The association between aortic regurgitation and increased arterial wave reflection
- DOI
- 10.1016/j.artres.2011.07.002How to use a DOI?
- Keywords
- Aortic regurgitation; Arterial wave reflection; Augmentation index
- Abstract
Background: Chronic Aortic Regurgitation (AR) increases left ventricular preload and afterload, which may enhance arterial wave reflection. The effects of AR on noninvasive measures of arterial wave reflection and central aortic pressure are unknown.
Methods: To determine the relation between AR and wave reflection, we prospectively studied 86 subjects with AR and 50 controls referred for echocardiography. Peripheral (P) blood pressures (BP) were measured using an automated sphygmomanometer. Central aortic systolic (CS) BP, central pulse pressure (CPP), central augmented pressure (AP), heart rate corrected augmentation index (AI75), AI, reflected wave systolic duration (ΔTr) and round trip travel time (Tr) were derived from the radial artery waveform obtained by applanation tonometry (Sphygmocor®, Atcor Medical). Pulse pressure amplification (PPA) was calculated as peripheral PP/central PP. There were 50 controls, 50 with mild, 25 with moderate, and 11 subjects with severe AR. Clinical characteristics were similar among the groups.
Results: AP, AI75, and CPP increased in a stepwise manner with increasing AR severity. On analysis of variance adjusted for age, gender, height, weight, mean peripheral BP, ejection fraction, and medication classes, AR severity was independently associated with increased AI75 (p<0.001), AP (p<0.001), CSBP (p=0.04). PPA decreased in a stepwise manner with increasing AR severity (p=0.001). Tr decreased and ΔTr increased.
Conclusions: AR is associated with increased amplitude and duration and earlier onset of the reflected pressure wave, which suggests arterial wave reflection to be a potentially important consideration in patients with AR.
- Copyright
- © 2011 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Chronic aortic regurgitation (AR) is a common valvular disorder, which poses volume and pressure overload on the left ventricle (LV).1 Chronic AR is characterized by increased forward stroke volume and systolic pressure in the aorta with an accompanying decline in the aortic diastolic pressure resulting from diastolic aortic run-off into the LV. Regurgitant volume into the LV may lead to cavity enlargement and a decline in ejection fraction (EF) and/or heart failure symptoms.2 Higher LV systolic pressure and larger cavity size contribute to increased wall stress and greater wall thickness.1,2 Although the prognosis of patients with AR is often benign relative to other valvular disorders, the development of LV dysfunction and/or heart failure symptoms occur at variable rates in individual patients.1,3,4 Although both LV mechanical properties and aortic impedance have been found to be the major determinants of outcome, the long protracted course of patients with AR has been attributed to lowered peripheral resistance caused by peripheral vasodilatation.5 However, chronic AR imposes heterogeneous vascular responses as some patients exhibit reduced arterial elastance and preserved LV function while others have increased arterial elastance and impaired LV function.6 Accordingly, vasodilators which have been expected to lower aortic impedance have yielded variable results in terms of clinical improvement.7,8 Therefore, the mechanisms of progressive LV dilation and dysfunction remain incompletely understood.9
In recent years, there has been a growing awareness of the importance of arterial wave reflection in understanding arterial function and ventricular-arterial interactions.10 Increased arterial pressure wave reflection imposes greater workload and higher oxygen demand on the LV. Increased wave reflection also contributes to systolic hypertension and LV hypertrophy, and has been found predictive of higher cardiovascular event rates.10 The effects of AR on noninvasive indices of arterial wave reflection and central aortic pressure have not been well studied. Accordingly, the objective of this study was to evaluate central aortic and wave reflection indices using applanation tonometry in subjects with varying severity of AR.
