Arterial stiffness and wave reflection: Biomarkers of cardiovascular risk
- DOI
- 10.1016/j.artres.2009.02.002How to use a DOI?
- Keywords
- Arterial stiffness; Aorta; Pulse pressure; Pulse wave velocity; Wave reflection
- Abstract
Arterial stiffness and excessive pressure pulsatility have emerged as important risk factors for cardiovascular disease. Arterial stiffness increases with age and in the presence of traditional cardiovascular disease risk factors, such as hypertension, diabetes and lipid disorders. Pathologic stiffening of large arteries with advancing age and risk factor exposure predominantly involves the elastic aorta and carotid arteries, whereas stiffness changes are relatively limited in muscular arteries. Aortic stiffening is associated with increased pulse wave velocity and pulse pressure, which are related but distinct measures of the pulsatile energy content of the pressure waveform. A dramatic increase in pulsatile energy content of pressure and flow waves in the arterial system places considerable pulsatile stress on the heart, large arteries and distal circulation. Large artery stiffening is associated with abnormalities in microvascular structure and function that may contribute to tissue damage, particularly in susceptible high flow organs such as the brain and kidneys. This brief review summarizes results of recent research on risk factors for and adverse effects of large artery stiffening.
- Copyright
- © 2009 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Interest in the role that arterial stiffening plays in the pathogenesis of cardiovascular disease (CVD) has increased dramatically in the past decade, in large part because of studies that have used pulse pressure as a measure of stiffness. In general terms, if the wall of the aorta stiffens, pulse pressure increases, although there are exceptions to this generality that will be discussed later in this review. Thus, pulse pressure is a widely accessible, if imperfect, indicator of arterial stiffness. Numerous studies performed over the past decade have shown that higher pulse pressures are associated with a moderate increase in the risk for major CVD events, such as myocardial infarction, heart failure, arrhythmia and stroke.1–6 In addition, excessive pressure pulsatility is associated with evidence of microvascular damage and dysfunction,7 which may explain associations between increased pulse pressure and a number of conditions common in older people that are thought to involve a microvascular insult, such as cognitive impairment, macular disease and chronic kidney disease.
The potential scope of the disease burden attributable to increased arterial stiffness is underscored by the change in pulse pressure with advancing age. Pulse pressure increases rapidly after 50 years of age at a time when incidence and prevalence of hypertension and CVD also increase markedly. An analysis of data from the National Health and Nutrition Survey (NHANES) has shown that increased systolic pressure is nearly universal in hypertensives after 50 years of age. In this age range, more than 80% of cases have isolated systolic hypertension (ISH), which represents an isolated or predominant abnormality in pulse pressure.8 Data from the Framingham study has shown that contrary to prior beliefs, ISH arises de novo on a background of normal or high normal blood pressure and is not the terminal phase of longstanding essential hypertension.9 On a population basis, pulse pressure increases by 10 mmHg per decade starting from about 50 mmHg at 50 years of age. Thus, average, but not optimal, pulse pressure in Western societies is roughly equal to age in middle-aged and older people. This level of pulse pressure is not optimal because even after adjusting for age and other potential confounders, each 10 mmHg increase in pulse pressure is associated with a 10–40% increase in risk for various major clinical events. Thus, a pulse pressure greater than 50 mmHg is reason for concern at all ages and should not be ignored in older people just because of the known increase in pulse pressure with advancing age.
Measures of arterial stiffness
Arterial stiffness can be evaluated using a variety of techniques and calculations that do not always change concordantly at a single site or in differing regions of the vasculature. Pulse pressure is a widely used measure of arterial stiffness, but is potentially confounded by factors related to cardiac function, such as heart rate, stroke volume and the pattern of ventricular ejection. Aortic pulse wave velocity (aPWV), which is widely regarded as the present gold standard measure of arterial stiffness,10 is less sensitive to cardiac function and thus may provide a better estimate of aortic stiffness. Measurement and interpretation of PWV is straightforward. Two pressure or flow waveforms are measured a known distance apart and distance between measurement sites is divided by the propagation time delay. As the arterial wall stiffens, waves in the lumen travel at a higher velocity. PWV can be measured along any accessible artery; however, studies have shown that the aorta is the predominant site of pathologic arterial stiffening. Measurements taken at the carotid and femoral arteries give carotid–femoral PWV (Fig. 1), which is a good surrogate of aPWV. This gold standard measure of stiffness has limitations. The distance traveled is not straightforward because as an advancing pressure wave travels up the brachiocephalic and carotid arteries, it also travels around the aortic arch (filled region in the aortic arch in Fig. 1). This parallel transmission complicates assessment of the carotid–femoral transit distance, which is generally estimated by using the suprasternal notch as a fiducial point for the bifurcation site (i.e., the takeoff of the brachiocephalic artery) where parallel transmission begins. Transit distance is then estimated by the notch-to-femoral distance minus the notch-to-carotid distance, i.e., the total distance distal to the bifurcation minus the length of parallel transmission.
