Arterial stiffness, wave reflection amplitude and left ventricular afterload are increased in overweight individuals
- DOI
- 10.1016/j.artres.2013.08.001How to use a DOI?
- Keywords
- Overweight; Arterial stiffness; Wave reflections; Wasted ventricular energy; Leptin; Endothelial dysfunction
- Abstract
Background: Reflected pressure waves from the lower body to the heart in overweight subjects return early and augment aortic systolic pressure and increase left ventricular (LV) afterload and wasted energy.
Methods: Central aortic pressure waves were generated from radial artery pressure waves recorded non-invasively in 176 subjects (age 52 ± 18 years). Augmentation index (Alx), an estimate of wave reflection, and LV wasted energy (Ew) were calculated from the generated aortic pressure wave. Data were collected in 88 non-diabetic, non-renal disease normotensive overweight (BMI 30 ± 5.0 kg/m2) subjects and compared to data collected from 88 normotensive normal weight (BMI 23 ± 2.1, P < 0.01) control subjects matched for gender, age, height, heart rate and ejection duration.
Results: Compared to controls, overweight subjects had higher aortic systolic (124 ± 17 vs 114 ± 14 mmHg, P < 0.001) and pulse (44 ± 16 vs 37 ± 12 mmHg, P < 0.001) blood pressures. Also, increased BMI was associated with an increase in Alx (28 ± 9.3 vs 20 ± 12%, P < 0.001), reflected wave duration (176 ± 28 vs 159 ± 30 msec, P < 0.004) and Ew (2527 ± 1732 vs 1498 ± 1375 dsc−2, P < 0.001) and a decrease in pressure wave travel time (133 ± 14 vs 143 ± 15 msec, P < 0.001). These modifications in wave reflection characteristics in overweight subjects were associated with a decrease in pulse pressure amplification (1.3 ± 0.14 vs 1.5 ± 0.29, P < 0.01).
Conclusions: Overweight subjects have a stiffer arterial system which decreases wave propagation time and causes the reflected pressure wave to arrive earlier at the heart with increased amplitude and duration. These changes in arterial function and wave reflection characteristics cause an increase in LV afterload, myocardial oxygen demand and wasted LV energy.
- Copyright
- © 2013 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
The prevalence of overweight (Body Mass Index, BMI > 25 kg/m2) in the United States has reached epidemic proportions and is continuing to increase.1 Numerous large observational epidemiology studies and clinical trials, including the Framingham Heart Study and the Third National Health and Nutrition Examination Survey (NHANES III) have shown a strong positive, continuous and graded association between BMI, blood pressure and mortality in both men and women in various racial and ethnic groups and from adolescence to old age.2–5 Furthermore, overweight and obesity increase renin–angiotensin system activity6 and cause a substantial increase in cardiovascular risk factors including hypertension,4,7 dyslipidemia,7 diabetes,7,8 obstructive sleep apnea,9 coronary artery disease (CAD),10,11 end stage renal disease (ESRD),12 endothelial dysfunction13,14 and LV hypertrophy (LVH)8,15,16 which lead to adverse cerebro- and cardiovascular (CV) events such as dementia,16 stroke,17,18 heart failure19 and death.2,3 Overweight and obesity also decrease mobility and increase inactivity.
Wildman et al.20 suggested that overweight exerts adverse effects on the CV system by increasing arterial stiffness, thus predisposing the individual to hypertension and premature aging of the arterial system. Excess body weight has been shown in numerous other reports to be related to both elastic and muscular arterial stiffness21–24 which in turn are positively associated with all the above CV risk factors.25,26 Although these studies measured central elastic and peripheral muscular artery stiffness, they did not investigate the stiffness-related changes in wave reflection characteristics and wasted LV energy that may accompany the increase in BMI. Results from previous reports that did study wave reflection characteristics in overweight subjects measured as BMI, body fatness or waist circumference have been inconsistent.27–33 Accordingly, we assessed the hypothesis that reflected pressure waves from the lower body to the heart in non-diabetic, non-renal disease, normotensive overweight individuals would return early and augment central aortic systolic pressure and increase LV afterload and wasted LV energy.
