Augmentation index and arterial stiffness in patients with type 2 diabetes mellitus
- DOI
- 10.1016/j.artres.2013.09.002How to use a DOI?
- Keywords
- Arterial stiffness; Type 2 diabetes mellitus; Augmentation index
- Abstract
Background: Augmentation index (AIx) is regarded as a marker of systemic arterial stiffness. Patients with type 2 diabetes mellitus (T2DM) have increased arterial stiffness, but not AIx, which suggests that mechanisms contributing to AIx in T2DM may differ from healthy individuals and be unrelated to arterial stiffness. The aim of this study was to examine the cardiovascular and clinical determinates of AIx (including arterial stiffness) in patients with T2DM compared with controls.
Methods: Clinical characteristics and haemodynamic variables (including aortic and brachial pulse wave velocity [stiffness], cardiac output, systemic vascular resistance and heart rate) and AIx (by radial tonometry) were recorded in 53 T2DM and 53 matched controls. Correlates of AIx unadjusted for heart rate were assessed by uni- and multi-variable analysis.
Results: Compared with controls, T2DM patients had significantly higher aortic stiffness (7.6 ± 1.6 vs 6.7 ± 1.9 m/s p = 0.016), cardiac output, heart rate, brachial and central BP; lower brachial stiffness and systemic vascular resistance, but no significant difference in AIx (27 ± 9 vs 24 ± 11% p = 0.184). AIx (adjusted or unadjusted) was not significantly related to aortic or brachial stiffness in either group (p > 0.198 all). Independent predictors of AIx in T2DM patients were height and heart rate, whereas in controls, AIx was independently related to height.
Conclusions: Determinants of AIx in patients with T2DM differ from healthy individuals. Moreover, AIx is not significantly related to regional large artery stiffness and should not be regarded as indicative of systemic arterial stiffness.
- Copyright
- © 2013 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Increased arterial stiffness is an independent predictor of cardiovascular events and total mortality in both healthy and diseased populations.1 Augmentation index (AIx) is defined as the difference between the second and first systolic peaks on the central (aortic) pressure waveform expressed as a percentage of pulse pressure. AIx represents the pressure over time that the heart is exposed to during each contraction and is, therefore, a measure of left ventricular afterload.2 AIx is inversely related to heart rate, and is purported to be a marker of systemic arterial stiffness. This is based on the notion that the magnitude and speed of arterial wave travel is increased in the presence of stiffened vasculature through increased wave reflection.3
Several studies have shown that patients with type 2 diabetes mellitus (T2DM) have generalized vascular dysfunction and increased arterial stiffness compared to non-diabetic, age-matched people.4–10 Specifically, patients with T2DM have been shown to have increased aortic stiffness (assessed by aortic pulse wave velocity),4,7–9 higher carotid intima media thickness5 and elevated cardio-ankle vascular stiffness index11; as well as decreased systemic arterial compliance6,7 and arterial distensibility.10 Taken all together these data lead to the expectation that AIx should be significantly elevated in patients with T2DM. Indeed, this has been reported in some cross sectional case–control comparison studies.6,12,13 On the other hand, several studies have shown that despite significant increases in arterial stiffness among people with T2DM, no significant differences in AIx were found when compared with healthy subjects, and this was observed with14,15 or without8,9 adjusting for heart rate.
The above information brings into question the concept that AIx is indicative of systemic arterial stiffness and necessitates further investigations to determine reasons for the inconsistency in these findings. Thus, the aim of this study was to examine the cardiovascular and clinical determinates of AIx (including arterial stiffness) in patients with T2DM compared with controls. We hypothesized that arterial stiffness would be significantly elevated in patients with T2DM but would not be related to AIx and that the determinants of AIx would differ from healthy individuals.