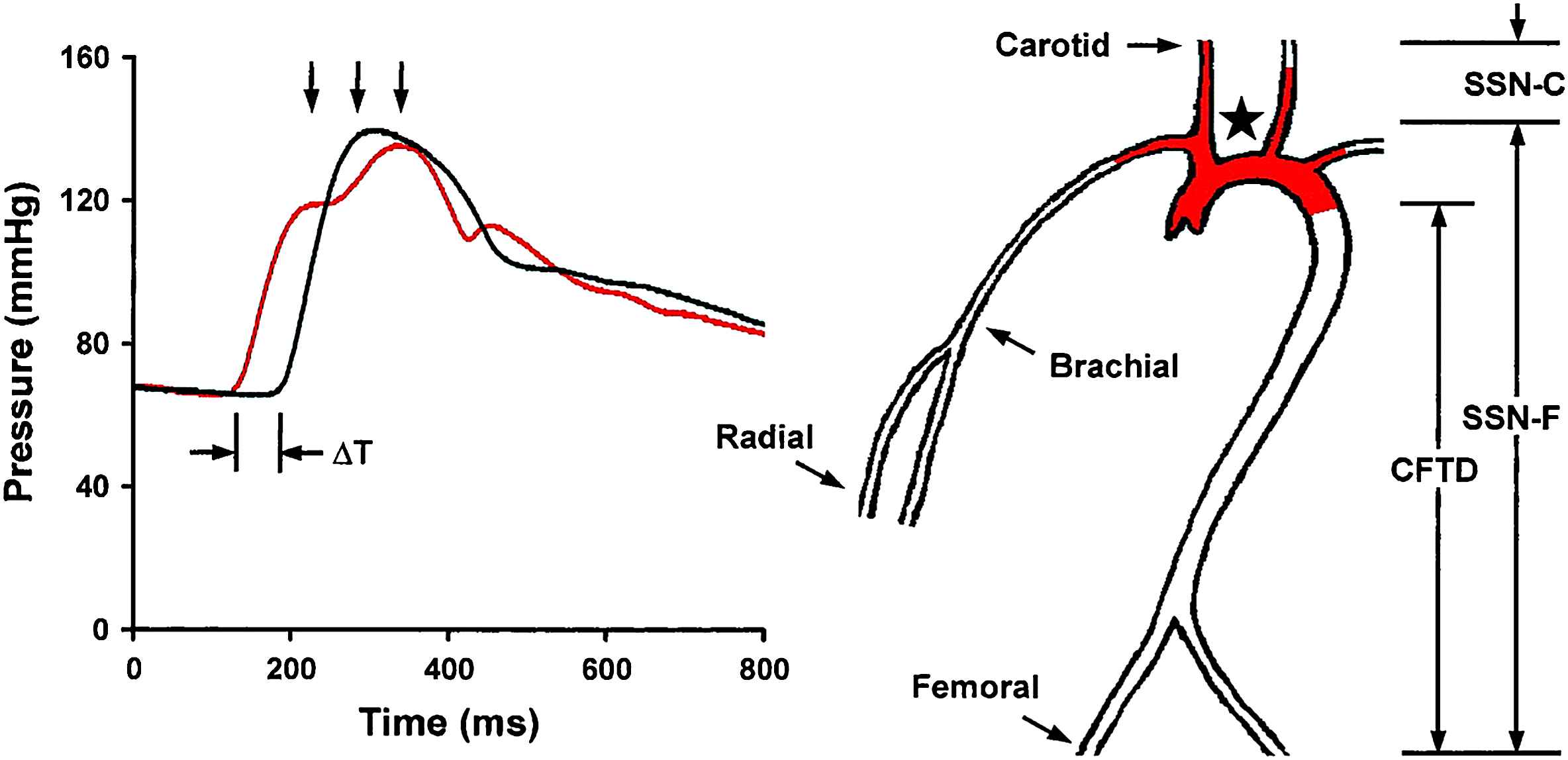
Measurement of PWV. Transit time, ΔT, between the foot of the carotid (red waveform) and femoral waveforms is measured with a tonometer. Carotid–femoral transit distance (CFTD) is estimated by measuring the distance from the suprasternal notch (SSN,) to the carotid (SSN-C) and femoral (SSN-F) sites and taking the difference to account for parallel transmission along the brachiocephalic and carotid arteries and around the aortic arch (red shading). This corrected distance is divided by transit time delay to give PWV. Note that carotid–femoral PWV fails to assess stiffness of the proximal aorta (red shading) (reproduced from Mitchell et al.62).