Methods
We prospectively studied 86 subjects with AR and 50 age and sex matched controls without AR who were referred for transthoracic echocardiography. Patients with more than mild aortic stenosis or mitral stenosis on echocardiography were excluded. All patients were in sinus rhythm without atrial fibrillation or paced rhythms. Clinical data were recorded from medical records at the time of echocardiographic evaluation. The study was approved by the institutional review board and written consent was obtained. Procedures followed were in accordance with institutional guidelines. CV risk factors and disease history including hypertension, diabetes, hypercholesterolemia, known coronary artery disease evidenced by a history of myocardial infarction, revascularization, or abnormal stress perfusion imaging, congestive heart failure history, smoking and current medications were identified by subject interview. Medication classes considered were angiotensin converting enzymes inhibitors or receptor blocking agents, calcium channel blocking agents, other vasodilators medications and diuretics. Subjects were excluded if pulses were not adequate to have arterial tonometry performed.
Applanation tonometry
After a 4-h fast (including caffeine, nicotine, and alcohol), studies were performed in a quiet, temperature controlled (22–24°C) room in the morning (8 a.m.–10 a.m.). Medications were not withheld. Participants were allowed to rest for 10min in the supine position. Baseline central arterial blood pressures (CBPs), heart rate corrected augmentation index (AI75) and augmentation pressure (AP) were obtained by applanation tonometry (Sphygmocor, Software version 8.2; AtCor Medical, New South Wales, Australia), according to previously published methods.11 The round trip travel time (Tr) of the pressure wave to and from the major reflecting sites in the lower body was determined from the aortic pressure waveform.12 The systolic duration of the reflected pressure wave (ΔTr) was determined from the inflection point to the incisura.10 (Tr+ΔTr) represents LV ejection duration (ED). Since the ED has been shown to be HR dependent, both the uncorrected ED and ED corrected for HR (EDc) are reported.13 Indices of LV workload and myocardial oxygen demand were also derived from the pressure waveform using the technique of pulse wave analysis.10 Wasted LV pressure energy (Ew) is defined as the extra energy that the LV must generate to overcome the augmented pressure, and does not contribute to blood flow production. Wasted energy is estimated from the equation Ew=2.09 AP(ED-Tr).14 The wasted energy index (EwI) is defined as the ratio of the Ew to the total energy generated by the LV in systole and is expressed as a percentage. The total energy was calculated as 1.33×(ED)×(mean pressure in systole) as reported previously.14 The systolic tension time index was obtained as the area under the systolic (AS) portion of the aortic pressure wave and that has shown to be related to work of the heart and to myocardial oxygen consumption.15 The area under the diastolic portion (AD) of the aortic waveform is associated with coronary perfusion and is an approximation of the energy supply of the heart. The ratio of supply and demand is termed the subendocardial viability ratio (SEVR) or Buckberg index (SEVR=AD/AS).16 Pulse pressure amplification (PPA) is defined as peripheral pulse pressure/central pulse pressure.
Echocardiographic data
Transthoracic Echocardiographic studies were performed using Phillips Sonos 5500 and 7500 echocardiography machines (Phillips, Andover, Mass.). Two observers blinded to hemodynamic and clinical data performed the consensus echo interpretation. Echocardiographic variables pertaining to the LV included end-diastolic diameter and ejection fraction (EF). LV mass was calculated using the American Society of Echocardiography (ASE) formula and the LV EF was calculated by biplane modified Simpson’s method.17 With regard to AR, the AR/LVOT ratio was measured at the level of the aortic valve from the most optimal long-axis view.18 For each patient, end-diastolic diameter, LV EF, and AR/LVOT ratio were measured. The severity of AR was determined from calculation of the ratio of the height of the AR color jet to the height of the LV outflow tract (AR/LVOT) according to ASE criteria: mild, <0.25; moderate, 0.25–0.64; severe, ≥0.65.18
Statistics
Data are expressed as mean±standard deviation (SD). Categorical variables are presented as absolute values (percentages). Continuous variables were compared using Student t-test and Fischer exact test was used to compare frequencies of dichotomous variables. GLM ANOVA (analysis of variance) adjusted for age, gender, height, weight, mean arterial pressure (MAP), EF, and medication classes was performed to determine independent association between indices of wave reflection and aortic regurgitation. Bonferroni correction was applied to the model for multiple comparisons. All statistical analyses were achieved using the Statistical Package for Social Sciences (SPSS) 18.0 software (SPSS Inc, Chicago, IL). A p value <0.05 was considered significant.
