A systematic appraisal of ventricular–aortic load in African American men
- DOI
- 10.1016/j.artres.2009.02.003How to use a DOI?
- Keywords
- Arterial stiffness; Wave reflection; Left ventricle; Hemodynamic
- Abstract
Background: We examined several measures of ventricular–vascular load as they relate to ECG-derived measures of left ventricular (LV) morphology in a cross-section of 19 young African American and 19 white men.
Methods: Measures of steady and pulsatile LV load derived from aortic blood pressure waveforms included: aortic characteristic impedance (Zc), effective arterial elastance (Ea), arterial compliance, aortic reservoir function, aortic wave reflection (AIx), and total peripheral resistance (TPR). Also derived from the pressure waveform were the rate pressure product (RPP), tension–time index (TTI), diastolic pressure–time index (DPTI), and the subendocardial viability ratio (SEVR). ECG was used to measure R-wave area, R-wave amplitude, and QRS duration as crude proxies of LV morphology.
Results: African American men had greater Ea, AIx, TPR and reduced aortic compliance compared with white men (all p < 0.05). There was a positive association between Ea, Zc, TPR and LV morphology (p < 0.05). There was an inverse association between arterial compliance and LV morphology (p < 0.05). AIx was not associated with LV morphology. There were no racial differences in aortic reservoir function, RPP, TTI, DPTI, or SEVR. Aortic reservoir function was positively associated with DPTI and SEVR (p < 0.05) and inversely associated with RPP (p < 0.05).
Conclusions: In young African American men, LV morphology is influenced by LV load stemming from aortic stiffness and vascular resistance more-so than augmented pressure from wave reflections. Aortic reservoir function is preserved in young African American men, balancing myocardial oxygen supply and demand in the presence of altered vascular–ventricular coupling and LV remodeling.
- Copyright
- © 2009 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
With aging and disease, aortic stiffness and wave reflections increase, contributing to an increase in late systolic load (i.e. afterload) and LV hypertrophy.1,2 Left ventricular ejection of stroke volume into a stiff aorta has also been shown to increase cardiac energetic demand and reduce myocardial perfusion.3–5 Aortic stiffness is greater in young African American men compared with young white men13 yet few studies have systematically examined ventricular–vascular load and cardiac work in young African American men.
Left ventricular (LV) hypertrophy is more common in African Americans.8 As such, the prevalence of heart failure, cerebrovascular disease, stroke, retinal damage and ultimately death related to concentric LV remodeling is higher in African Americans compared with other racial/ethnic groups.6–9 However, alterations in LV geometry are apparent in young normotensive African American men10,11 with racial differences manifesting during early adolescence/early childhood.12 These alterations are so prevalent that it has been stated that cardiac hypertrophy is not only common, but epidemic in African Americans, irrespective of the presence or absence of hypertension.10
Examination of ventricular–vascular coupling and LV load in young African American men is important as this may provide physiologic insight into the pathogenesis of LV hypertrophy evident in this population. Hence the purpose of this investigation was to examine novel measures of aortic function and LV load in young African American and white men. We hypothesized that young African American men would have greater aortic elastance, and reduced aortic reservoir function when compared with a group of young white men and this would be associated with racial differences in LV morphology and cardiac work.
Methods
Subjects
From our previous data set of 30 white and 25 African American men,13 we selected 19 white and 19 African American men matched for factors that have been shown to affect arterial stiffness and wave reflection including: age, body weight, body fat, cardiorespiratory fitness, total cholesterol, LDL cholesterol, triglycerides, glucose, glomerular filtration rate, family history of CVD, inflammatory markers (C-reactive protein and white blood cell count), height and heart rate. Subjects were not taking any medications. Subjects were self-defined as African American if reporting that both parents were of African descent. All subjects were recruited from the local university student population. All subjects gave written consent. This study was approved by the Institutional Review Board of the University of Illinois at Urbana-Champaign.