Parallel transmission of the pressure waveform in the brachiocephalic system and aortic arch implies an additional limitation of aPWV. When measuring carotid–femoral PWV, the advancing pressure waveform has already traversed the aortic arch by the time the waveform is sensed in the carotid artery. As a result, carotid–femoral PWV provides a measure of stiffness of the descending and distal aorta but has a relative blind spot for changes in the aortic arch (filled region in Fig. 1). This is a potentially important limitation because the aortic arch normally provides nearly half of total arterial compliance. The aortic arch can be assessed directly by measuring pressure and flow and using the waveforms to compute aortic input impedance. When pressure and flow are both known, summary measures such as characteristic impedance of the aorta can be derived. Characteristic impedance is analogous to resistance but represents the impedance to pulsatile rather than steady flow. Thus, characteristic impedance defines the change in pressure in the proximal aorta that results from a given change in flow. It is important to recognize that this linear relation between pressure and flow applies only in the absence of wave reflection. Thus, characteristic impedance must be computed from the change in pressure and flow within the first third of systole, before the wave has time to travel down the aorta to branch points and regions of increased stiffness or resistance that act as reflecting sites and return a portion of the wave to the central aorta as a reflected wave. Characteristic impedance, like aPWV, is related directly to stiffness of the arterial wall and inversely to lumen diameter. However, characteristic impedance is five times more sensitive to diameter as compared to aPWV. As a result of these differing relations to geometry, characteristic impedance and aPWV may not always change concordantly.11,12 Pulse pressure is closely related to characteristic impedance, particularly in middle-aged and older people. Thus, pulse pressure and aPWV may not convey the same information regarding aortic stiffness and should be considered complementary rather than redundant.
A number of additional variables have been proposed as measures of arterial stiffness, but they are either too complex to implement in the clinic or have complex relations with arterial stiffness that complicate interpretation. For example, high resolution wall tracking systems have been developed that allow for precise assessment of the pressure–diameter relation of a superficial artery, such as the carotid. However, these systems require a highly skilled operator and expensive equipment. In addition, many older studies employing the wall tracking approach are confounded by using brachial pulse pressure to compute stiffness of the carotid artery. Central and brachial pulse pressure may differ because of the effects of variable timing of wave reflections, leading to a biased estimate of local arterial stiffness when brachial pulse pressure is used to compute central stiffness.
Similarly, measures of wave reflection have been proposed as potential surrogates of arterial stiffness. One such measure is augmentation index, which is the proportion of central pulse pressure that is attributable to a late systolic rise in pressure due to overlap between the forward and reflected pressure waves. The rationale behind augmentation index as a measure of aortic stiffness is that as aPWV increases, reflected waves should return to the heart earlier, leading to progressively more overlap between forward and reflected wave and greater pressure augmentation.13 As we shall see, this seemingly straightforward relation between augmentation index and aortic stiffness may apply in young adults but breaks down after about 60 years of age, at a time when augmentation falls, whereas aPWV, pulse pressure and CVD risk increase dramatically.14,15 These observations suggest that augmentation index may have limited utility as a marker of CVD risk, particularly after 60 years of age.
Risk factors for increased arterial stiffness
Age is an important risk factor for arterial stiffening. Data from the Framingham Heart Study has shown that the prevalence of abnormal carotid–femoral pulse wave velocity increases from a few percent prior to 50 years of age to nearly 70% after 70 years of age.16 Other CVD risk factors are also associated with increased aortic stiffness, whether assessed as carotid–femoral PWV or as forward pressure wave amplitude determined from a calibrated carotid pressure waveform. Thus, diabetes or elevated fasting glucose, obesity, higher heart rate, hypertension and lipid disorders (particularly higher triglycerides and lower HDL) are associated with a stiffer aorta.16 Traditional CVD risk factors as well as measures of arterial stiffness are also related to abnormal endothelial function, raising the possibility of common mechanisms or crosstalk in the relations between arterial stiffness, endothelial function and risk factor exposure. These relations are likely to be bidirectional in that increased stiffness and excessive pressure pulsatility have been shown to impair endothelial function and the endothelium has been shown to modulate arterial properties. If either stiffness or endothelial function is impaired, the potential for feedback exists and a vicious cycle of progressive arterial stiffening and endothelial dysfunction may ensue.