Results
Patient characteristics are shown in Table 1. There were 50 controls who were age and gender matched. Among patients with AR, 50 subjects had mild, 25 had moderate, and 11 subjects had severe AR. All patients were in sinus rhythm (86±10beats/min). Clinical characteristics were similar between controls and the 3 groups of AR patients. LV mass increased and LVEF decreased in a stepwise manner as the severity of AR increased from controls to mild to moderate to severe AR. Table 2 summarizes the hemodynamic differences among the different categories of aortic regurgitation. All analyses were performed using GLM ANOVA (analysis of variance) adjusted for age, gender, MAP, height, weight, and LVEF.
| Variables | Control | Mild | Moderate | Severe | p Value |
|---|---|---|---|---|---|
| Age (years) | 68 (67–69) | 71 (67–75) | 68 (60–76) | 64 (51–76) | 0.26 |
| Males (%) | 45 | 44 | 48 | 67 | 0.54 |
| Weight (kg) | 83.2 (76–90) | 74.9 (71–79) | 73.2 (66–80) | 80.6 (68–93) | 0.10 |
| Height (m) | 1.66 (1.63–1.68) | 1.68 (1.65–1.70) | 1.66 (1.63–1.70) | 1.72 (1.65–1.78) | 0.23 |
| BMI (kg/m2) | 27.9 (24–32) | 26.4 (25–28) | 26.4 (24–29) | 26.8 (24–30) | 0.61 |
| LV mass (g) | 165.9 (138–194) | 200.4 (178–223) | 206.0 (174–237) | 278.5 (229–328) | <0.01 |
| LVMI | 87.7 (68–108) | 109.6 (98–121) | 114.3 (100–128) | 141.2 (119–163) | <0.01 |
| EF (%) | 58 (53–64) | 46 (41–51) | 47 (41–53) | 36 (26–46) | <0.01 |
| SV (ml) | 81 (75–87) | 66 (50–83) | 128 (78–178) | 115 (83–147) | <0.01 |
| HTN (%) | 80 | 83 | 89 | 92 | 0.67 |
| CHOL (%) | 55 | 46 | 48 | 25 | 0.30 |
| DM (%) | 44 | 28 | 33 | 58 | 0.31 |
| CAD (%) | 26 | 27 | 41 | 42 | 0.39 |
| CHF (%) | 32 | 30 | 30 | 67 | 0.09 |
| Smoker (%) | 10 | 6 | 4 | 9 | 0.71 |
BMI, body mass index; LV mass, left ventricular mass; LVMI, left ventricular mass indexed to body surface area; EF, ejection fraction; SV, stroke volume; HTN, hypertension; CHOL, cholesterol; DM, diabetes mellitus; CAD, coronary artery disease; CHF, congestive heart failure, kg; kilograms, m, meters, g, grams; ml, milliliters. Data presented as mean (95% confidence interval).
Clinical and demographic information.
| Variable | Control | Mild | Moderate | Severe | p Value |
|---|---|---|---|---|---|
| AP (mmHg) | 5 (1–9) | 12 (10–14) | 13 (10–16) | 19 (14–24) | <0.001 |
| AI75 (%) | 12 (7–16) | 21 (18–24) | 23 (19–27) | 34 (28–41) | <0.001 |
| CSBP (mmHg) | 116 (112–120) | 120 (118–123) | 123 (119–126) | 125 (119–131) | 0.04 |
| CPP (mmHg) | 38 (31–45) | 44 (40–48) | 47 (42–53) | 56 (47–65) | 0.02 |
| CDBP(mmHg) | 76 (73–79) | 76 (75–78) | 75 (73–77) | 75 (72–79) | 0.89 |
| Tr (ms) | 146 (138–154) | 133 (129–138) | 137 (131–143) | 125 (114–136) | 0.02 |
| ΔTr (ms) | 133 (115–151) | 159 (149–170) | 170 (156–184) | 184 (160–209) | <0.01 |
| EW (dynes/cm2) | 1272 (505–3048) | 4559 (3506–5611) | 5251 (3836–6666) | 7794 (5321–10266) | <0.001 |
| PPA | 1.48 (1.40–1.56) | 1.35 (1.31–1.40) | 1.34 (1.28–1.39) | 1.22 (1.12–1.32) | 0.001 |
| ED (ms) | 278 (261–294) | 288 (278–297) | 307 (294–320) | 309 (288–331) | 0.01 |
| EDc (ms) | 407 (393–420) | 405 (397–412) | 425 (415–436) | 423 (406–441) | 0.009 |
AP, augmented pressure; AI75, augmentation index corrected to heart rate of 75 beats/min; CSBP, central systolic blood pressure; CPP, central pulse pressure; CDBP, central diastolic blood pressure; Tr, round trip travel time; ΔTr, systolic duration of reflected wave; EW, wasted energy effort; PPA, pulse pressure amplification ratio; ED, ejection duration; EDc, heart rate corrected ejection duration.
