Central haemodynamics reveal significant potential for prevention in Black hypertensive patients born and living in sub-Saharan Africa
On behalf of the NOAAH investigators.
- DOI
- 10.1016/j.artres.2011.11.002How to use a DOI?
- Keywords
- Arterial stiffness; Augmentation index; Black Africans; Prevention; Pulse wave velocity
- Abstract
Background: Few studies assessed arterial stiffness in Black hypertensive patients born and living in sub-Saharan Africa, where cardiovascular disease reaches epidemic proportions.
Methods: The Newer versus Older Antihypertensive Agents in African Hypertensive Patients (NOAAH) trial is currently recruiting native African patients to compare the efficacy of various antihypertensive drugs given once daily as single-pill combinations. Two centres engaged in pulse wave analysis and measured carotid–femoral pulse wave velocity (PWV). Statistical methods included single and multiple linear regressions.
Results: Of 172 patients screened, 116 entered the ancillary study on central haemodynamics (51.3% women; mean age 52.7 years; untreated blood pressure 147.6/87.1mmHg). The augmentation indexes were higher (p<0.0001) in women than men, both peripherally (pAI, 11.1 vs. −10.6%) and centrally (cAI, 39.0 vs. 28.0%). PWV (8.91m/s) and central pulse pressure (cPP, 48.7mmHg) were similar (p>0.844) in both sexes. pAI and cAI increased with female sex and mean arterial pressure, but decreased with heart rate and body mass index. cPP increased with age and mean arterial pressure. PWV increased with age and mean arterial pressure. Patients with measurements above the age-specific thresholds determined in healthy Black South Africans amounted to 0 for cAI, 1 (1.2%) for cPP, and 11 (18.3%) for PWV.
Conclusion: NOAAH patients have measures of arterial stiffness similar to those of a healthy Black reference population with determinants as reported in the literature. Our observations highlight the potential for the prevention of irreversible arterial damage by timely treating sub-Saharan hypertensive patients to target blood pressure levels.
- Copyright
- © 2011 Published by Elsevier B.V. on behalf of Association for Research into Arterial Structure and Physiology. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
The prevalence of hypertension is dramatically increasing in sub-Saharan Africa. From almost being nonexistent nearly 70 years ago, more than 35% of the adult population is currently hypertensive in many African countries.1,2 Out of the 57 million deaths worldwide in 2008, 63% were from non-communicable disorders and principally from cardiovascular disorders.3 Most cardiovascular complications of hypertension in Africa occur at younger ages compared to that in the developed countries.3 Hypertension is a reversible risk factor,4 but studies of antihypertensive drugs done in African Americans cannot be readily extrapolated to Blacks born and living in Africa, because of differences between African and American Blacks in natural selection,5 ethnic admixture,6 living environments and life style.7 To address this issue, we mounted the Newer versus Older Antihypertensive Agents in African Hypertensive Patients (NOAAH) trial to compare in native African patients a single-pill combination of newer drugs, not involving a diuretic, with a combination of older drugs including a diuretic.8
Hypertension accelerates the age-related9,10 increase in arterial stiffness, which is an independent predictor of cardiovascular outcome.11 Applanation tonometry allows measuring several indexes of arterial stiffness and central haemodynamic variables in a non-invasive way,12,13 but most studies in Blacks were done outside Africa, principally in African Americans14,15 and Caribbeans.15,16 In a report involving indigenous South Africans, Shiburi and coworkers17 proposed thresholds for central haemodynamic indexes pending their validation in prospective outcome studies. We studied the applicability of these thresholds in untreated patients with mild-to-moderate hypertension, who were enrolled in the run-in phase of the NOAAH trial.8
Methods
Recruitment of patients
The Ethics Committees of the University of Kinshasa and all participating centres approved the protocol of the Newer versus Older Antihypertensive Agents in African hypertensive Patients (NOAAH) trial (NCT010130458).18 NOAAH is an investigator-led clinical trial and complies with the Helsinki declaration.19 Patients aged 30–69 years with uncomplicated hypertension (140–179/90–109mmHg) and 2 or fewer associated risk factors are eligible. After a 4-week run-in period off treatment, 180 patients will be randomised to once daily bisoprolol/hydrochlorothiazide 5/6.25mg or amlodipine/valsartan 5/160mg. To attain and maintain blood pressure below 140/90mmHg during 6 months of follow-up, the doses of bisoprolol and amlodipine in the combination tablets will be increased to 10mg/day with the possible addition of α-methyldopa or hydralazine. NOAAH is powered to demonstrate a 5-mmHg between-group difference in sitting systolic pressure with a 2-sided p-value of 0.01 and 90% power.