Methods
Study participants
Exclusion criteria for participation in the study included; pregnancy or a clinical history of cardiovascular disease including cardiac arrhythmia. A total of 152 eligible participants responded to community advertisement and were examined between June 2010 and February 2011. The sample comprised 53 patients with T2DM (51% male), and each of these were matched with one healthy control participant selected from the remaining 99 healthy participants. Matching was made on the basis of the same sex and the nearest age (total n = 106). Diabetes mellitus was determined by self-reported diagnosis by a physician. Hypertension was defined as: clinic brachial BP ≥ 140/90 mmHg; use of antihypertensive medications or self-reported diagnosis of hypertension by a physician. Participant characteristics are summarized in Table 1.
| Healthy | T2DM | p Value | |
|---|---|---|---|
| Male, n (%) | 27 (51%) | 27(51%) | 1.00 |
| Age (years) | 58 ± 6 | 61 ± 8 | 0.082 |
| Waist:hip (ratio) | 0.92 ± 0.10 | 0.96 ± 0.16 | 0.196 |
| Weight (kg) | 75 ± 14 | 88 ± 16 | <0.001 |
| Height (cm) | 171 ± 10 | 169 ± 10 | 0.159 |
| Body mass index (kg/m2) | 25.4 ± 3.5 | 30.8 ± 5.0 | <0.001 |
| Ambulatory day-time systolic BP (mm Hg) | 136 ± 13 | 141 ± 15 | 0.105 |
| Ambulatory day-time diastolic BP (mm Hg) | 83 ± 8 | 80 ± 9 | 0.092 |
| Antihypertensive medication, n (%) | 3 (6.1%) | 30 (56.6%) | <0.001 |
| Statins, n (%) | 1 (1.9%) | 30 (56.6%) | <0.001 |
| Glucose (mmol/L) | 4.8 ± 0.6 | 7.6 ± 1.9 | <0.001 |
| Cholesterol (mmol/L) | 5.7 ± 1.0 | 4.6 ± 1.1 | <0.001 |
| Triglycerides (mmol/L) | 1.0 ± 0.4 | 1.5 ± 0.7 | <0.001 |
| High density lipoprotein (mmol/L) | 1.7 ± 0.5 | 1.4 ± 0.4 | <0.001 |
| Low density lipoprotein (mmol/L) | 3.5 ± 1.0 | 2.5 ± 0.8 | <0.001 |
| Haemoglobin A1c (%) | 5.3 ± 1.0 | 7.2 ± 0.8 | <0.001 |
Data expressed as mean ± standard deviation or %. p value is for between group analyses. BP, blood pressure.
Participant characteristics between healthy participants (n = 53) and patients with type 2 diabetes mellitus (T2DM; n = 53).
Study protocol
Participants attended the research clinic for assessment on two occasions. At visit one, all standard anthropometric (including height, weight, waist and hip circumference) and BP variables were measured in a temperature controlled room (23 °C ± 1 °C). Prior to this visit, participants were asked to refrain from alcohol consumption and exercise on the day of testing and to avoid consuming heavy meals (i.e. were in a post-absorptive state), smoking and caffeine containing products in the three hours prior to testing. At visit two, fasting blood samples were taken and all participants were fitted with a 24-h ambulatory BP monitor. All participants signed informed consent and the study was approved by the Tasmanian Health and Medical Human Research Ethics Committee.
Arterial stiffness
After the participant had been resting supine on a bed for 10 min, duplicate measures of brachial pulse wave velocity (PWV) were measured in the carotid-to-radial arterial segments using ECG-gated hand held applanation tonometry (SphygmoCor 8.1, AtCor Medical, Sydney, Australia). Aortic PWV was measured in duplicate from the carotid-to-femoral arterial segments using the same tonometry apparatus. Arterial length was estimated by subtracting the transcutaneous distance between the sternal notch and carotid sampling site from the distance between the sternal notch and the radial sampling site (for brachial PWV) and femoral sampling site (for aortic PWV).16
Brachial and central blood pressure
After supine measures, participants were moved into a seated position with feet flat on the floor, back supported by the chair and with a pillow placed under the arm so that the BP cuff was at the same height as the heart. After 10 min of rest, duplicate brachial BP measurements were recorded by a validated automatic device (Omron HEM-907; OMRON Europe B.V. (OMCE), Hoofddorp, The Netherlands)17 using an appropriately sized cuff in accordance with guidelines.18 Central BP was measured in duplicate by radial applanation tonometry (SphygmoCor 8.1, AtCor Medical, Sydney, Australia) immediately following the brachial BP measurements. A validated19 generalized transfer function was applied to the measured radial artery pressure waveforms to allow for the reconstruction of the central (aortic) pressure waveform. Pulse pressure amplification was calculated as the ratio of brachial to central pulse pressure and heart rate was determined from the electrocardiogram recording during the radial waveform measurement by the device.