Methods
One-hundred and seventy-six healthy volunteers were included in the study which was approved by the Institutional Review Board of the University of Florida. Data were collected and analyzed in 88 normotensive, non-diabetic, non-renal disease, overweight (BMI > 25 kg/m2) subjects (43 males and 45 females) and compared to data collected from 88 normal weight (BMI < 25 kg/m2) control subjects matched for gender, age, height, and heart rate. No one in either group was taking cardiovascular drugs or smoked; data were collected at least two hours after a meal and intake of coffee.
Pulse waveform analysis
Assessment of wave reflection characteristics – estimates of LV afterload and myocardial oxygen demand – were performed non-invasively using the commercially available SphygmoCor system (AtCor Medical, Sydney, Australia). Radial artery pressure waveforms were recorded at the wrist, using applanation tonometry with a high-fidelity micromanometer (Millar Instruments, Houston, Texas). After 20 sequential waveforms had been acquired and averaged, a validated generalized mathematical transfer function was used to synthesize the corresponding central aortic pressure waveform.34–36 Indices of afterload and oxygen demand were derived from the pressure waveform using the technique of pulse wave analysis.37–39 The merging point of forward and reflected waves (the inflection point, Pi) is identified on the pressure waveform (Fig. 1). P1 and AP are estimates of forward and reflected wave amplitudes, respectively. The aortic augmentation index (AIx) is defined as the reflected wave amplitude divided by pulse pressure (PP) and expressed as a percentage.40 Larger values of AIx indicate increased wave reflection from the lower body or earlier return of the reflected wave to the heart as a result of increased pulse wave velocity (attributable to increased elastic and/or muscular artery stiffness).34 The time, Tr, from the beginning upstroke of the synthesized aortic systolic pressure waveform to the beginning upstroke of the reflected wave (Pi) is the round-trip travel time of the pressure wave to and from the major “effective” reflecting site in the lower body40 and the time, (ED − Tr), from the inflection point to the incisura, is the systolic duration of the reflected wave34; ED is ejection duration. Amplification of the pressure wave from the central aorta to the radial artery was calculated as the ratio of peripheral artery pulse pressure and central aortic pulse pressure.34,41 Elevated systemic artery PWV leads to a shorter Tr, a longer (ED − Tr) and a decreased pulse pressure amplification and indicates early return of the reflected pressure wave from the lower body to the heart.38 When the reflected wave returns during systole, as seen in Fig. 1, the aortic pressure is augmented (AP