The recruiting centres are located in Cameroon (Doula and Yaoundé), Gabon (Libreville) Nigeria (Enugu and Ilorin) and Senegal (Dakar). Two centres (Doula and Enugu) opted to take part in the ancillary study on arterial stiffness and central haemodynamics. On 21 July 2011, of 172 patients screened for entry at these 2 centres, 116 (67.4%) had entered the ancillary study on central haemodynamics, but 32 had no measurements before randomisation. The remaining patients, 84 had pulse wave analysis and 60 had pulse wave velocity measurements before randomisation while untreated.
Haemodynamic measurements
To ensure steady state, participants were invited for the haemodynamic measurements in the morning and asked to refrain from smoking or drinking any alcoholic beverage or coffee for 2h before the examination. After they had rested in the supine position for 10–15min, we recorded during an 8-s period the radial waveform at the dominant arm by applanation tonometry using a high-fidelity SPC-301 micromanometer (Millar Instrument, Inc., Houston, Texas, USA) interfaced with a laptop computer running SphygmoCor software, version 7.1 (AtCor Medical Pty. Ltd., West Ryde, New South Wales, Australia). We discarded recordings when the systolic or diastolic variability of consecutive waveforms exceeded 5% or the amplitude of the pulse wave signal was less than 80mV. We calibrated the pulse wave by blood pressure measured using a validated20 oscillometric OMRON 705IT recorders (OMRON Healthcare Europe BV, Nieuwegein, Netherlands) according to the guidelines of the European Society of Hypertension21 immediately before the recordings. From the radial signal, the SphygmoCor software calculates the aortic pulse wave by means of a validated and population-based generalized transfer function. The peripheral (radial) augmentation index was defined as the ratio of the second to the first peak of the pressure wave expressed in percent. The central (aortic) augmentation index was the difference between the second and the first systolic peak given as a percentage of the aortic pulse pressure. Central pulse pressure was the difference between systolic and diastolic blood pressure derived from the transfer algorithm of the SphygmoCor. Mean arterial blood pressure was diastolic pressure plus one-third of central pulse pressure.
Aortic pulse wave velocity was measured by sequential recordings of the arterial pressure waveform at the carotid and femoral arteries. Distances from the suprasternal notch to the carotid sampling site (distance A) and from the suprasternal notch to the femoral artery (distance B) were measured. The pulse travel path was distance B minus distance A. Pulse transit time was averaged over 10 consecutive beats. Aortic pulse wave velocity was the ratio of the distance in meters to the transit time in seconds.
In each centre, a single observer (M.K.K. and B.C. A.) did all the SphygmoCor measurements. Based on repeat examination of 10 patients in each centre, we computed the coefficient of variation as the standard deviation of the pairwise differences between duplicated measurements divided by its mean. The intra-observer variability was around 8.1% for cAI, 10.0% for pAI and 9.6% for PWV, respectively.
Other measurements
Using a standardised questionnaire, we obtained information on each subject’s medical history, smoking and drinking habits, and use of medications. Fasting venous blood was collected to measure the concentration of total cholesterol, blood glucose, and serum creatinine.
Statistical methods
For database management and statistical analyses, we used the SAS software, version 9.2 (SAS Institute Inc, Cary, North Carolina, USA). Departure from normality was evaluated by Shapiro–Wilk’s statistic.22 Skewness is the third moment about the mean divided by the cube of the standard deviation.22 Kurtosis is the fourth moment about the mean divided by the square of the variance of the distribution.22 The central tendency and spread of the data are reported as mean±SD or as median (interquartile range). Our statistical methods also included Student’s t-test for unpaired observations to compare means and single and stepwise multiple regressions to identify correlates of the central haemodynamic measurements. In stepwise regression, P-values for covariables to enter and stay in the models were set at 0.15. The covariables considered were sex (1, 0 for women and men, respectively), age, body mass index, mean arterial pressure, smoking (0, 1), alcohol intake (0, 1), serum cholesterol, blood glucose, and serum creatinine.
Results
Characteristics of the participants
The 84 participants included 43 women (51.2%) and 28 treatment naïve patients (33.3%). No patient had diabetes mellitus. The participants’ age ranged from 33 to 68 years, averaging 51.7±8.1 (SD) years. Brachial blood pressure measured in the supine position averaged 143.8±15.9mmHg systolic and 84.0±10.6mmHg diastolic.