Data presented as mean (95% confidence interval) adjusted for height, weight, age, gender, ejection fraction and mean arterial pressure. Bonferroni correction was used for multiple comparisons.
Hemodynamic parameters for categories of aortic regurgitation.
Characteristics of reflected wave
There was a stepwise increase in AI75, AP, CSBP, and CPP with increasing severity of AR (Fig. 1). PPA decreased in a stepwise manner with the severity of AR (p=0.001). These relationships did not change after adjusting for medication classes. Both ED and ΔTr increased progressively (p=0.01 and p<0.01 respectively), while Tr decreased (p=0.02) with increasing AR severity.
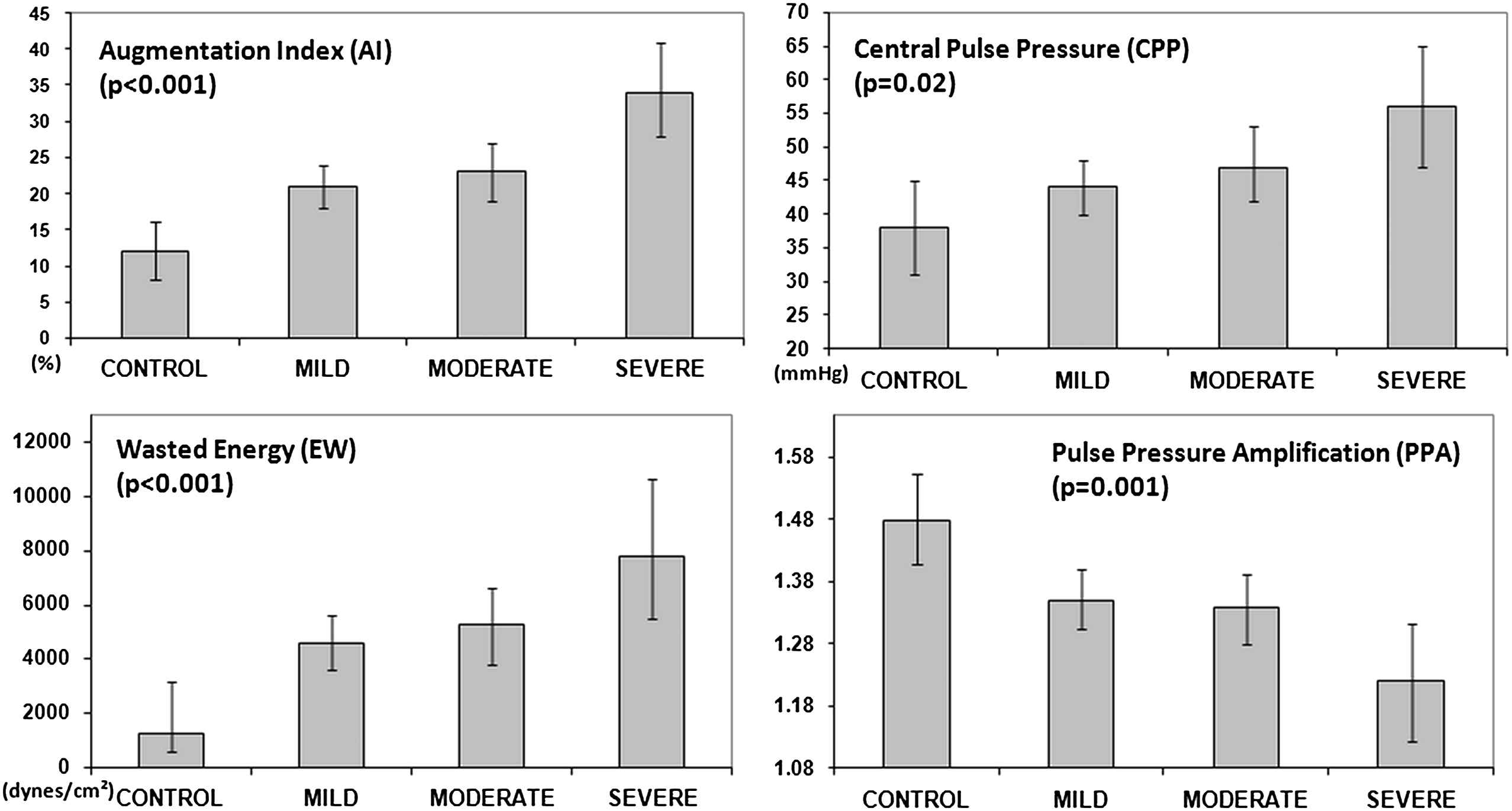
Central pressure and wave reflection indices in control, mild, moderate and severe aortic regurgitation groups. Data is presented as mean values after adjustment of covariates. Error bars represent 95% confidence interval.
Measures of LV workload and oxygen demand
Both Ew and EwI increased in a stepwise manner with progressive degrees of AR (both p<0.001). SEVR also was related to the severity of AR (p=0.04).
Discussion
This study characterized changes in noninvasively determined central arterial BPs and arterial wave reflection indices in patients with varying severities of AR, and compared them to age and gender matched controls. The major finding was that AR was associated with an increase in the amplitude and duration of the reflected pressure wave. Patients with AR also had a lower round trip travel time indicating earlier arrival of the reflected wave. Dose response relationships were observed between AR severity and indices of wave reflection, LV load and wasted energy. These findings collectively suggest enhanced wave reflection with increasing AR contributes to higher LV workload and indices of myocardial oxygen demand.
To our knowledge, this is the first study to characterize wave reflection indices obtained noninvasively in the setting of AR. Prior studies that have characterized hemodynamic alterations associated with AR have used invasive cardiac catheterization. Few prior studies have evaluated arterial stiffness, a distinct property of the arterial system, in the setting of AR. Razzolini compared 11 patients with AR to 23 normal subjects and found markedly reduced aortic compliance using a mono-exponential diastolic decay methodology.19 Similarly, Segers et al. used a computer model of heart–arterial