Augmentation index
AIx was determined from the radial (radial AIx) and aortic pressure wave (central AIx) and was calculated as the difference in pressure between the second and first systolic peaks (augmented pressure on the central waveform) expressed as a percent of pulse pressure. Because AIx is significantly influenced by heart rate,20 it was also adjusted to a heart rate of 75 beats per minute using SphygmoCor software.
Cardiothoracic bioimpedance
Cardiac output, stroke volume and systemic vascular resistance were measured throughout the assessment by cardiothoracic bioimpedence (Physio Flow; Manatec Biomedical; Macheren, France). This device has previously been validated against invasive measures21 and has good reproducibility.22
Blood biochemistry
Venous blood samples were taken from the antecubital fossa following an overnight fast in order to assess blood biochemistry (including glucose, insulin, total cholesterol, triglycerides and glycated haemoglobin [HBA1c]) in all participants. Analytical biochemistry was performed using accredited hospital pathology laboratory methodologies.
Statistical analysis
All data were analysed using SPSS for windows software version 19.0 (IBM SPSS Statistics, New York, USA). Data are presented as mean ± standard deviation unless otherwise stated and p < 0.05 was considered statistically significant. Data were assessed for normality and all variables were normally distributed. Independent t-tests assuming unequal variance were performed for continuous variables to compare characteristics between control participants and patients with T2DM and Chi Square tests were performed for dichotomous variables. Univariable associations between variables were assessed by Pearson’s correlations. Analysis of covariance was additionally undertaken to assess between group differences in AIx (correcting for age, gender, height and heart rate) and aortic PWV (correcting for age, gender and mean arterial pressure). Multivariable regression analyses for the predictors of AIx were performed separately in patients with T2DM and controls. Models examined variables that significantly correlated with AIx and variables of clinical relevance (including age, height, heart rate, body mass index [BMI], antihypertensive medication and statin use). These variables were added separately into the regression model.
Results
Participant characteristics
As shown in Table 1, there was no significant difference between patients with T2DM and healthy participants with respect to sex, age, waist to hip ratio, height or 24 h ambulatory determined day-time BP. Patients with T2DM were significantly heavier, had higher BMI, were more likely to be taking medication for hypertension (including angiotensin receptor blockers, beta blockers and angiotensin converting enzyme inhibitors) and hyperlipidemia (statins), had lower total cholesterol and high and low density lipoprotein and had poorer glycemic control compared to healthy participants.
Arterial stiffness
Compared to healthy participants, patients with T2DM had significantly increased aortic PWV and significantly lower brachial PWV (p < 0.05 for both, Table 2). Furthermore, after adjusting aortic PWV for age, gender and mean arterial pressure, aortic PWV remained significantly higher in patients with T2DM (p < 0.005).