increased) and, therefore, the LV must generate enough energy to overcome this added boost in pressure.42–44 This energy (or effort) is wasted since it does not contribute to blood flow production and can be estimated, as the area enclosed by the boost in late systolic pressure curve. Wasted energy represents that component of tension-time-index (TTI) (or systolic pressure time index, SPTI) that is attributable to wave reflection and late systolic pressure augmentation and is directly related to LV mass16 and likely myocardial oxygen demand.43,45
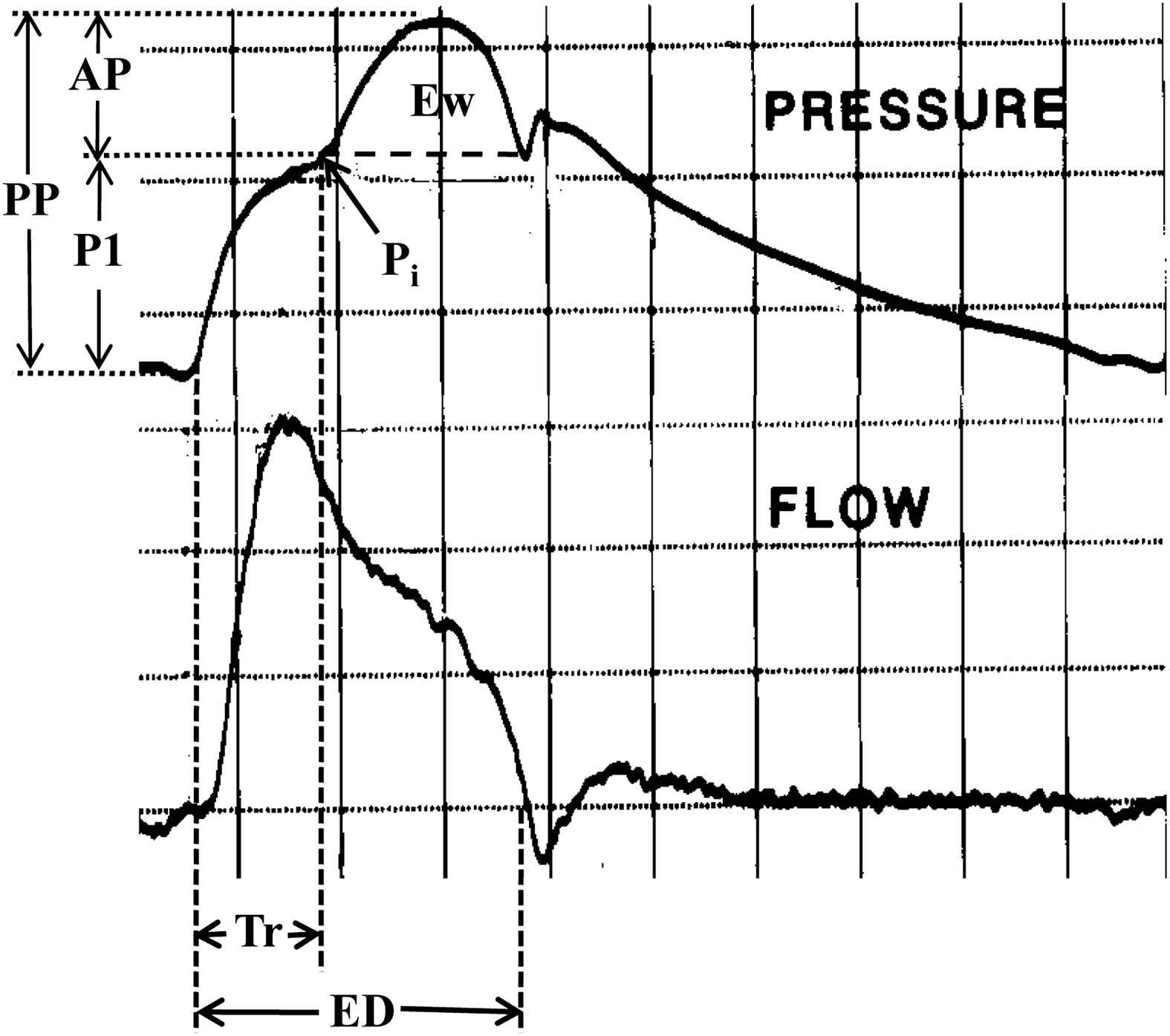
High-fidelity pressure and flow velocity waveforms measured invasively in the ascending aorta of a middle-aged patient. Pi is an inflection point that indicates the beginning upstroke of the reflected pressure wave with amplitude AP and duration (ED − Tr). This enclosed area (Ew) represents energy wasted by the ventricle during ejection. Tr is the round-trip travel time of the forward (or incident) wave (amplitude P1) from the ascending aorta to the major “effective” reflecting site in the lower body and back. The forward and reflected waves add together to give the measured pressure wave with pulse pressure (PP) = (P1 + AP). ED is ejection duration.
Only high-quality recordings, defined as an in-device quality index of >80% (derived from an algorithm including average pulse height, pulse height variation, diastolic variation, and the maximum rate of rise of the peripheral waveform) and acceptable curves on visual inspection, were included in the analysis. All measurements were taken in the supine position in a quiet, temperature-controlled room after a brief rest period of at least 5.0 min. Brachial artery blood pressure measurements (average of three) were performed with a validated, automated oscillometric blood pressure monitor (Omron R3, Omron Healthcare) and cuff. An appropriate size blood pressure cuff was chosen to avoid over estimation of blood pressure in the overweight subjects. In all subjects the cuff was placed on the forearm approximately midway between the shoulder and elbow 2–3 cm above the antecubital fossa. In most normal weight subjects (93%) a standard adult blood pressure cuff (bladder dimension, 24 × 10 cm, for arm circumference 22–32 cm) was used, while in the overweight subjects (95%) a large adult cuff (bladder dimension, 30 × 12 cm, for arm circumference, 32–42 cm) was used.34
Data analysis
Statistical analysis was performed using an unpaired, two-tailed Student’s t-test and regression analysis. Results are presented as means ± SD. The statistical tests were used to compare overweight and control subjects regarding age, height, weight, heart rate, pressure components (brachial and aortic) and wave reflection variables. A P value of <0.05 was considered statistically significant.