In all participants, the peripheral augmentation index was skewed to the left (P=0.032; skewness: −0.256; kurtosis: −0.871; Kolmogorov–Smirnov statistic: 0.102) with similar findings for the central augmentation index (P=0.006; skewness: −0.382; kurtosis: −0.369; Kolmogorov–Smirnov statistic: 0.117). The central pulse pressure was normally distributed (P=0.20; skewness: −0.255; kurtosis: −0.141; Kolmogorov–Smirnov statistic: 0.067). The distribution of pulse wave velocity was skewed to the right (P<0.001; skewness: 0.702; kurtosis: 0.215; Kolmogorov–Smirnov statistic: 0.180).
Table 1 lists the characteristics of the 84 participants by sex. Women were smaller and weighed less than men did, but body mass index was similar in both sexes (P=0.52). The peripheral and central augmentation indexes were higher in women than in men, but all other haemodynamic measurements were similar in women and men. The subgroup of 60 patients, who underwent a measurement of pulse wave velocity in addition to pulse wave analysis had similar characteristics as the whole study group.
| Characteristic | Women | Men | P-value |
|---|---|---|---|
| Anthropometrics | |||
| Women, n | 43 | 41 | 0.74 |
| Age, years | 52.0±8.1 | 51.4±8.2 | 0.72 |
| Height, cm | 161.0±6.3 | 170.6±6.1 | <0.0001 |
| Weight, kg | 76.0±17.0 | 83.3±16.6 | 0.051 |
| Body mass index, kg/m2 | 29.3±6.6 | 28.5±4.9 | 0.52 |
| Peripheral haemodynamics | |||
| Systolic pressure, mmHg | 141.4±17.2 | 146.5±14.1 | 0.15 |
| Diastolic pressure, mmHg | 83.7±11.1 | 84.2±10.1 | 0.85 |
| Mean arterial pressure, mmHg | 103.4±12.7 | 105.2±10.5 | 0.48 |
| Heart rate, beats/min | 63.8±9.9 | 66.6±9.1 | 0.19 |
| Augmentation index, % | 11.1±14.7 | −10.6±14.8 | <0.0001 |
| Central haemodynamics | |||
| Systolic pressure, mmHg | 133.9±17.3 | 134.5±12.3 | 0.86 |
| Diastolic pressure, mmHg | 85.1±11.1 | 85.8±10.1 | 0.76 |
| Pulse pressure, mmHg | 48.8±9.5 | 48.6±9.3 | 0.94 |
| Mean arterial pressure, mmHg | 101.4±12.7 | 102.1±9.9 | 0.79 |
| Augmentation index, % | 39.0±8.5 | 28.0±8.8 | <0.0001 |
| Aortic pulse wave velocity, m/s | 8.87±1.92 | 8.97±1.83 | 0.84 |
| Measurements on blood | |||
| Glucose, mmol/l | 4.99±0.74 | 4.96±0.96 | 0.89 |
| Cholesterol, mmol/l | 4.64±0.95 | 4.32±0.92 | 0.12 |
| Creatinine, μmol/l | 83.5±18.4 | 105.3±36.9 | 0.0018 |
| Lifestyle | |||
| Current smoking, n (%) | 0 | 2 (2.38) | 0.14 |
| Drinking, n (%) | 10 (23.2) | 23 (56.1) | 0.0013 |
Values are mean±SD. P-values are for the sex differences. Peripheral systolic and diastolic blood pressures were measured in the supine position immediately before the central hemodynamic measurements.
Characteristics of participants.
Among the 84 participants, 2 men (2.38%) were current smokers, and 33 subjects (39.3%; 10 women and 23 men) reported use of alcohol. Median tobacco use was 12.5 cigarettes per day (interquartile range, 10–15). In drinkers, the median alcohol consumption was 72.0g per week (interquartile range, 36.8–94.4).