| Haemodynamic variable | Healthy | T2DM | p Value |
|---|---|---|---|
| Arterial stiffness | |||
| Aortic pulse wave velocity (m/s) | 6.7 ± 1.9 | 7.6 ± 1.6 | 0.016 |
| aAortic pulse wave velocity (m/s) | 6.8 ± 1.8 | 7.6 ± 1.8 | 0.023 |
| Brachial pulse wave velocity (m/s) | 8.5 ± 1.0 | 8.1 ± 0.9 | 0.037 |
| Haemodynamics | |||
| Brachial systolic blood pressure (mm Hg) | 117 ± 11 | 124 ± 13 | 0.004 |
| Brachial diastolic blood pressure (mm Hg) | 68 ± 8 | 71 ± 9 | 0.178 |
| Mean arterial pressure (mm Hg) | 80 ± 22 | 88 ± 16 | 0.039 |
| Brachial pulse pressure (mm Hg) | 49 ± 5 | 54 ± 10 | 0.004 |
| Radial augmentation index (%) | 76 ± 16 | 80 ± 12 | 0.171 |
| Central systolic blood pressure (mm Hg) | 107 ± 12 | 114 ± 13 | 0.004 |
| Central diastolic blood pressure (mm Hg) | 69 ± 8 | 72 ± 9 | 0.147 |
| Central pulse pressure (mm Hg) | 37 ± 6 | 43 ± 10 | 0.001 |
| Heart rate (bpm) | 57 ± 7 | 64 ± 9 | <0.001 |
| Stroke volume (ml) | 78 ± 13 | 85 ± 14 | 0.006 |
| Cardiac output (l/min) | 4.49 ± 0.72 | 5.54 ± 1.15 | <0.001 |
| Systemic vascular resistance (d s.cm−5) | 1562 ± 281 | 1326 ± 249 | <0.001 |
| Augmented pressure (mm Hg) | 10 ± 5 | 12 ± 5 | 0.032 |
| Central augmentation index (%) | 24 ± 11 | 27 ± 9 | 0.184 |
| Central augmentation index (heart rate 75 bpm) | 15 ± 11 | 21 ± 7 | 0.002 |
| bCentral augmentation index (%) | 24 ± 9 | 27 ± 9 | 0.043 |
| Pulse pressure amplification (ratio) | 1.33 ± 0.15 | 1.27 ± 0.14 | 0.043 |
Data expressed as mean ± standard deviation. p value is for between group analyses.
Aortic pulse wave velocity was adjusted for age, gender and mean arterial pressure.
Central augmentation index was adjusted for age, gender, height and heart rate.
Haemodynamic comparison between healthy participants (n = 53) and patients with type 2 diabetes mellitus (T2DM; n = 53).
Augmentation index
There was no significant difference in AIx between groups (radial or central; Table 2), however, when AIx was normalized to a heart rate of 75 beats per minute, patients with T2DM had significantly increased AIx compared to the healthy controls. Furthermore, AIx remained significantly higher in patients with T2DM after adjusting further for age, gender, height and heart rate.
Haemodynamics
Patients with T2DM had significantly increased brachial systolic BP, mean arterial pressure, pulse pressure, central systolic BP, central pulse pressure, heart rate, stroke volume, cardiac output and augmented pressure, but significantly lower systemic vascular resistance and pulse pressure amplification (p < 0.05 for all, Table 2) compared to healthy participants. There was no difference in brachial or central diastolic BP between groups.
Univariable associations with augmentation index
Table 3 summarizes the univariable associations between AIx and different haemodynamic variables. AIx was not significantly correlated with either aortic PWV (Fig. 1a) or brachial PWV (Fig. 1b) in patients with T2DM or healthy controls (p > 0.05 for both, Table 3). Moreover, after adjusting AIx for a heart rate of 75 beats per minute, there was still no significant association between AIx and aortic or brachial PWV in either patients with T2DM (r = −0.091, p = 0.527 and r = 0.090, p = 0.527 respectively) or healthy controls (r = −0.023, p = 0.872 and r = −0.015, p = 0.311 respectively). In patients with T2DM, AIx significantly correlated with age, height, HBA1c, central systolic BP, heart rate, cardiac output and systemic vascular resistance (p < 0.05 for all, Table 3). In healthy participants, AIx did not significantly correlate with age or heart rate (p > 0.05), but was significantly correlated with height (p = 0.002) and central systolic BP (p < 0.001, Table 3).
| Independent variable | Healthy | T2DM | ||
|---|---|---|---|---|
| r | p Value | r | p Value | |
| Age (years) | 0.11 | 0.427 | 0.47 | <0.001 |
| Height (cm) | −0.42 | 0.002 | −0.46 | <0.001 |
| Body mass index (kg/m2) | 0.14 | 0.310 | 0.06 | 0.673 |
| Hemoglobin A1c (%) | −0.24 | 0.193 | 0.33 | 0.042 |
| Arterial stiffness | ||||
| Aortic pulse wave velocity (m/s) | −0.04 | 0.776 | −0.19 | 0.198 |
| Brachial pulse wave velocity (m/s) | −0.14 | 0.327 | 0.03 | 0.828 |
| Haemodynamics | ||||
| Brachial systolic blood pressure (mm Hg) | 0.26 | 0.057 | 0.07 | 0.596 |
| Brachial diastolic blood pressure (mm Hg) | 0.35 | 0.010 | −0.18 | 0.211 |
| Central systolic blood pressure (mm Hg) | 0.58 | <0.001 | 0.33 | 0.015 |
| Heart rate (bpm) | 0.07 | 0.631 | −0.63 | <0.001 |
| Stroke volume (ml) | −0.14 | 0.320 | −0.23 | 0.101 |
| Cardiac output (l/min) | −0.18 | 0.194 | −0.60 | <0.001 |
| Systemic vascular resistance (d s.cm−5) | 0.23 | 0.104 | 0.54 | <0.001 |
R refers to Pearson’s correlation coefficient and p value is for the correlation between augmentation index and variables.