All subjects reported to the laboratory for 2 days of testing. The first visit consisted of fasting blood draws and body composition assessment via air displacement plethysmography. The second visit comprised vascular measures followed by an exercise test until volitional fatigue on a cycle ergometer with concomitant metabolic gas measurement for the assessment of peak oxygen uptake (i.e. cardiorespiratory fitness). For vascular measures, all subjects were at least 3-h postprandial and did not consume caffeine, alcohol or exercise for 24 h prior to testing. Participants rested in the supine position for a period of 10 min in a temperature-controlled room prior to testing.
Ventricular–vascular measures derived from pulse waveform analysis.
Brachial blood pressure (BP) was measured in the supine position using an automated oscillometric cuff. All brachial BP measurements were made in duplicate, following established guidelines. If these values deviated by more than 5 mmHg, a third measurement was conducted. The average of the two closest values was recorded and used for subsequent analysis.
Radial artery pressure waveforms were attained in the supine position from a 10-s epoch using applanation tonometry and a high-fidelity strain gauge transducer (Millar Instruments, Houston, TX). Using a generalized validated transfer function,14 a central aortic pressure waveform was reconstructed from the aforementioned radial artery pressure waveform (SphygmoCor, AtCor Medical, Sydney, Australia).15 Left ventricular (LV) systolic ejection duration was taken as the time from the foot of the pressure wave upstroke to the incisura of the dicrotic notch (also the point at which end systolic pressure was calculated). Diastolic time was calculated as total pulse period − LV ejection duration. Augmented pressure (AP) was defined as the difference between central SBP and the pressure at the forward/primary wave peak (P1). P1 was defined as the pressure at the first inflection point − central/aortic diastolic blood pressure. Augmentation index (AIx) was calculated as the ratio of amplitude of the pressure wave above its systolic shoulder (i.e. the difference between the early and late systolic peaks of the arterial waveform), to the total pulse pressure expressed as a percentage (P2 − P1/PP × 100). Travel time of the forward pressure wave from the aorta to the peripheral reflection site and back (Tr) was determined from the time from the initial upstroke of the pressure wave to the foot of the reflection wave.16 Wasted LV pressure effort (ΔEw), the energy required by the LV to overcome augmented pressure from wave reflections, was calculated as 2.09 × AP(ED − Tr) as previously described.2
Diastolic run-off (DR), the portion of SV that is stored in the aorta during systole and then flows into peripheral arteries during diastole by means of the cushioning properties of the vessel, was calculated as:
SAC was calculated as:
Tau was calculated as:
The systolic tension–time index (TTI) is the area under the systolic portion of the aortic pressure wave and was taken as an index of myocardial oxygen demand. Myocardial oxygen consumption was also examined using the rate pressure produce (aortic SBP × HR). The diastolic pressure time index (DPTI) is the area under the diastolic portion of the aortic pressure wave and was used as an index of coronary perfusion/cardiac oxygen supply. Cardiac oxygen supply potential was also assessed by calculating the diastolic time fraction (DTF) as the ratio of diastolic time and pulse interval.3 The ratio of DPTI to systolic TTI represents the subendocardial viability ratio (SEVR) and was used as an index of subendocardial perfusion.
Beat-to-beat blood pressure was recorded for a 15-min epoch using finger plethysmography (Finometer, FMS, The Netherlands). The Modelflow method was used to reconstruct an aortic flow waveform by simulating a non-linear time-varying three-element windkessel model of the aortic input impedance from the arterial pressure wave.19 Age, sex, height, weight and mean arterial pressure are entered to estimate the aortic area–pressure relationship using the arctangent model of Langewouters et al.20 From this model, aortic characteristic impedance (Zc) and stroke volume (computed by integrating the systolic area under the flow pulse) were derived. Cardiac output (Q) was calculated as HR × SV.