Correlates of the haemodynamic measurements
In single regression analysis (Table 2), the peripheral and central augmentation indexes were inversely correlated with height, weight and heart rate as measured during the recording of these arterial measurements. The peripheral augmentation index also increased with the blood glucose concentration. Central pulse pressure was positively correlated with age and the serum creatinine level. Pulse wave velocity slightly increased with age, but this relation did not reach formal significance (P=0.065). Both central pulse pressure and pulse wave velocity were positively with mean arterial pressure.
| Variable | Peripheral augmentation index | Central augmentation index | Central pulse pressure | Pulse wave velocity |
|---|---|---|---|---|
| Age | +0.125 (0.26) | +0.152 (0.17) | +0.245 (0.026) | +0.240 (0.065) |
| Height | −0.491 (<0.0001) | −0.442 (<0.0001) | −0.0589 (0.60) | −0.181 (0.17) |
| Weight | −0.386 (0.0003) | −0.392 (0.0003) | −0.117 (0.29) | +0.148 (0.26) |
| Heart rate | −0.346 (0.0013) | −0.433 (<0.0001) | −0.116 (0.29) | +0.161 (0.22) |
| Mean arterial pressure | + 0.0298 (0.79) | +0.0241 (0.83) | +0.385 (0.0003) | +0.384 (0.0024) |
| Glucose | +0.279 (0.0126) | +0.141 (0.21) | +0.00773 (0.95) | −0.171 (0.21) |
| Cholesterol | −0.130 (0.25) | −0.0327 (0.77) | +0.141 (0.21) | +0.737 (0.59) |
| Creatinine | −0.184 (0.10) | −0.0852 (0.45) | 0.237 (0.0345) | −0.0334 (0.80) |
Values are single correlation coefficients (P-value). The number of patients is 60 for pulse wave velocity and 84 for the other haemodynamic measurements.
Single correlation coefficients.
In stepwise multiple regression analysis (Table 3), the peripheral (R2=0.548) and central (R2=0.509) augmentation indexes increased with female sex and mean arterial pressure, but decreased with heart rate and body mass index. The peripheral augmentation index also increased with the blood glucose concentration. Central pulse pressure (R2=0.226) independently increased with age and mean arterial pressure but decreased with heart rate. Pulse wave velocity (R2=0.218) increased with age and mean arterial pressure.
| Variable | Sex (0,1) | Age (years) | BMI (kg/m2) | Heart rate (beats/minute) | Mean arterial pressure (mm Hg) | Glucose (mmol/l) |
|---|---|---|---|---|---|---|
| Peripheral augmentation index | ||||||
| Model R2 | 0.367 | – | 0.496 | 0.522 | 0.548 | 0.443 |
| Regression coefficient±SE (P-value) | +23.5±3.0 (<0.0001) | – | −0.587±0.261 (0.0067) | −0.381±0.161 (0.050) | +0.311±0.131(0.0457) | +4.70±1.79 (0.0020) |
| Central augmentation index | ||||||
| Model R2 | 0.324 | – | 0.477 | 0.437 | 0.509 | – |
| Regression coefficient±SE (P-value) | +10.9±1.7 (<0.0001) | – | −0.359±0.150 (0.0223) | −0.395±0.093 (0.0002) | +0.173±0.076(0.0328) | – |
| Central pulse pressure | ||||||
| Model R2 | – | 0.173 | – | 0.226 | 0.115 | – |
| Regression coefficient±SE (P-value) | – | +0.278±0.110 (0.0237) | – | −0.217±0.098 (0.0278) | +0.302±0.080 (0.0024) | – |
| Aortic pulse wave velocity | ||||||
| Model R2 | – | 0.144 | – | – | 0.218 | – |
| Regression coefficient±SE (P-value) | – | +0.0693±0.0251 (0.0055) | – | – | +0.0506±0.0188(0.0362) | – |
Values are coefficients of multiple determination (R2) and (partial) regression coefficients±SE (P-value given). “–” Indicates a non-significant parameter. P-values for covariables to enter and stay in the models were set at 0.15. The covariables considered were sex (1 vs. 0 for women vs. men), age, body mass index (BMI), heart rate (mean of 2 measurements in the supine position during the arterial examination), mean arterial pressure (measurements in the supine position immediately before the central hemodynamic examination), smoking (0,1), alcohol intake (0,1), serum cholesterol, blood glucose, and serum creatinine.
Stepwise multiple regression.
Comparison with a healthy reference population
The number of patients with haemodynamic measurements above the age-specific thresholds determined in healthy South Africans of African ancestry18 amounted to 0 (0.00%) for the central augmentation index, 1 (1.19%) for the central pulse pressure, and 11 (18.3%) for aortic pulse wave velocity (Fig. 1). Among patients younger than 50 years, these numbers were 0, 0, and 3 (5%), respectively.