Univariable associations of augmentation index in healthy participants (n = 53) and patients with type 2 diabetes mellitus (T2DM; n = 53).
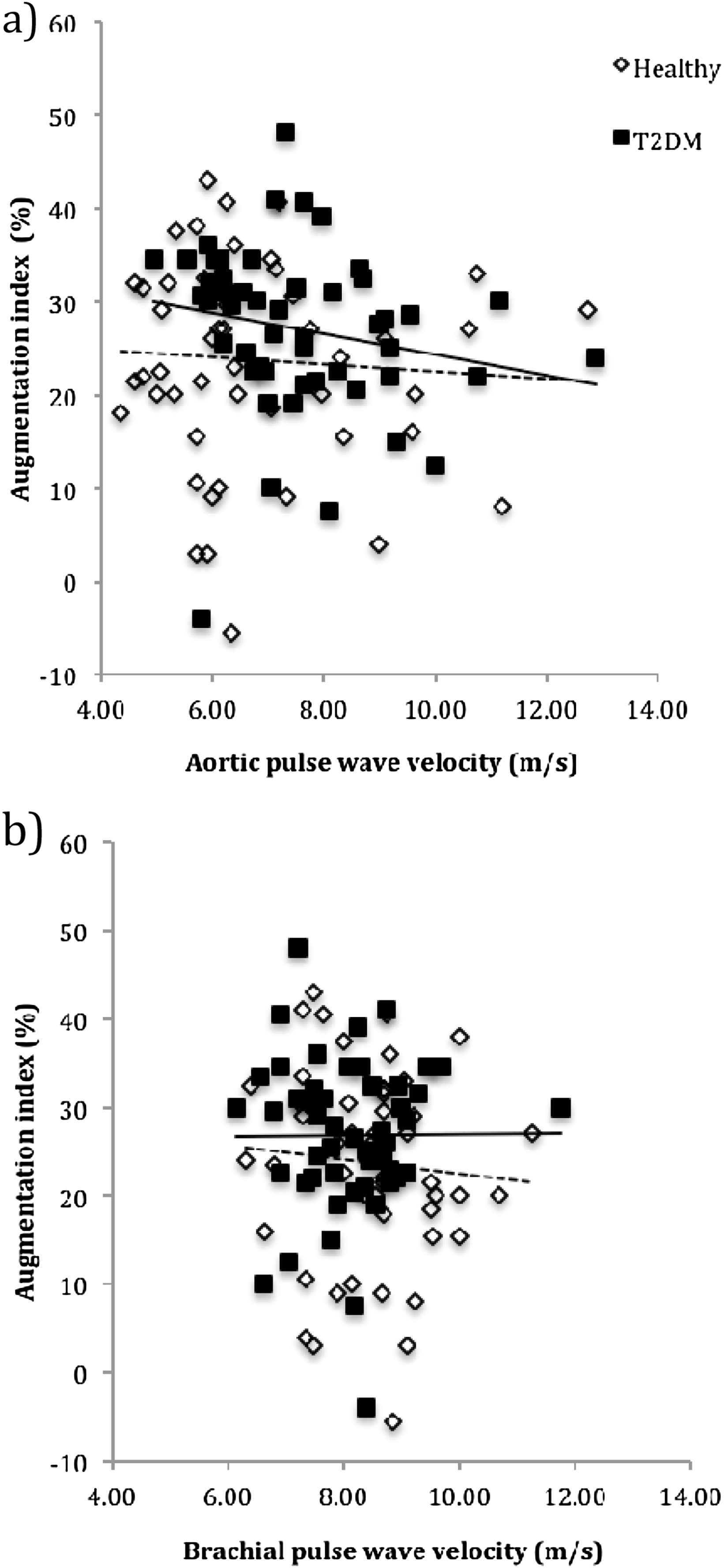
The univariable relationship between augmentation index and arterial stiffness (aortic pulse wave velocity (a) and brachial pulse wave velocity (b)) in healthy participants (dashed line) (r = −0.03, p = 0.863 and r = −0.13, p = 0.373 respectively) and patients with type 2 diabetes mellitus (T2DM).