SV/Aortic PP ratio was used as an index of arterial compliance.21–23 Effective arterial elastance (Ea) was estimated as end systolic pressure/stroke volume.24
Carotid ultrasound
Carotid artery diameter was measured by ultrasonography (SSD-5500, Aloka, Tokyo, Japan). Carotid artery pressure was obtained using applanation tonometry and calibrated against brachial mean arterial and diastolic pressure. A one-point carotid pulse wave velocity was calculated, as previously described in detail.25 Carotid characteristic impedance was then calculated by re-arranging the water hammer equation as Zc = (PWV × ρ)/A, where ρ is blood density (assumed constant 1.055 kg/cm3) and A is carotid area. Carotid Zc has been used as a surrogate for aortic Zc 26 and in the present study was used to corroborate measures of aortic Zc derived from peripheral pulse waveforms.
Electrocardiography (ECG)
Heart rate (HR) was recorded continuously for 15 min using ECG with a single lead CM5 configuration (Biopac Systems, Santa Barbara, CA, USA). The ECG was collected on-line at a sampling rate of 1000 Hz, in real time, and stored on a computer and analyzed off-line. Data were visually and automatically inspected for ectopic beats (premature, supraventricular, ventricular) and interpolated to provide a continuous data stream. HR peaks were automatically detected from a stable 5-min epoch via an established detection algorithm (WinCPRS, Turku, Finland). A digital filter was used to detect QRS complexes. The complex candidates were found by calculating how many times the signal’s derivative crossed a threshold level. One crossing signifies a baseline shift; 2–4 crossings signify that it is a real complex; five or more crossings are indicative of a noise block. The slope range/threshold setting was set to assure that very short RR-intervals were not accepted during the detection process. This was used to prevent false detections on very high T-waves. Ensemble averaged R-peaks were determined by a maximum of the absolute value of the peak value of the signal. R-wave amplitude, R-wave area, and QRS duration were taken as crude proxies of LV morphology with previous studies noting associations of these ECG parameters with LV mass and LV chamber internal dimensions.27–29
Statistics
All data are reported as means ± SEM. A priori significance was set at p < 0.05. Analysis of variance (for normally distributed data) and Mann Whitney U-tests (for non-normally distributed data) were used to assess differences in continuous outcome variables. Chi-square tests were used to compare categorical variables (family history of hypertension, family history of diabetes). Pearson’s correlation coefficients (for normally distributed data) and Spearman’s correlation coefficients (for non-normally distributed data) were used to assess relationships between variables of interest. Data analysis was carried out using Statistical Package for the Social Sciences (SPSS, v 12.0.1, SPSS, Inc., Chicago, IL).
Results
As seen in Table 1, groups were well matched for all other demographic variables. African American and white men had similar systolic BP (130 ± 2 vs. 129 ± 2 mmHg, p = 0.46), diastolic BP (75 ± 2 vs. 73 ± 1 mmHg, p = 0.27), and mean arterial pressure (92 ± 2 vs. 90 ± 1 mmHg, p = 0.36) and aortic pulse pressure (34 ± 2 vs. 33 ± 1 mmHg, p = 0.59).
| Variable | White | African American | p-value |
|---|---|---|---|
| Age (years) | 23 ± 1 | 22 ± 1 | 0.10 |
| Height (m) | 178.8 ± 1.5 | 177.2 ± 1.3 | 0.38 |
| Weight (kg) | 82.8 ± 3.2 | 85.5 ± 3.9 | 0.59 |
| Body mass index (kg/m2) | 25.8 ± 1.0 | 26.9 ± 1.1 | 0.46 |
| Body fat (%) | 20.0 ± 2.2 | 16.7 ± 2.9 | 0.37 |
| Total cholesterol (mg/dl) | 160.4 ± 7.3 | 161.8 ± 4.4 | 0.87 |
| HDL cholesterol (mg/dl) | 38.7 ± 1.8 | 44.6 ± 2.3 | 0.05 |
| LDL cholesterol (mg/dl) | 100.1 ± 6.2 | 100.9 ± 4.8 | 0.92 |
| Triglyceride (mg/dl) | 88.5 ± 9.1 | 82.2 ± 8.4 | 0.61 |
| Glucose (mg/dl) | 88.7 ± 1.5 | 87.4 ± 1.8 | 0.59 |
| White blood cell count (mg/dl) | 6.3 ± 0.3 | 5.7 ± 0.3 | 0.22 |
| C-reactive protein (mg/l) | 1.6 ± 0.7 | 1.9 ± 0.6 | 0.72 |
| eGFR (ml/min per 1.73 m2) | 95.3 ± 3.0 | 102.2 ± 3.9 | 0.17 |
| Peak oxygen uptake (ml/kg per min) | 30.9 ± 1.0 | 31.8 ± 1.5 | 0.61 |
| Family history hypertension (%) | 63 | 68 | 0.74 |
| Family history diabetes (%) | 52 | 42 | 0.52 |
Subject characteristics.