Relations between aortic stiffness, pulse pressure and age
Aortic stiffness increases considerably and nonlinearly with age. Carotid–femoral PWV, a close surrogate for aortic wall stiffness, increases monotonically from young adulthood throughout the entire human lifespan. The age trajectory of carotid–femoral PWV is mildly concave, with the slope increasing modestly at midlife. Augmentation index, a measure of relative wave reflection, increases in parallel with aPWV through midlife, but then reaches a plateau or falls slightly after 60 years of age.14,15 In contrast, pulse pressure and characteristic impedance fall slightly between maturation and midlife, even though aPWV increases,17 and then increase steeply after 60 years of age, at a time when aPWV continues to increase and augmentation index begins to fall. These simple observations suggest a minimal contribution of wave reflection to the prominent increase in pulse pressure after 60 years of age.12,14,18 Furthermore, since cardiac output is known to fall with age in older people, the increase in pulse pressure is attributable primarily to an increase in characteristic impedance of the aorta, leading to increased forward wave amplitude despite constant or falling flow.12,18
The nominal pattern of increasing pulse pressure with advancing age after midlife is accelerated in some individuals, who develop a markedly elevated pulse pressure and systolic hypertension, often with a normal or only mildly elevated mean pressure. The phenomenon of increasing systolic and falling diastolic blood pressure leading to isolated systolic hypertension was once thought to represent the end result of elastin fragmentation in the aorta following decades of “essential” or diastolic hypertension. However, recent work has shown that the majority of cases of isolated systolic hypertension arise on a background of normal blood pressure, without antecedent diastolic hypertension. Indeed, the converse is true — risk for developing mixed hypertension is markedly elevated (7.1-fold) in those with antecedent ISH, suggesting that abnormalities in large artery function (and pulse pressure) may actually precede changes in microvascular function (and mean pressure) in some cases.9 Thus, an accelerated increase in pulse pressure appears to arise de novo in a relatively large subset of individuals who develop ISH.
An early reduction in pulse pressure and characteristic impedance, at a time when aPWV is increasing, followed by a transition into concordant increases in pulse pressure, characteristic impedance and aPWV, suggests that aortic diameter modulates the effects of aortic wall stiffening on pulse pressure.12,17–21 Characteristic impedance and aPWV are similarly related to aortic wall stiffness but characteristic impedance has a markedly (five-fold) higher inverse dependence on aortic diameter.11,12 Thus, when aortic properties change, the effects on characteristic impedance and aPWV may dissociate if a change in diameter is involved. PWV increases whenever a change in wall stiffness exceeds the relative change in diameter. In contrast, if wall stiffness and diameter both increase, characteristic impedance will increase only if the relative change in wall stiffness is more than five times the change in diameter. For example, if one acutely titrates mean arterial pressure across a wide range in an animal, starting from low pressure, aPWV increases monotonically and the increase accelerates at high pressure.11 This pattern provides evidence that changes in wall stiffness exceed the change in lumen diameter across the full range of distending pressure. In contrast, the distending pressure relation is mildly U-shaped for characteristic impedance, which initially falls moving from low to average mean pressure because the effect of distention on wall stiffness is less than five-fold greater than the effect on diameter.22 As distending pressure continues to increase, wall stiffening enters a nonlinear phase where stiffening exceeds diameter change by more than five-fold and characteristic impedance increases. This simple analogy recapitulates age-related change in characteristic impedance and aPWV, but does not explain the changes because the trajectory of mean pressure with age is flat after 60 years of age when characteristic impedance and pulse pressure increase substantially. Furthermore, it is important to recall that carotid–femoral PWV (the usual surrogate for aPWV) and characteristic impedance are measured in differing segments of the aorta. Thus, differential change in characteristic impedance and carotid–femoral PWV may represent differing effects of aortic geography rather than geometry.