Predictors of augmentation index
Separate regression models were performed for healthy participants and patients with T2DM and are shown in Table 4. The models included the covariates of age, height, BMI, HBA1c, heart rate, cardiac output antihypertensive medication and statin use. In patients with T2DM, the strongest predictors of AIx (model adjusted R2 = 0.47, p = 0.001) were height and heart rate, which accounted for 13% and 15% of the variance in AIx respectively. The strongest predictor of AIx in controls (model adjusted R2 = 0.17, p = 0.012) was height, which explained 20% of the variance in AIx. Only a few of the healthy participants were being treated for hypertension (n = 3) or hyperlipidemia (n = 1), and the addition of these variables in the multivariable analysis did not affect the model.
| Independent variable | Unstandardized β (95% CI) | p Value |
|---|---|---|
| Healthy | ||
| Age (years) | −0.06 (−0.40, 0.53) | 0.782 |
| Height (cm) | −0.53 (−0.83, −0.23) | 0.001 |
| Body mass index (kg/m2) | 0.66 (−0.16, 1.48) | 0.110 |
| Heart rate (bpm) | −0.19 (−0.61, 0.22) | 0.353 |
| T2DM | ||
| Age (years) | 0.14 (−0.97, 0.37) | 0.240 |
| Height (cm) | −0.32 (−0.53, −0.10) | 0.005 |
| Body mass index (kg/m2) | 0.01 (−0.46, 0.47) | 0.961 |
| Hemoglobin A1c (%) | 1.82 (−0.12, 3.78) | 0.066 |
| Heart rate (bpm) | −0.43 (−0.67, −0.13) | 0.004 |
| Cardiac output (l/min) | 0.24 (−2.29, 2.77) | 0.846 |
| Antihypertensive medication | 0.24 (−2.63, 6.05) | 0.427 |
| Statin use | 0.90 (−3.13, 4.95) | 0.649 |
Models were performed separately for healthy participants and patients with T2DM. Data are unstandardized β coefficient and p value relates to the independent variable in the model.
Multivariable regression for associations between augmentation index and cardiovascular and clinic characteristics in healthy participants (n = 53) and patients with type 2 diabetes mellitus (T2DM; n = 53).
Discussion
The main finding of this study was that despite patients with T2DM having significantly increased arterial stiffness (aortic PWV), there was no difference in AIx unadjusted for heart rate, compared to age and sex matched healthy controls. Secondly, whether adjusted for heart rate or otherwise, AIx was not significantly related to aortic or brachial artery stiffness in healthy individuals or patients with T2DM. Furthermore, the determinants of AIx in patients with T2DM were significantly different to those in healthy individuals. In patients with T2DM, the independent predictors of AIx were height and heart rate whilst in healthy participants only height was independently related to AIx. Our findings suggest that AIx is not a reliable marker of arterial stiffness in patients with T2DM or healthy individuals and separate factors are likely to influence AIx between these populations.
Arterial stiffness in patients with T2DM
Our observation of increased aortic PWV in patients with T2DM is consistent with numerous studies showing that patients with T2DM have accelerated arterial stiffening compared to non-diabetic matched controls.4–9 The Strong Heart Study23 also found that arterial stiffness assessed using the ratio of pulse pressure to stroke volume, was significantly increased in diabetic patients compared to normoglycemic individuals. AIx has been heralded as a surrogate marker of systemic arterial stiffness24,25 however studies examining the association between AIx and measures of vascular stiffness in patients with T2DM are not conclusive. Indeed, similar to our study, no difference in AIx has been observed between patients with T2DM and healthy participants.8,9,14,15 On the other hand, others6,12,13 have reported AIx to be significantly increased in patients with T2DM compared with non-diabetic individuals, but this was only after adjusting for heart rate or only evident in male study participants. A possible explanation for these discrepancies may be that AIx is influenced by a multitude of factors beyond arterial stiffness that result in altered waveform patterns in patients with T2DM and contribute to inconsistent findings in terms of the overall effect on AIx.