Groups did not differ in heart rate, total heart period duration, systolic ejection duration, diastolic duration, pressure at the inflection point, end systolic pressure or aortic reservoir function (Tables 2 and 3). Group differences in total peripheral resistance (slightly higher in African American men), characteristic impedance (slightly higher in African American men), reflection time (slightly lower in African American men) and diastolic run-off (slightly lower in African American men) approached but did not attain significance (Table 2). Stroke volume, systemic arterial compliance, and SV/PP ratio were significantly lower and effective aortic elastance, AIx, and augmented pressure as higher in African American men compared with white men (p < 0.05, Table 2). There was a significant group difference in LV pressure effort (African American: −290 ± 259 dyn s/cm2 vs. white: −1100 ± 190 dyn s/cm2, p < 0.05).
| Variable | White | African American | p-value |
|---|---|---|---|
| Stroke volume (ml) | 108 ± 4 | 96 ± 4 | 0.03 |
| End systolic pressure (mmHg) | 91 ± 2 | 96 ± 2 | 0.12 |
| Total peripheral resistance (mmHg/l per min) | 0.88 ± 0.04 | 1.03 ± 0.06 | 0.06 |
| Systemic arterial compliance (ml/mmHg) | 4.1 ± 0.3 | 2.6 ± 0.7 | 0.04 |
| Stroke volume/pulse pressure ratio | 3.3 ± 0.1 | 2.9 ± 01 | 0.03 |
| Diastolic run-off (ml) | 68 ± 3 | 61 ± 3 | 0.09 |
| Reservoir function (%) | 64 ± 2 | 64 ± 2 | 0.81 |
| Aortic Zc (mmHg s/ml) | 46 ± 1 | 48 ± 1 | 0.15 |
| Carotid Zc (dyn s/cm5) | 1309 ± 60 | 1392 ± 47 | 0.29 |
| Effective elastance (mmHg/ml) | 0.87 ± 0.04 | 1.04 ± 0.05 | 0.02 |
Vascular parameters.
| Variable | White | African American | p-value |
|---|---|---|---|
| Heart rate (bpm) | 58 ± 2 | 58 ± 2 | 0.97 |
| Heart period (ms) | 1065 ± 39 | 1046 ± 29 | 0.69 |
| LV ejection duration (ms) | 336 ± 5 | 328 ± 3 | 0.13 |
| Diastolic duration (ms) | 728 ± 38 | 718 ± 28 | 0.83 |
| P1 (mmHg) | 33 ± 1 | 34 ± 1 | 0.45 |
| Augmented pressure (mmHg) | −3 ± 2 | −1 ± 4 | 0.03 |
| Augmentation index (%) | −9 ± 2 | −3 ± 2 | 0.04 |
| Reflection time (ms) | 178 ± 6 | 166 ± 3 | 0.09 |
LV, left ventricular; P1, pressure at the inflection point − diastolic pressure.
LV pressure effort and associated components.