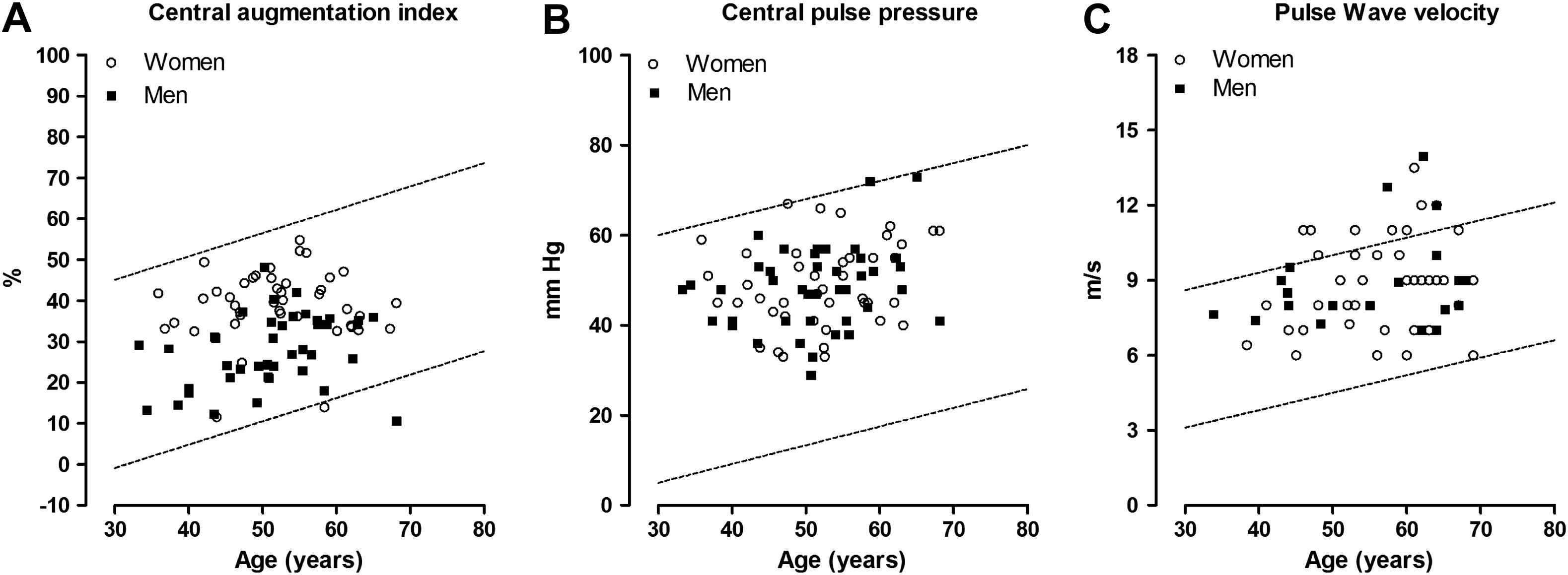
Scatter plots of the central haemodynamic measurements versus age. Circles indicate women and squares are used for men. The lines delineate the 95% confidence interval for the prediction of individual values based on age and were derived in a normal South African reference population of Black ethnicity published in Pruijm et al.18
Discussion
The key finding of our study was that hypertensive black patients living in sub-Saharan Africa had central haemodynamic indexes of arterial stiffness, which were below values derived in a healthy reference population of Black South African origin. These observations indicate that in sub-Saharan Africa, a large number of hypertensive patients had not yet progressed to intermediate stages of target organ damage, as exemplified by stiffening of the large arteries. This reveals a significant potential for prevention of cardiovascular complication, if these patients would be treated to target with antihypertensive drugs.
Lemogoum23 and colleagues observed that isoproterenol increased heart rate, systolic blood pressure and pulse pressure and decreased diastolic blood pressure to a similar degree in 21 healthy Black volunteers and 20 matched White subjects. However, isoproterenol decreased carotid–femoral pulse wave velocity in Whites from 5.9 to 5.7m/s, but not in Black subjects (6.2–6.6m/s). These results are compatible with the hypothesis of ethnic differences in β-adrenergic sensitivity. Because of these ethnic differences, we compared our current results with those in healthy native Black South Africans.
In our current study, the correlates of the central hemodynamic indexes were in line with the literature.24–28 However, with the exception of one report in South Africans,17 most studies in Blacks were done outside Africa, principally in African Americans14,15 and Caribbeans.15,16 In addition, most of these studies in Blacks were hospital based and included patients with multiple cardiovascular risk factors, in particular diabetes mellitus. Salciccioli15 and coworkers studied 449 African Americans and 454 African Caribbeans, using applanation tonometry. In both ethnic groups, the mean augmentation index and carotid-to-radial pulse wave velocity were similar (23 vs. 24% and 9.0 vs. 9.0m/s, respectively; P≥0.20). In multivariable-adjusted analyses, sex, age, weight, mean arterial pressure, heart rate, and family history of coronary artery disease were independently associated with the augmentation index and combined explained close to 50% of its variance.