interaction to determine that arterial elastance, calculated as the ratio of LV end-systolic pressure and stroke volume (a measure of arterial stiffness), is determined by aortic leak severity rather than by arterial system properties.20 Devlin also evaluated arterial elastance and found individual patients to have heterogeneous responses to AR with some patients exhibiting increased and others decreased arterial elastance.6 He observed greater AR severity was accompanied by higher arterial elastance. Kopel et al. found increased carotid arterial distensibility in a group of younger patients with chronic AR and postulated that greater arterial compliance to be a compensatory mechanism aimed at lessening the impact of systolic volume ejected into conduit arteries.21 Varying conclusions in these studies likely result from differing study methods and patient populations. In addition, there is a complex interplay between altered loading conditions due to AR, LV function, and the arterial system. A combination of these factors may serve to increase wave reflection in patients with AR.
The present study expands upon prior hemodynamic observations in patients with aortic regurgitation by examining arterial wave reflection noninvasively using applanation tonometry. Additionally, the use of wave reflection indices in valvular heart disease lies outside the usual application of wave reflection measurements in distinguishing central from peripheral artery pressures in the management of hypertension and in the evaluation of cardiovascular risk. In recent years, there has been a growing use of this technique to noninvasively measure central pressures and indices of arterial stiffness and waveform reflection.10 In general, aging and atherosclerosis are associated with arterial stiffening, which increases the amplitude and velocity of the reflected wave.22,23 The characteristics of reflected wave are dependent upon a complex interplay of factors including: LV function, large artery elasticity, small artery compliance, wave velocity, reflective sites distance, and heart rate. The reflected wave occurs earlier in the cardiac cycle and may shift from diastole to systole, causing an increase in late systolic pressure. Therefore, higher amplitude and earlier wave reflection impact negatively on ventricular–arterial coupling by contributing to isolated systolic hypertension and reducing diastolic coronary perfusion.