African American men had significantly greater R-wave amplitude and QRS duration than white men (p < 0.05, Table 4). There were no group differences in R-wave area, TTI, DPTI, DTF, SEVR or RPP (Table 4).
| Variable | White | African American | p-value |
|---|---|---|---|
| R-wave amplitude (ms) | 1.75 ± 0.1 | 2.11 ± 0.1 | 0.04 |
| R-wave area (ms) | 0.040 ± 0.002 | 0.050 ± 0.004 | 0.10 |
| QRS duration (ms) | 93 ± 9 | 131 ± 15 | 0.04 |
| Systolic TTI (auc) | 2025 ± 84 | 1953 ± 63 | 0.50 |
| Diastolic PTI (auc) | 3349 ± 73 | 3495 ± 75 | 0.18 |
| Diastolic TF | 0.68 ± 0.01 | 0.68 ± 0.01 | 0.72 |
| SEVR (%) | 171 ± 9 | 181 ± 7 | 0.35 |
| RPP (mmHg/min) | 6137 ± 214 | 6381 ± 227 | 0.44 |
TTI, tension–time index; PTI, pressure–time index; TF, time fraction; RPP, rate pressure product; auc, area under the curve.
Measures of LV morphology and myocardial perfusion.
The inter-relationship between measures of aortic function in African American and white men are provided in Tables 5 and 6. Of importance, the various measures of aortic function (compliance, elastance, impedance) were correlated with each other in both African American and white men. Measures of aortic elastance, impedance, and reservoir function were associated with R-wave properties in African American men, but not in white men. QRS duration was not associated with any measure of aortic function in either group of men (p > 0.05). There was no association between LV pressure effort, AIx, end systolic pressure and ECG parameters in either group of men (p > 0.05). There was a significant inverse association between end systolic pressure and stroke volume in African American men (r = −0.40, p = 0.05) but not white men (r = −0.22, p = 0.18). The correlation between aortic Zc and carotid Zc approached statistical significance (r = 0.28, p = 0.055). In African American men, carotid Zc was associated with R-wave amplitude (r = 0.46, p < 0.05) and inversely associated with SV/PP ratio (r = −0.43, p < 0.05). In white men, carotid Zc was associated with elastance (r = 0.49, p < 0.05) and inversely associated with SV/PP ratio (r = −0.41, p < 0.05). Carotid Zc was not associated with any other parameter. The association between aortic reservoir function and measures of cardio-metabolic energetics are shown in Table 7.
| Variable | R-amp | R-area | Ea | Aortic Zc | Reservoir | SAC | TPR |
|---|---|---|---|---|---|---|---|
| R-area | 0.94* | ||||||
| Ea | 0.43* | 0.43* | |||||
| Aortic Zc | 0.38* | 0.32 | 0.68* | ||||
| Reservoir | −0.40* | −0.44* | 0.28 | 0.13 | |||
| SAC | −0.38* | −0.22 | −0.56* | −0.35 | 0.44* | ||
| TPR | 0.55* | 0.47* | 0.78* | 0.54* | −0.05 | −0.51* | |
| SVPP ratio | −0.48* | −0.45* | −0.65* | −0.49* | −0.04 | 0.30 | −0.65* |
Significant correlation (p < 0.05).
Correlations between LV morphology and aortic function in African American men.
| Variable | R-amp | R-area | Ea | Aortic Zc | Reservoir | SAC | TPR |
|---|---|---|---|---|---|---|---|
| R-area | 0.88* | ||||||
| Ea | 0.05 | −0.01 | |||||
| Aortic Zc | 0.24 | 0.07 | 0.56* | ||||
| Reservoir | 0.26 | 0.22 | 0.49* | 0.19 | |||
| SAC | −0.12 | −0.11 | −0.62* | −0.16 | −0.41* | ||
| TPR | 0.14 | 0.21 | 0.65* | 0.44* | 0.36 | −0.25 | |
| SVPP ratio | −0.09 | −0.08 | −0.47* | −0.40* | −0.13 | 0.20 | −0.57* |
Significant correlation (p < 0.05).
Correlations between LV morphology and aortic function in white men.
Discussion
There were several novel findings in the present study. African American men have greater effective aortic elastance than young white men and this adaptation was associated with racial differences in LV morphology. Although there were racial differences in AIx, this was not associated with LV morphology. There were no racial differences in aortic reservoir function, and reservoir function was associated with myocardial oxygen consumption and coronary perfusion in both African American and white men.
