces widespread fragmentation of the medial elastic fibre network,18 (Table 1 and Fig. 2) and there is an inverse relationship between the calcium and desmosine contents of the aortic wall.39 Desmosines are cross-linking amino acids specific to elastin. Thus elastocalcinosis involves both elastic fibre fragmentation and a loss of elastin cross-linking. Factors involved in osteogenetic vascular calcification such S-100 calcium-binding proteins6,7 are found in the medial calcium deposits.38,39
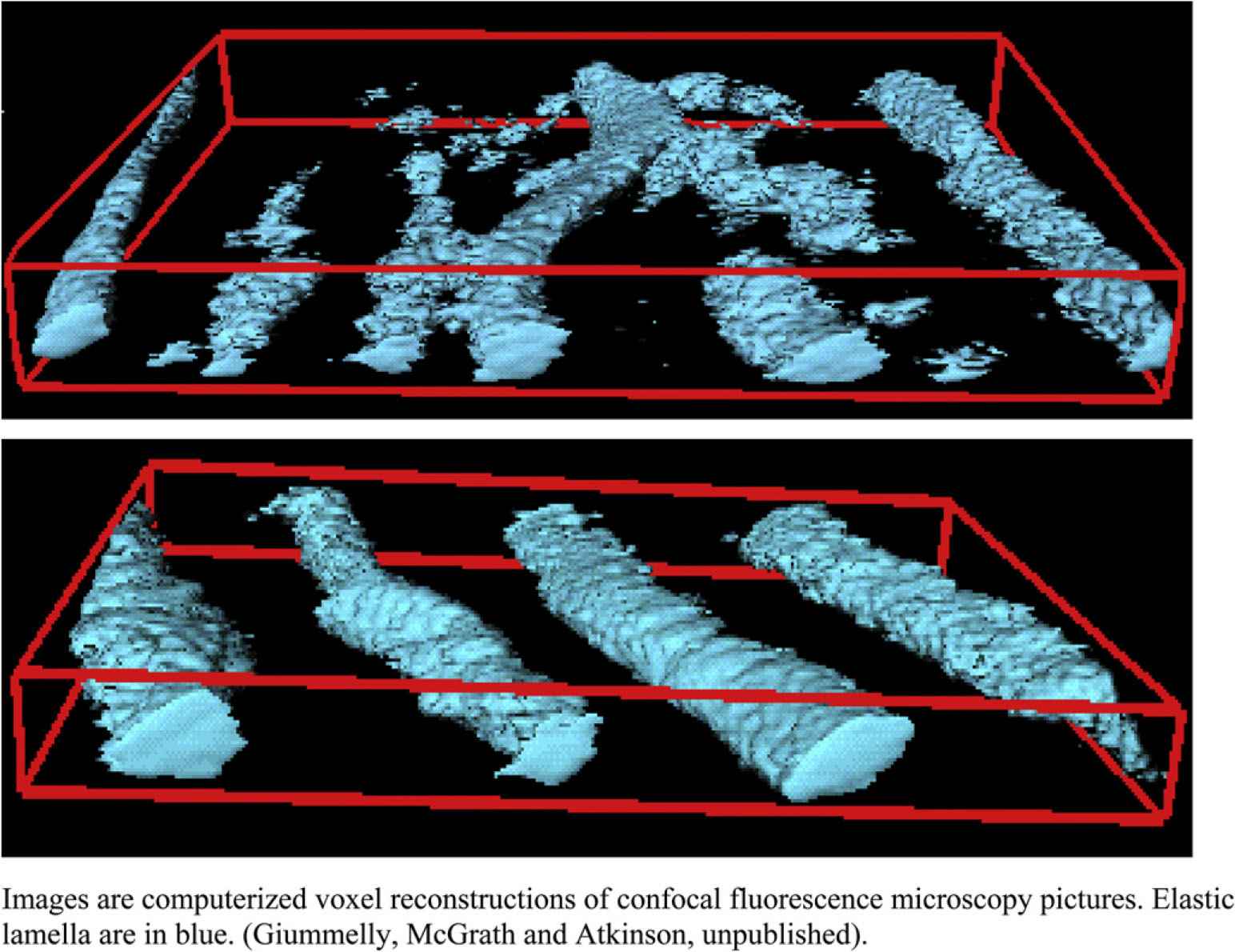
Rupture of the elastic lamellar network in the aortic wall of the VDN model (upper) compared to control (lower panel).