Results
Of the 88 overweight subjects studied 43 were males and 45 were females; the BMI was >25 ≤ 30 kg/m2 in 51, >30 ≤ 35 kg/m2 in 25 and >35 kg/m2 in 12 subjects (average for total 30 ± 5.0 kg/m2). The matched normal weight control group of subjects had an average BMI of 23 ± 2.1 kg/m2. There was no significant difference (NS) in age, gender, height, heart rate, ejection duration or diastolic blood pressure between the two groups (Table 1). As expected, older subjects in each group had stiffer arteries and increased augmentation indices and decreased wave reflection travel time. In the overweight group, the age-AIx relationship was shifted upward and to the left compared to the normal control group indicating stiffer arteries and increased wave reflection in the overweight subjects for a given age.
| Normal weight (N = 88) | Overweight (N = 88) | P | |
|---|---|---|---|
| Age (years) | 50 ± 19 | 53 ± 17 | 0.12 |
| Sex (male/female) | 49/39 | 43/45 | |
| Height (cm) | 171 ± 10 | 170 ± 10 | 0.67 |
| HR (beats/min) | 70 ± 11 | 68 ± 10 | 0.11 |
| Weight (kg) | 67 ± 12 | 87 ± 14 | 0.01 |
| BMI (kg/m2) | 23 ± 2.1 | 30 ± 5.0 | 0.01 |
| Brachial SP (mmHg) | 126 ± 15 | 134 ± 18 | 0.002 |
| Brachial DP (mmHg) | 75 ± 6.7 | 76 ± 9.8 | 0.24 |
| Brachial PP (mmHg) | 50 ± 12 | 58 ± 19 | 0.007 |
| P1 (mmHg) | 29 ± 7.0 | 31 ± 9.7 | 0.03 |
| Tr (msec) | 143 ± 15 | 133 ± 14 | 0.001 |
| Aortic SP (mmHg) | 114 ± 14 | 124 ± 17 | 0.001 |
| Aortic mean pressure (mmHg) | 94 ± 9.8 | 98 ± 11 | 0.003 |
| Aortic PP (mmHg) | 37 ± 12 | 44 ± 16 | 0.001 |
| Pulse pressure amplification | 1.5 ± 0.29 | 1.3 ± 0.14 | 0.01 |
| Ejection duration (msec) | 302 ± 25 | 308 ± 25 | 0.63 |
| AP (mmHg) | 8.2 ± 6.5 | 13 ± 7.8 | 0.001 |
| AIx (%) | 20 ± 12 | 28 ± 9.3 | 0.001 |
| (ED − Tr) (msec) | 159 ± 30 | 176 ± 28 | 0.004 |
| Wasted LV energy (dsc−2) | 1498 ± 1375 | 2527 ± 1732 | 0.001 |
HR, heart rate.
SP, systolic pressure.
DP, diastolic pressure.
PP, pulse pressure.
P1, unaugmented pressure.
Tr, time of wave travel to and from periphery.
BMI, body mass index.
AP, amplitude of reflected wave.
AIx, aortic augmentation index.
(ED − Tr), systolic duration of reflected wave.
ED, ejection duration.
Pertinent variables of the normal weight and overweight subjects.