Aortic properties and LV morphology
Consistent with previous echocardiographic and electrocardiographic findings,10,30–32 we noted racial differences in measures of LV morphology despite comparable brachial BP in young African American and white men. We have recently shown that brachial BP does not reflect racial differences in vascular burden as aortic BP is higher in young African American men.13 Thus, we hypothesized that LV load would be higher in African American men. To test this hypothesis, we examined several novel measures of vascular load that incorporate varying aspects of current arterial hemodynamic theory.
Effective arterial elastance (Ea) is a measure of the net load imposed on the LV due to systemic functional properties of the vascular tree.24 Unlike other measures of LV afterload which only account for steady-state pressure–flow relationships, Ea takes into account the pulsatile component of blood pressure and flow due to vascular stiffness.33 Integrating such measures as vascular resistance, compliance, characteristic impedance and systolic/diastolic time intervals, Ea correlates well with other measures of arterial load derived invasively from vascular input impedance.24 In support of this, we noted an association between Ea and Zc.
We noted greater effective arterial elastance in African American men compared with young white men. Upon examination of the factors that govern Ea, it can be seen that the African American men had significantly lower arterial compliance, greater total peripheral resistance, and slightly higher characteristic impedance, all contributing to greater LV load. Moreover, Ea was correlated with LV morphology in African American men, as were all components of Ea (i.e. compliance, resistance, impedance), suggesting that differences in large artery function (compliance, impedance) and small vessel tone (total peripheral resistance) may begin to modulate LV morphology at a young age in African American men.
Wave reflection and LV morphology
Ea is based on windkessel theory which treats the cardiovascular system as a closed hydraulic chamber with capacitive (arterial compliance), resistive (vascular resistance), and local inertia (characteristic impedance) components. However, a limitation of this three-element model is that it does not take into account the well established epiphenomena of wave reflection. We noted significant racial differences in AIx. However, this was not associated with measures of LV morphology in our group of young normotensive men. This is likely related to waveform contour. Mean values for AIx and AP were negative signifying that pressure waves arrived during late systole/early diastole (i.e. Type C waveform, see Fig. 1) and the timing/magnitude of reflected waves did not alter absolute peak pressure in the majority of subjects. Therefore the LV did not have to overcome any added pressure from wave reflection. Indeed, the LV pressure effort was negative in both groups of young men and although there were racial differences, there was no “wasted” LV energy per se. Previously, we noted that augmented pressure from arterial wave reflection was not a correlate of another form of target organ damage (carotid intima-media thickness) in young African American men while arterial stiffness was correlated with IMT.13 Our recent findings expand upon this and suggest that arterial stiffness has a greater modulatory influence on LV morphology than wave reflection in young African American men. This is supported by recent findings demonstrating that experimentally reducing compliance via aortic banding can induce LV hypertrophy via alterations in Zc, and this can occur independently of augmented pressure from reflected waves.34