Returning to the age-related decline in characteristic impedance prior to 60 years of age, it is possible that the known increase in mean arterial pressure during this period recapitulates the first half of the experiment described above, leading to a seemingly discrepant increase in aPWV yet a fall in characteristic impedance. However distending pressure does not explain the pattern after 60 years of age when mean pressure stabilizes or falls. Attenuation of the rate of increase in aortic diameter with age as the aortic wall continues to stiffen, however, could allow characteristic impedance to increase, although at a slower rate than the increase in aPWV. The early increase in aortic diameter and reduction in characteristic impedance may represent adaptation to other hemodynamic stresses, such as increasing cardiac output in parallel with an increase in body weight. Arteries are well known to remodel to a larger diameter if ambient flow increases and the aorta behaves similarly in this regard.23–25 Alternatively, diameter enlargement in young and middle-aged adults may represent an active adaptation to stiffening of the arterial wall that serves to limit the increase in pulse pressure. This potentially compensatory response may be attenuated in certain individuals or may reach a limit beyond which the increase in diameter abates, possibly in part because of the same mechanisms that cause wall stiffening to accelerate at about the same age.26 Importantly, a modest shift in the age–diameter trajectory of the aorta is amplified by the strong dependence of characteristic impedance on diameter and could lead to a major age-related increase in characteristic impedance and pulse pressure (Fig. 2). Thus, regardless of whether aortic enlargement with age is pathologic or compensatory, blunted enlargement in those with high pulse pressure, or vice versa, may contribute to the observed inverse association between aortic diameter and pulse pressure. Blunted aortic enlargement alone cannot explain a disproportionate increase in characteristic impedance and pulse pressure that exceeds the relative increase in aPWV, as may be seen in some forms of hypertension,12 suggesting that pathologic inward wall thickening or remodeling of the aorta to a smaller lumen diameter may also occur in some cases. Prior work has shown that smaller aortic diameter is related to presence of an atherogenic lipid profile,27 raising the possibility that abnormal endothelial function and inappropriate sensing of resting flow in the aorta may contribute to mismatch between aortic diameter and flow with advancing age and may contribute to the observed inverse relation between aortic diameter and pulse pressure. If this is the case, interventions that improve endothelial function may promote aortic remodeling and restoration of the balance between flow and diameter, potentially reducing pressure pulsatility.
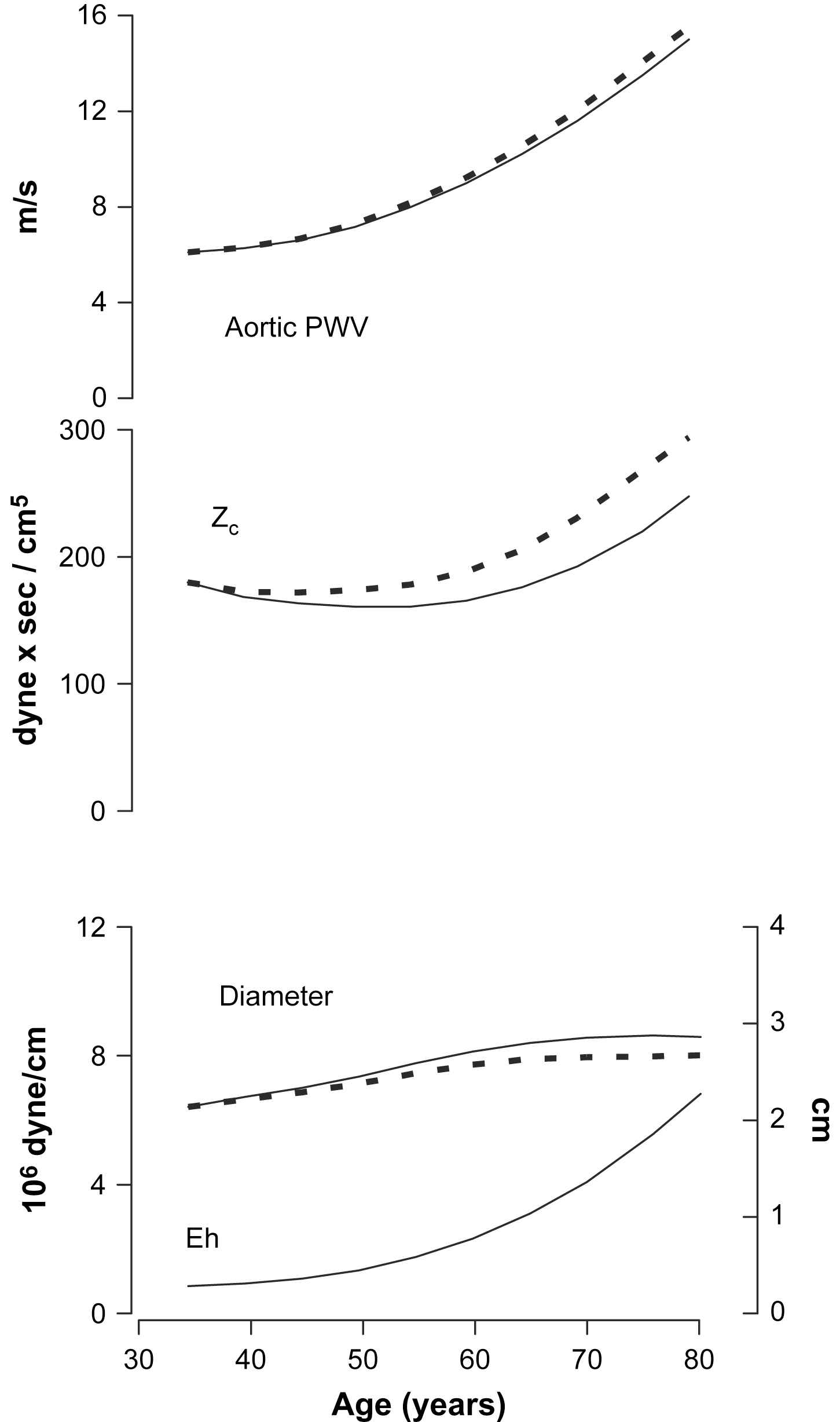
Potential effects of alterations in aortic diameter on pulsatile hemodynamics. PWV increases monotonically during the full lifespan, whereas characteristic impedance (Zc) falls slightly during early adulthood and then increases to high levels in older people. Changes in aortic diameter may mediate these disparate relations between PWV and Zc. The solid line in the aortic diameter plot approximates the known change in aortic diameter with advancing age. A rapid increase in aortic diameter prior to 60 years of age may account for the reduction in Zc even as PWV increases. The broken line in the diameter plot represents a hypothetical aortic diameter trajectory that would produce the accelerated rise in Zc shown with a broken line in the Zc plot. Thus, attenuation of an age-related increase in aortic diameter could manifest as increased Zc and pulse pressure in older individuals, giving rise to the inverse relation between pulse pressure and aortic diameter. Eh, elastance–wall thickness product.