Determinants of AIx in patients with T2DM
In both healthy participants and patients with T2DM, AIx was significantly and inversely related to height. This supports previous findings and conventional theory suggests this is because people of shorter stature have reduced distance to arterial pressure reflecting sites and this influences the timing and magnitude of arterial wave travel, causing early return to the heart (during systole) and resulting in an increase in AIx.3,26 In our study, patients with T2DM had significantly increased heart rate compared to healthy participants and, similar to previous studies6,27 heart rate was significantly related to AIx in patients with T2DM. An increase in heart rate shortens the ejection duration and corresponding lower AIx is purported to be due to reflected pressure waves being moved into the diastolic (rather than systole) phase,3 however, aortic reservoir function should also be considered when interpreting physiological mechanisms of AIx.28,29 In our findings, the effect of heart rate on AIx was greater than the effect of arterial stiffness (aortic PWV) because AIx was significantly increased in patients with T2DM after correcting for heart rate. Other studies have demonstrated the same effect whereby significantly increased AIx compared to healthy people was only seen after adjusting for heart rate.6,20,27 On the other hand, Lacy et al.15 found no difference in AIx between people with and without diabetes even after adjustment for heart rate. Potentially, this may be due to the study population which included patients with both type 1 and type 2 diabetes mellitus and additionally, the majority of the patients with diabetes were receiving insulin, which has a diminishing effect on AIx.30
In our study, patients with T2DM had significantly increased cardiac output (due to a rise in both heart rate and stroke volume) and decreased systemic vascular resistance, compared to healthy participants. Elevated cardiac output has previously been observed in people with insulin resistance and patients with T2DM.31,32 Although not independent predictors of AIx, both cardiac output and systemic vascular resistance were significantly correlated with AIx in patients with T2DM, but not in healthy participants. The increase in left ventricular flow output together with the reduction in systemic vascular resistance could together be contributing to the relative reduction in AIx in diabetic individuals. The high flow output may be suggestive of increased dilation of the proximal aorta (among other possibilities) but would need to be assessed in future studies. Interestingly, patients with T2DM had significantly lower brachial PWV, which may also be suggestive of muscular peripheral artery dilation beyond that of controls.
Limitations
This is a relatively small case–control comparison study that cannot attribute causality and further studies are required in order to determine the exact mechanisms contributing to AIx in patients with T2DM. In our study, we determined arterial stiffness via PWV, however, the addition of other markers of arterial stiffness would strengthen the findings with respect to the relation between AIx and systemic arterial stiffness. Finally, more than half the patients with T2DM were taking medication for hypertension or hyperlipidaemia, and the vasoactive properties of these medications could have influenced the results. Future studies in drug naïve participants could overcome this problem.
Conclusions
The main finding of this study was that AIx (whether adjusted or unadjusted for heart rate) was not related to arterial stiffness in patients with T2DM and that the determinants of AIx in these patients were significantly different to that of healthy participants. Our findings indicate that AIx should not be referred to as a surrogate maker of arterial stiffness and further work is needed in order to understand the disparate systemic haemodynamics that may explain the difference in AIx between people with and without T2DM.
Grants
Associate Professor Sharman was supported by a
Disclosure
The authors have nothing to disclose.
References
Cite this article
TY - JOUR AU - Rachel E.D. Climie AU - Sonja B. Nikolic AU - Petr Otahal AU - Laura J. Keith AU - James E. Sharman PY - 2013 DA - 2013/10/02 TI - Augmentation index and arterial stiffness in patients with type 2 diabetes mellitus JO - Artery Research SP - 194 EP - 200 VL - 7 IS - 3-4 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2013.09.002 DO - 10.1016/j.artres.2013.09.002 ID - Climie2013 ER -