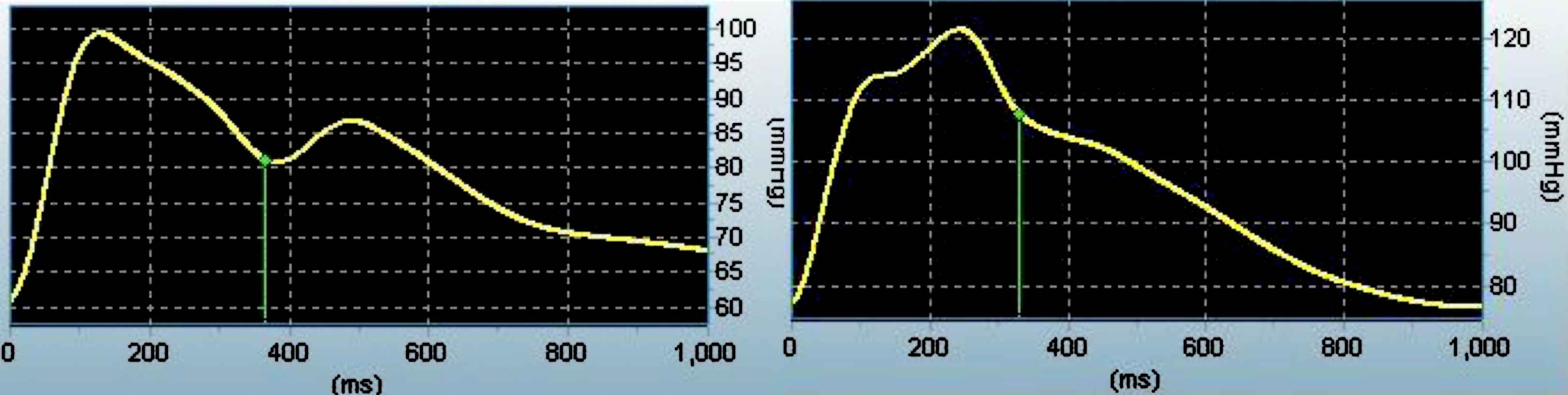
Left: Young white male with a Type C waveform (AI = −11.0%). Right: Young African American male with a Type A waveform (AIx = 17%).
Pulsatile pressure–flow relationships
Zc is defined as the ratio of pulsatile pressure to pulsatile flow in the proximal aorta during early systole before the arrival of reflected pressure waves and quantifies the mechanical reaction of the aorta in opposing the ejection of blood by the LV.35 Zc was slightly higher in African American men, suggesting slightly higher early systolic load. Moreover, pressure at the inflection point (P1), an index of peak LV ejection velocity that is associated with Zc,36–38 was also slightly higher in African American men. Primary wave pressure (P1) increases with age and is associated with aortic stiffness.37,38 Indeed, looking within our data set, we noted an association between aortic PWV and P1 in both white and African American participants (unpublished observation). However in the present study, we did not note any significant racial differences in P1 despite differences in aortic stiffness. Examination of the biophysical/mathematical inter-relations of vascular parameters provides insight into the lack of difference in P1 and Zc between white and African American men.
In general, Zc = (Eh/r5)1/2, where r is radius, E is Young’s elastic modulus and h is wall thickness.39,40 As can be seen from this equation, Zc is modulated substantially by radius (raised to an approximate power of 2.5) and less so by E and h (owing to the square root function). According to the Moens–Korteweg equation, E = (PWV2×D×ρ)/h. There may not be racial differences in vessel diameter.13 However, it is well established that African American men have higher pulse wave velocity (PWV) and slightly greater wall thickness.13 Teleologically, P1 and Zc should be higher in African American men, however owing to the square root relationship of E and h to Zc, changes in vascular stiffness and vascular wall thickness have a relatively smaller effect on impedance. A 20% increase in E or h will produce only a 10% increase in Zc. Therefore, although P1 and aortic stiffness are related in young men, the higher PWV and wall thickness witnessed in young African American men may not be great enough to substantially alter Zc and in turn P1 and it is likely our study was underpowered to detect such subtle differences.
End systolic pressure was 5 mmHg higher in African American men, suggesting that reflected pressure waves likely arrived to a greater extent during late systole in this group, augmenting late systolic load. We noted an association between end systolic pressure and SV in African American but not white men. Systolic tissue velocities vary inversely with arterial afterload, with late systolic load having the greatest influence.26 Rather than contributing to aortic outflow, LV energy was likely used to overcome pressure from wave reflections during late systole in young African American men, contributing to a reduction in ejected stroke volume during the deceleration phase of ventricular outflow.41 In other clinical states, increased late systolic load has been shown to reduce cardiac output,42 and therapeutic interventions that reduce this load increase cardiac output.43 Thus whilst wave reflections may not contribute substantially to LV morphology, they may still impact the systemic circulation in young African American men via modulation of pulsatile pressure–flow associations.