Ferreira and colleagues29 investigated 82 White (49 normotensive and 33 untreated hypertensive) and 38 Black (24 normotensive and 14 untreated hypertensive) adult Brazilian men aged from 19 to 50 years. They measured carotid–femoral pulse wave velocity by means of the Complior device (Artech Medical, Paris, France). 30 In the normotensive group, White men presented higher mean values of pulse wave velocity than Black men did, whereas the opposite was found in the hypertensive group. In age-adjusted analyses, the differences in pulse wave velocity between Whites and Blacks were significant in the normotensive (8.15 vs. 7.75m/s; P<0.001) and hypertensive (8.88 vs. 9.30m/s; P<0.001) participants. The slope of pulse wave velocity on age was more than twice as steep in Blacks compared with Whites. These observations suggest that there might be a greater pressure-dependent increase in aortic stiffness in Blacks than in Whites.30
Din-Dzietham and coworkers14 reported on 268 African Americans and 2459 whites, who were aged 45 to 64 years at baseline examination in 1986 to 1989, free of coronary heart disease and stroke/transient ischaemic attack, from Forsyth County, North Carolina. The β-stiffness index and pulsatile arterial diameter change were derived from brachial blood pressure and from echo-tracked systolic and diastolic carotid arterial diameters. African Americans had stiffer carotid arteries than their White counterparts did, with a rightward shift of the distribution. In adjusted analyses, the β-stiffness index was 9% higher in African Americans.14
The present study must be interpreted within the context of its potential limitations. Clinical research in developing countries differs in several ways from that in developed countries, partly because of the cultural differences, relatively poor health care and research infrastructure, wide socioeconomic divide within the society, illiteracy of patients, and lack of sufficient numbers of trained investigators and support personnel in these countries.31 We introduced SphygmoCor measurement of the central haemodynamics as an optional ancillary study at 4 centres, of which 2 succeeded after an investigators’ training meeting held in Douala on 27 August 2010, to implement the technique. Compared with experienced European9 and Chinese10 observers, the investigators at these 2 centres reached an intra-observer variability of similar accuracy. Furthermore, our study has a small sample size and we might have lacked power to pick up physiologically important correlates of the central haemodynamic measurements, such as age. We recruited only at 2 centres and our results might therefore not be representative for all Black patients in sub-Saharan Africa or for patients with more severe or advanced hypertension.
In conclusion, estimates of the central haemodynamic variables and their determinants in Black hypertensive patients living in sub-Saharan Africa are in line with those reported in South Africans17 and other ethnicities.9,10 Most NOAAH patients have measures of arterial stiffness similar to those of a healthy reference population. This observation reveals a significant potential for the prevention of irreversible arterial structural damage by timely treating sub-Saharan hypertensive patients to target blood pressure levels.17
Conflict of interest
None of the authors declares a conflict of interest with regard to the information presented in this manuscript.
Acknowledgements
The Belgian Hypertension Committee endorsed the NOAAH trial. Prof. M. O’Rourke (Saint Vincent ’s Clinic, University of New South Wales, Sidney, Australia) helped in raising support for the ancillary substudy on arterial stiffness. The authors gratefully acknowledge the expert clerical and secretarial support of Ms. Barbara Andries, Ms. Sandra Covens, Ms. Ya Zhu and Ms. Sonja Zuba.
References
Cite this article
TY - JOUR AU - Birinus Ezeala-Adikaibe AU - Yan-Ping Liu AU - Daniel Lemogoum AU - Benedict C. Anisiuba AU - Marius K. Kamdem AU - Joseph Kaptue AU - Chinwuba K. Ijoma AU - Lutgarde Thijs AU - Augustine N. Odili AU - Kei Asayama AU - Jan A. Staessen AU - Jean-René M’Buyamba-Kabangu AU - Ifeoma I. Ulasi PY - 2011 DA - 2011/12/14 TI - Central haemodynamics reveal significant potential for prevention in Black hypertensive patients born and living in sub-Saharan Africa JO - Artery Research SP - 41 EP - 48 VL - 6 IS - 1 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2011.11.002 DO - 10.1016/j.artres.2011.11.002 ID - Ezeala-Adikaibe2011 ER -