Large artery stiffness and microvascular function
Elevated pulse pressure is a risk factor for several conditions and diseases of aging, such as cognitive impairment,28–30 white matter lesions,31,32 macular degeneration,33 and kidney dysfunction.34,35 These disorders share microvascular abnormalities as a common element of their pathophysiology, suggesting a relation between large artery stiffness and microvascular function.33,34,36–40 Local control of blood flow, particularly in high flow organs such as the brain and kidney, is mediated in part by myogenic tone in the resistance vessels. When perfusion pressure is increased, the vessels constrict to increase resistance and maintain flow at a relatively constant level. Over time, if pressure is persistently elevated, tone is replaced by inward eutrophic or hypertrophic remodeling, leading to a relatively fixed increase in resistance. Microvascular remodeling was once thought to represent a long-term adaptation to elevated mean pressure that limits hyperperfusion, particularly in autoregulated organs such as the brain and kidney. However, work in animal models has shown that myogenic tone and inward eutrophic or hypertrophic remodeling of small resistance vessels may be sensitive to pulse pressure as well as mean pressure.41,42 If this is the case, a disproportionate increase in pulse pressure with no change in mean pressure, as often occurs in older people, could trigger an increase in resistance that compromises resting flow and flow reactivity in flow-sensitive organs such as the brain and kidney.
In order to investigate potential relations between large and small artery function, we evaluated aortic stiffness and forearm hyperemic flow reserve in the Framingham offspring cohort.7 In this middle-aged sample, baseline flow was related to a number of traditional cardiovascular disease risk factors, although the relations were largely positive at baseline and negative during reactive hyperemia, meaning that risk factor exposure was associated with higher resting flow but impaired reactivity. For example, higher levels of heart rate, body mass index, fasting glucose and total/HDL cholesterol ratio and especially active smoking were associated with increased resting flow velocity while higher fasting glucose and mean arterial pressure and antihypertensive treatment were associated with blunted flow reserve. In contrast, prevalent cardiovascular disease and increasing age were associated with lower resting flow and markedly impaired flow reactivity. These observations suggest that microvascular perfusion may be over-driven at baseline in the presence of many risk factors whereas reactivity is impaired. Possible mechanisms for high resting flow include the vasodilatory effects of persistent hyperinsulinemia or hypertriglyceridemia.37,43–45 Smoking was associated with markedly higher resting flow despite the known acute vasoconstrictor effects of nicotine. Nicotine acting through nonneuronal peripheral nicotinic acetylcholine receptors may stimulate diffuse neovascularization and increased flow by increasing growth factor production and release.46 A potential liability of elevated resting flow, particularly in the presence of factors that stiffen the aorta, is that lower impedance may allow additional pulsatility to penetrate into and damage the microcirculation.7 For example, hyperperfusion of the kidney, as is seen in the early stages of diabetic- or smoking-related kidney disease, may increase susceptibility to pulsatile damage, leading to glomerular loss and subsequent progressive loss of kidney function. A similar phenomenon in the brain or in muscular beds may contribute to microvascular damage and tissue loss in those distributions.