The observed findings were not unexpected. Years before the widespread use of applanation tonometry in clinical studies, Razzolini suggested AR to be accompanied by a large reflection wave, which augmented aortic pressure.19 Similar changes in carotid Doppler spectral waveforms have been reported in patients with AR.24 In addition to a greater forward LV stroke volume, the LV would be expected to simultaneously generate a higher amplitude pressure wave, which would be transmitted and reflected to yield a larger reflected wave. In support of our findings, we previously found water immersion to increase the AI.25 The stepwise prolongation ΔTr is consistent with the longer ED and shortened Tr that we found with increasing AR. In addition, the presence and severity of AR was associated with increases in Ew and SEVR. In addition, ED lengthened, suggesting increased LV load and augmented function. Therefore, enhanced wave reflection in the setting of AR may contribute to increased ventricular afterload.
The ability to noninvasively measure wave reflection indices may allow for better evaluation of ventricular–vascular coupling and physiologic assessment in patients with AR. This may also have implications for medical therapy. Studies that have evaluated vasodilators in the setting of AR have provided conflicting results.1–3 As arterial stiffness and wave reflection have been proposed as therapeutic targets in the treatment of hypertension, these arterial properties might serve a similar role in the setting of AR. Further studies are required to establish proof of concept.
Several additional observations merit consideration. Age was not an independent determinant of wave reflection indices as generally expected. Therefore, the presence of AR may attenuate age-related changes in arterial wave reflection. Also, the inverse relationship between PPA and AR severity initially appears counterintuitive as this implies a higher aortic PP relative to brachial artery PP. AR has classically been described as producing findings of peripheral arterial pressure amplification, this notion based on the presence of overshoot in femoral artery sheath pressure.26 However, evaluation of the aortic waveform shows there is an increase in CPP to a greater degree than PPP with increasing AR severity, thus lowering the PPA. There may be several reasons for this, such as increased aortic stiffening due to degenerative wall changes and decreased elastin content in those with more severe AR, endothelial dysfunction, or functional changes such reduced effective aortic diameter and higher impedance due to altered pressure-flow relationships.27 Also, peripheral arterial pressure amplification has been attributed to high LV ejection velocities, which may not be present in this study population as depression of LV function was evident by reduction of mean LV EF in the AR groups. Lastly, although mean LV EF was lower in patients with more severe AR, the higher AI75 in patients with AR was most likely related to the AR rather than the LV EF, given that lower LV EF is associated with decreased wave reflection.28
Limitations
This study is subject to the limitations of a cross-sectional study. Small sample size as well as uncertainty of AR etiology limited our ability to detect whether there were differences in wave reflection indices in patients with leaflet versus aortic root causes of AR. Although no patient was known to have acute severe AR, we could not assess the duration of AR. We determined the ΔTr in the standard manner, from the inflection point to the incisura, however this may not represent the entire reflected wave systolic duration. We conclude that the presence and severity of AR is associated with a greater magnitude and longer duration of the reflected arterial wave. The specific mechanism responsible for these findings as well as the prognostic value of wave reflection measures and their potential in guiding therapy in patients with AR merits further study.
References
Cite this article
TY - JOUR AU - Haroon Kamran AU - Louis Salciccioli AU - Carl-Frederic Bastien AU - Abhishek Sharma AU - Jason M. Lazar PY - 2011 DA - 2011/08/11 TI - The association between aortic regurgitation and increased arterial wave reflection JO - Artery Research SP - 49 EP - 54 VL - 6 IS - 1 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2011.07.002 DO - 10.1016/j.artres.2011.07.002 ID - Kamran2011 ER -