Regarding the upstream cardiac consequences of increased aortic wall stiffening there is little global change in cardiac performance at base and under volume overload.28 A number of compensatory mechanisms presumably maintain cardiac performance. Left ventricular hypertrophy occurs and ventricular mass is positively correlated to aortic wall isobaric elasticity.39 There is mild ventricular fibrosis and a shift in myosin isoforms to the beta form28 (Table 1). Preload recruitable stroke work and end-systolic elastance are both elevated in VDN and this lowers the ratio of arterial elastance over end-systolic elastance and so increases efficiency.24 The VDN model may prove useful for the study of cardiac adaptation to increased pulse pressure following increased arterial stiffness.
Regarding the downstream impact of increased pulse pressure we have studied renal function in the VDN model. Clinical studies suggest a strong link between chronic renal failure and vascular calcification, the latter leading to arterial wall stiffening and hyperpulsatility. An increase in pulse pressure in the renal circulation could increase pulsatile wall stress and so damage endothelial and smooth muscle cells.8,42 The kidneys of VDN rats show extensive damage to glomeruli and vasa recta. Glomerular filtration rate decreases and albuminuria increases. There are significant linear relationships between albuminuria or glomerular filtration rate and central aortic pulse pressure.16 These preliminary results suggest a link between increased central aortic pressure pulsatility and downstream microvascular function (in the kidney).
In summary, vascular calcification may be species-specific to man. As laboratory animals such as the rat grow old they suffer from only mild arterial calcification. Different animal models of induction of massive arterial calcification by pharmacological and other means exist. Although extrapolation from such models to the clinical situation in terms of aetiology is difficult, such models could be useful in the non clinical study of the pathophysiological consequences of vascular calcification. Thus the VDN model reproduces the structural and functional aspects of medial elastocalcinosis of arteries seen with ageing in man. A proviso has to be added. All surgical or pharmacological “injury” models involve an acute challenge to a relatively young animal. Does this mimic what happens during senescence of the human aortic wall elastic fibre over decades? The fibre is subject to slowly developing mechanical failure following the cumulative effect, over a very long time, of repetitive systolic shocks to the wall41 and this in an environment that may include hypertension, atheroma, diabetes and other cardiovascular complications.
What is the evidence in man that aortic calcification is linked to increased wall stiffness? Asymptomatic hypertensive patients with aortic pulse wave velocity values above normal show abdominal aortic calcification.32,46 The calcium antagonist, nitrendipine, lowers pulse wave velocity in patients with aortic calcification but has no effect in those with non-calcified vessels.33 An increase in aortic pulse wave velocity is related to aortic calcification in patients suffering from end-stage renal failure30,31 and arterial medial calcification is a strong prognostic marker for cardiovascular mortality in haemodialysis patients.31 In diabetic patients also, arterial medial calcification is related to cardiovascular mortality, coronary heart disease and stroke.29
Finally, the VDN model may provide a useful tool to evaluate potential anticalcinotic therapy with drugs such as calcium entry blockers22 or angiotensin I converting enzyme inhibitors.21,47 Recent evidence suggests that anti-inflammatory PPAR-gamma ligands such as the thiazolidinediones (glitazones) are anticalcinotic.15 Other potential molecular candidates are compounds that compete with calcium for binding sites on the elastic fibre or those that chelate calcium.
References
Cite this article
TY - JOUR AU - Jeffrey Atkinson PY - 2009 DA - 2009/11/12 TI - Arterial calcification and its physiological consequences: Ideas from animal models JO - Artery Research SP - 132 EP - 136 VL - 3 IS - 4 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2009.10.183 DO - 10.1016/j.artres.2009.10.183 ID - Atkinson2009 ER -