After defining correlates of forearm blood flow, we next evaluated relations between arterial stiffness and forearm vascular resistance. We evaluated two measures of aortic stiffness, forward wave amplitude (which is closely related to characteristic impedance of the aorta) and carotid–femoral PWV, which provide information on the amplitude and momentum, respectively, of an advancing pressure wave. Adjusting for potentially confounding risk factors that were related to arterial stiffness and forearm blood flow, we found that both measures of aortic stiffness were associated with higher forearm vascular resistance at rest and particularly during hyperemia. Those individuals in the highest as compared to the lowest tertiles for both stiffness measures had a two-fold higher forearm vascular resistance during hyperemia, indicating markedly impaired flow reactivity in participants with the stiffest aortas. In light of the moderately severe stimulus used in these studies (5 min of ischemia), we suspect that alternations in several vasodilatory pathways are involved in the blunted hyperemic response. In addition, as noted above, increased arterial stiffness may contribute to microvascular structural alterations that cannot be reversed acutely in response to a metabolic or other stimulus. The net effect was markedly impaired flow reactivity in those individuals in this middle-aged and older cohort who had a stiffened aorta. Since increased aortic stiffness is also associated with blood pressure lability, older people with stiffened arteries and impaired microvascular reactivity are a setup for repeated episodes of transient ischemia that may insidiously damage target organs such as the brain and kidneys.40
Arterial stiffness and clinical events
Studies in high-risk47–49 and community-based50–52 samples have demonstrated that pulse pressure and aPWV are important predictors of CVD events. The associations with CVD risk persist after adjustment for potential confounding by conventional CVD risk factors that are also associated with increased arterial stiffness, such as hypertension, diabetes and lipid disorders, suggesting a separate component of risk that is directly attributable to aortic stiffness. Because of the repeatedly demonstrated, consistent and proportional association between aPWV and adverse events in hypertensive individuals, assessment of aPWV was added as a recommended test in the most recent European guidelines for management of hypertension.53,54 The only limitation noted in those guidelines was the lack of widespread availability of aPWV measurements in clinical practice.
Recent studies have suggested that central pulse pressure may be a better predictor of risk than peripheral (brachial) pulse pressure.55,56 However, this assertion has not been demonstrated conclusively. Central and peripheral pulse pressures are highly correlated, particularly in older people at increased risk for CVD. In light of this high collinearity, it will be difficult to demonstrate that knowledge of the small, variable difference between central and peripheral pulse pressure, which contributes relatively little to the total observed variance in pulse pressure, adds significantly to risk prediction. In contrast, differential change in central versus peripheral systolic and pulse pressure in response to therapy, where the full signal is just the change from baseline, may be a different story. In this setting, a 4–6 mmHg differential change in central as compared to peripheral systolic and pulse pressure, because of changes in timing or amplitude of wave reflection, often represents a substantial fraction or even all of the therapeutic effect. As a result, change in central and peripheral systolic pressure may correlate poorly and the differential change in central pulse pressure could potentially provide important insights into what were previously considered the “nonhemodynamic” effects of therapy.57,58
There are several potential mechanisms that could contribute to higher risk in individuals with elevated aPWV. As noted above, increased stiffness is associated with abnormal microvascular structure and function. Abnormal microvascular reactivity may increase susceptibility to intermittent microvascular ischemia and tissue damage. In addition, marked stiffening of the aorta with no change in muscular artery stiffness eliminates the steep ascending stiffness gradient that is present in the normal arterial system. An ascending stiffness gradient moving from heart to periphery creates wave reflection and therefore limits transmission of pulsatility into the periphery. When aortic stiffness reaches and then exceeds stiffness of the muscular arteries, wave reflection at this interface is diminished. We have proposed that this “impedance matching” between aorta and muscular arteries after midlife may contribute to the seemingly paradoxical reduction in measures of wave reflection, such as augmentation index, even as aPWV and pulse pressure increase in older people.14,59 Reduced wave reflection in the larger muscular arteries means that more pulsatile energy penetrates into the small arteries and microcirculation where excessive dissipation of pulsatile energy may cause damage. Thus, a fall in augmentation index after 60 years of age may be a marker of increased transmission of pulsatility into the periphery and increased vascular risk. High flow organs, such as the brain and kidneys are particularly susceptible to this type of pulsatile barotrauma because a greater fraction of pulsatility already penetrates into the microvessels of high flow, low impedance vascular beds.