Aortic reservoir and cardio-metabolic efficiency
With each cardiac contraction, energy is imparted to the aorta causing expansion. This potential energy of pressure, along with blood, is briefly stored and upon cardiac relaxation the vessel recoils. Kinetic energy and blood stored in the vessel wall is imparted back to systemic blood flow. This cushioning function of the artery ensures adequate run-off during diastolic decay to the periphery and may also aid in coronary perfusion. Heart–lung preparations have shown that when the reservoir function of the systemic circulation is removed, flow during diastole drops to almost zero. Wave-only theory (i.e. the blood pressure wave is comprised of overlapping incident and reflected pressure waves) neglects the reservoir function of the aorta.44 Aortic reservoir function may be impaired with aging45 but has been shown to be preserved with hypertension17 and in young patients after arterial switch operation,18 despite marked reductions in aortic distensibility.
Diastolic run-off was lower in African American men and this was likely due to greater peripheral vascular resistance preventing adequate run-off to the peripheral microcirculation. Although diastolic run-off was significantly lower in African American men, when expressed as a percentage of total stroke volume, there were no group differences. These findings suggest that there are no racial differences in aortic reservoir function, despite marked differences in arterial elastance and compliance, in young men. Interestingly, aortic reservoir function was associated with measures of myocardial energetics. Aortic reservoir function was inversely associated with myocardial oxygen consumption and positively associated with coronary perfusion/cardiac oxygen supply, cardiac oxygen supply potential and subendocardial perfusion. Aortic reservoir function has been shown to be an important determinant of coronary blood flow.18 Our findings would suggest that aortic reservoir function is preserved in young African American men, possibly as a means of balancing myocardial oxygen supply and demand despite altered ventricular–aortic interactions and LV remodeling.
Limitations of this study should be noted. The Model Flow method has not been validated in different racial/ethnic groups. Although an aortic flow velocity waveform can be reasonably predicted from this model, for the accurate derivation of SV, aortic diameter is also required.19,46 Aortic diameter estimated from prediction models were originally developed from the aortas of white individuals.20 Although studies directly assessing aortic diameter in African Americans are currently lacking, previous studies have alluded to potential racial differences. Fox et al. have noted that the prevalence of aortic regurgitation is greater in the African American population compared with the white population and this is associated with aortic root diameter.47 In light of this, care should be taken when interpreting present findings as several vascular measures were derived from Model Flow SV.
It is worth mention that there is an association between aortic diameter and carotid diameter.48,49 We and others have previously reported no racial differences in carotid diameter,13,50 arguing against variation in vascular geometry in young African American and white men. Moreover, our vascular measures derived from SV noting racial differences are consistent with findings in the literature employing other measures of vascular stiffness/compliance (i.e. ultrasound, pulse wave velocity). Finally, results obtained from aortic Zc using the Model Flow method were similar to those obtained from carotid Zc obtained from ultrasound measures that did not use the Model Flow method. Additional research is warranted to substantiate present findings using more accepted measures of SV and Zc.
In conclusion, LV load is increased in African American men due to greater effective arterial elastance (i.e. reductions in large artery compliance, increases in peripheral artery resistance, slight increases in characteristic impedance) and augmented pressure from wave reflections. In young African American men, LV morphology may be influenced by arterial elastance but not augmented pressure from wave reflections. Aortic reservoir function is preserved in young African American men, possibly as a means of balancing myocardial oxygen supply and demand in the presence of ventricular–vascular uncoupling and LV concentric remodeling.
Acknowledgements
Funding for this project was provided by the American Heart Association (greater mid-west pre-doctoral fellowship) and the American College of Sports Medicine (graduate student research award). We thank all participants for their time and arteries.
References
Cite this article
TY - JOUR AU - Kevin S. Heffernan AU - Bo Fernhall PY - 2009 DA - 2009/03/14 TI - A systematic appraisal of ventricular–aortic load in African American men JO - Artery Research SP - 65 EP - 72 VL - 3 IS - 2 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2009.02.003 DO - 10.1016/j.artres.2009.02.003 ID - Heffernan2009 ER -