Brachial artery systolic (134 ±18 vs 126 ± 15 mmHg. P < 0.002), mean (98 ± 11 vs 94 ± 9.8 mmHg, P < 0.003) and pulse (58 ± 19 vs 50 ± 12 mmHg, P < 0.007) pressures were greater in the overweight group compared to the control group. Also, central aortic systolic (124 ± 17 vs 114 ± 14 mmHg, P < 0.001) and pulse (44 ± 16 vs 37 ± 12 mmHg, P < 0.001) pressures were greater in the overweight group compared to the control group. Table 1 and Fig. 2 summarize the results of the study. The amplitude of the forward wave (P1) was greater in the overweight (31 ± 9.7 vs 29 ± 7.0 mmHg, P < 0.03) than in the control group suggesting an increase in peak flow or central aortic stiffness. The reflected pressure wave from the lower body arrived at the heart earlier (i.e. Tr was reduced; 133 ± 14 vs 143 ± 15 msec, P < 0.001) with greater amplitude (13 ± 7.8 vs 8.2 ± 6.5 mmHg, P < 0.001) and longer duration (176 ± 28 vs 159 ± 30 msec, P < 0.004) in the group of overweight subjects compared to control group subjects (See example Fig. 2). These differences in wave reflection characteristics produced a higher AIx (28 ± 9.3 vs 20 ± 12%, P < 0.001) and caused the LV to generate more wasted energy (2527 ± 1732 vs 1498 ± 1375 dyne-sec/cm2, P < 0.001) in the overweight subjects than in controls. These differences in arterial properties and wave reflection characteristics were still highly significant after correcting for the elevated mean arterial pressure measured in the overweight group. There were more “Murgo” type “A” waves (AIx > 12%) (80 vs 61) in overweight subjects compared to control subjects and fewer type “B” (12% ≥ AIx > 0.0%) (8 vs 22) and “C” (AIx ≤ 0.0%) (0 vs 5) waves.34
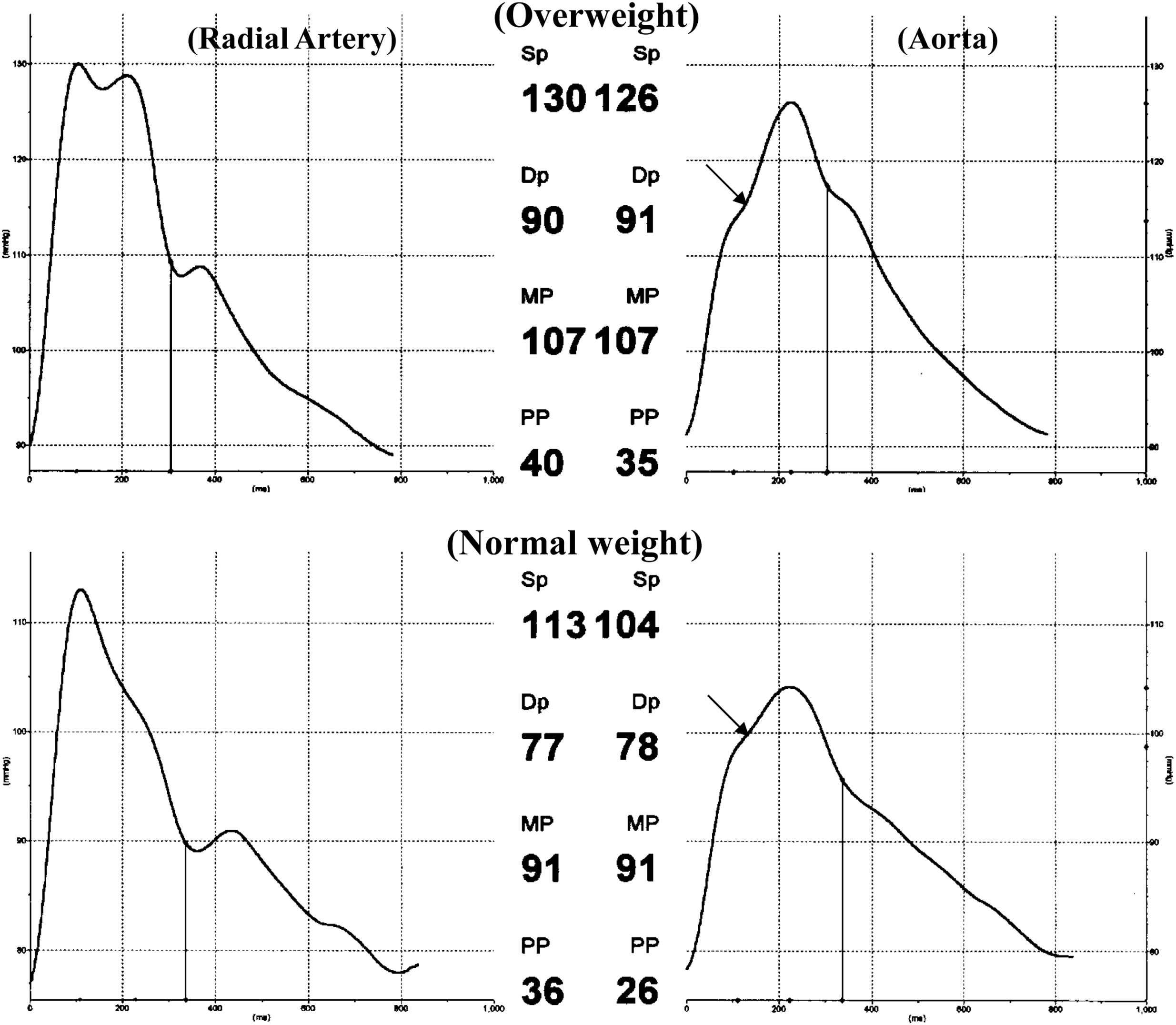
Illustration showing the alterations in radial artery and aortic pressure waves in an overweight subject (above; age 46 yrs and BMI 35 kg/m2) compared to a control subject (below; age 47 yrs and BMI 22 kg/m2). Travel time of the reflected wave was reduced (130 vs 148 msec) and reflected wave amplitude and systolic duration were increased (12 vs 6.0 mmHg; 180 vs 162 msec, respectively) resulting in elevated wasted LV energy (2107 vs 993 dsc−2) in the overweight subjects. In this example pulse pressure amplification and AIx were 1.14 and 35% in the overweight subject and 1.38 and 21% in the normal weight subject.