Arterial stiffening is associated with and likely contributes to the pathogenesis of atherosclerotic disease and ventricular pathology and ultimately to clinical events through these pathways. Increased pulsatile load on the heart promotes ventricular hypertrophy and may culminate in systolic or diastolic heart failure. Resulting strain on the left atrium promotes hypertrophy and fibrosis and increases risk for atrial fibrillation.6 Arterial stiffness, whether assessed as pulse pressure or aPWV, is associated with subclinical and symptomatic atherosclerotic vascular disease and with elevated levels of circulating inflammatory markers.60 It is easy to envision that the inflammation and fibrosis of advanced atherosclerosis might stiffen the wall of larger arteries. However studies in primate models have shown that the onset of diet-induced atherogenesis triggers remodeling that initially reduces aortic wall stiffness as assessed by aPWV.61 In contrast, pulse pressure is more closely related to lumen diameter than wall stiffness as noted above and thus could potentially be increased by atherogenesis if the process compromises the lumen of the aorta to even a modest degree. In support of this hypothesis, aortic lumen area has been shown to be reduced and inversely related to pulse pressure and the presence of an atherogenic risk factor profile.27 Conversely, aortic stiffening and excessive pressure pulsatility enhance regional stresses and flow abnormalities in the central aorta and proximal large arteries and may contribute to the propensity for focally severe atherosclerosis in these regions. Thus, excessive aortic stiffness and increased pressure pulsatility contribute to damage and inflammation in the arterial wall and may represent both a cause and a consequence of atherogenesis. Increased local pulsatile pressures and strains increase the likelihood of plaque rupture and thereby contribute to increased risk of overt clinical events in individuals with atherosclerotic disease.
Future directions
Although much has been learned in recent years, a number of key research priorities remain. We need to refine the arterial stiffness phenotype. Easy-to-use tools for assessing aortic stiffness and wave reflection, and harmonized guidelines for interpretation of these measures, are needed so that validated measures can be included routinely in large scale community-based studies and clinical trials. Our knowledge of molecular processes that contribute to aortic stiffening remains limited, in part because of the lack of widely available tools for assessing stiffness in vivo in humans and in animal models. When these tools are widely available, we will be able to identify additional correlates of aortic structure and function and determine the mechanisms of stiffening associated with traditional and novel vascular risk factors. Repeated measures of risk factors and stiffness over time in community-based studies will help to determine directionality of these associations.
The relation between aortic stiffness and microvascular function requires further study. Does aortic stiffening damage the microcirculation or are the associations confounded by other as yet undetermined factors? Does a primary, diffuse abnormality in microvascular function simultaneously affect aortic function and local control of blood flow in target organs? Additional studies in humans and animal models are needed that relate aortic stiffness to markers of microvascular target organ damage in the heart, brain and kidneys.
Existing data support a valuable role for measures of arterial stiffness, such as aPWV, as markers of CVD risk. Furthermore, several studies have shown that aortic stiffness is modifiable, although a systematic parallel evaluation of the hemodynamic effects of various classes of antihypertensive drugs has not been attempted and is sorely needed. Such a study may help to elucidate mechanisms of increased stiffness and would provide a foundation upon which to construct algorithms for the optimal treatment of hypertension based on the hemodynamic profile of an individual patient. A clearer understanding of the effects of vasoactive drugs on measures of stiffness would facilitate optimal design of an intervention trial that specifically targets arterial stiffness in order to demonstrate the predictive value of a change in arterial stiffness as an indicator of a reduction in clinical events. Such a study would further validate arterial stiffness as a modifiable risk factor for CVD and would provide a rationale for targeting arterial stiffness as a primary endpoint of therapy.
Acknowledgements
Dr. Mitchell’s work has been supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195), by National Institutes of Health grants HL076784, AG028321, HL070100, HL060040, HL080124, HL071039, HL077447 and HL073551, and by grants from Bristol-Myers Squibb, AstraZeneca and the Donald W. Reynolds Foundation.
References
Cite this article
TY - JOUR AU - Gary F. Mitchell PY - 2009 DA - 2009/03/18 TI - Arterial stiffness and wave reflection: Biomarkers of cardiovascular risk JO - Artery Research SP - 56 EP - 64 VL - 3 IS - 2 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2009.02.002 DO - 10.1016/j.artres.2009.02.002 ID - Mitchell2009 ER -