Discussion
We found in this study, using pulse wave analysis of the non-invasively obtained central aortic blood pressure waveform,38,46,47 that excess body weight as assessed by the BMI adversely alters measures of arterial stiffness, wave reflections and wasted LV energy generation. In this age group reflected wave amplitude is directly related to arterial stiffness and inversely related to major reflecting site distance and travel time of the reflected pressure wave from the lower body to the heart.34,40 Increased arterial stiffness causes an increase in transmission velocity of both forward and reflected waves which in-turn causes the reflected wave to arrive earlier with greater amplitude and duration in the central aorta.40,42 These changes in wave reflection characteristics augment aortic and LV pressure in late systole and increase LV afterload and wasted pressure energy which lead to LVH and cardiac failure.16,43,48 Also, recent studies have shown that central aortic stiffness and wave reflections are directly and positively associated with adverse cardiovascular events.49 Changes in arterial stiffness can alter the aortic pressure waveform by two mechanisms depending on whether the change occurs in the central elastic arteries or the peripheral muscular arteries. The early part (first systolic shoulder) of the aortic pressure wave (or forward pressure wave) with amplitude (P1) is generated by the LV ejection wave (see Fig. 1) through the “water hammer” principle and is dependent upon the elastic properties of the ascending aorta and is not influenced by wave reflections.34,50 An increase in ascending aortic stiffness not only increases P1, it decreases diastolic pressure, and thus, leads to a compromise in coronary blood flow.51 The increase in (P1) in our overweight subjects, therefore, could be due to either increased peak LV ejection velocity or elevated aortic stiffness or both. Although this mechanism increases both central and peripheral systolic and pulse pressure through a dual mechanism, the increase in central pressure is minor compared to the increase caused by amplitude and timing of wave reflection.34,41,52 The later part of the aortic pressure wave (or second systolic shoulder) with amplitude AP is inversely related to Tr and is generated by the reflected wave arriving during systole and adding to the forward traveling pressure wave by the superposition principle [i.e. (P1 + AP) = PP]. This augmentation of the central aortic pressure wave is an indicator of wave reflection severity and can be estimated as an augmentation index (AIx) which is dependent upon the elastic properties of the entire arterial tree, the velocity of the forward and reflected waves and distance to the major “effective” reflection site.34,40 These mechanisms are the major cause of increasing central aortic systolic and pulse blood pressure and decreasing pulse pressure amplification with advancing age and in patients with hypertension and other cardiovascular diseases.34,50,52 These modifications in wave reflection characteristics indicate that overweight subjects have stiffer arteries than normal weight subjects and support data from several previous investigations.20–24 The systolic pressure augmentation resulting from increased arterial stiffness increases circumferential arterial wall stress, LV afterload, wasted pressure energy and myocardial oxygen demand and causes a mismatch in ventricular/vascular coupling. Increased arterial wall stress initiates fracture of elastic load-bearing elements of the arterial wall and causes it to stretch, so that stress is transferred to the stiff collagen elements in the wall while increased LV afterload induces an increase in LV mass.16,34 Increased LV mass, which is associated with overweight even in the absence of increased brachial BP,15 is a powerful independent predictor of heart failure48 and coronary heart disease mortality.53 Also, the observed increase in central pulse pressure exposes the small vessels of the heart, brain and kidney to highly pulsatile pressure which may cause microvascular damage and result in decreased coronary flow reserve,51 renal insufficiency, intellectual deterioration54 and stroke.18
The association between aortic stiffness (aPWV) and body weight have been documented in young, middle-aged and older subjects in the SardiNIA Study,22 the Whitehall II Study,23 the Cardiovascular Health Study55 and the Health ABC Study56 as well as among younger hypertensives.57 Toto-Moukouo et al.57 found that obesity was associated with a 60 cm/s higher aPWV in hypertensive men and a 50 cm/s higher aPWV in hypertensive women compared with non-obese subjects. This increase is similar in magnitude to the 47 cm/s increase in aPWV reported in young obese subjects compared to normal-weight subjects by Wildman et al.20 Also, Danias et al.,58 using magnetic resonance imaging, found that the pressure-strain elastic modulus of the aorta was greater in young obese subjects compared to normal weight subjects. Most previous studies report changes in aortic stiffness only. Our study reports measurements that are influenced by the entire arterial tree (i.e. wave reflections and aortic stiffness).
There are a number of mechanisms by which excess body weight might contribute to increased arterial stiffness modification of wave reflection characteristics. Insulin resistance has been shown to accompany obesity and be associated with arterial stiffness in people with type 2 diabetes.59 This was discussed in detail by Wildman et al.20 but probably does not apply in our study since all our subjects were non-diabetic. The following cardiovascular abnormalities, not necessarily in order of importance, in overweight subjects may be responsible for the BMI-related increase in arterial stiffness and pulse pressure and changes in wave reflection characteristics. The first mechanism by which excess body weight might contribute to aortic stiffening is through increased local activation of the renin-angiotensin-aldosterone system (RAAS). All the components of local RAAS have been found in vascular smooth muscle cells as well as in renal and endothelial cells.6 Secondly, increased body weight has been associated with low-grade inflammation and endothelial dysfunction.60,61 The presence of higher levels of circulating immune system cells may increase movement of these cells into the artery wall, resulting in increased stiffness. C-reactive protein, a measure of inflammation, was found to be positively associated with central and peripheral arterial stiffness62 and AIx.63 Thirdly, obesity might increase aortic stiffening through the hormone leptin, which is secreted mainly from adipocytes. Circulating leptin increases in proportion to body fat and acts on the central nervous system to increase sympathetic activity and inhibit nitric oxide synthesis.64,65 Elevated serum levels of leptin was shown to be associated with increased arterial stiffness in a group of 294 healthy adolescents, independent of fat mass.66 Finally, excess body weight, especially in younger subjects, was found to be positively associated with hypercholesterolemia in the WHO MONICA Project.67 Although assessments of the relationships between lipid profile abnormalities and arterial stiffness have produced some conflicting results, the recent study by Wilkinson et al. found that subjects with hypercholesterolemia had an increased central aortic pulse pressure and AIx.68 Most published reports34,50,52 imply that an increase in arterial stiffness (central elastic and/or peripheral muscular) is associated with an increase in wave reflection strength in middle-aged populations (e.g. 35–65 yrs), which is the age range of subjects in our study. However, in younger and older subject this may not be true. The results of previous studies on overweight and arterial wave reflection characteristics have not been consistent. Three of these studies27,31,33 reported an increase in central augmentation index (AIx), which is comparable to results from our study, two reported no change29,32 and one28 reported a decrease. The reason for these differences is not immediately clear.
As suggested by Wildman et al.,20 the effects of weight loss on the process of arterial stiffening and wave reflection characteristics need to be evaluated in a future clinical trial so that the relative contributions of acute vs chronic effects can be determined. Indeed, recent reports69,70 have shown that weight loss reverses the arterial stiffening process, a focus on early interventions to accomplish weight loss would be wise, along with strategies for the prevention of initial weight gain.
Disclosure of interest
There is no potential conflict of interest.
References
Cite this article
TY - JOUR AU - Wilmer W. Nichols AU - John W. Petersen AU - Scott J. Denardo AU - Demetra D. Christou PY - 2013 DA - 2013/08/29 TI - Arterial stiffness, wave reflection amplitude and left ventricular afterload are increased in overweight individuals JO - Artery Research SP - 222 EP - 229 VL - 7 IS - 3-4 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2013.08.001 DO - 10.1016/j.artres.2013.08.001 ID - Nichols2013 ER -
