Application of non-invasive central aortic pressure assessment in clinical trials: Clinical experience and value
- DOI
- 10.1016/j.artres.2016.10.154How to use a DOI?
- Keywords
- Arterial stiffness; Applanation tonometry; Central aortic pressure; Clinical outcomes; Hemodynamics; Pulse waveform analysis; Wave reflection
- Abstract
Pressure measured with a cuff and sphygmomanometer in the brachial artery is accepted as an important predictor of future cardiovascular (CV) events. However, recent clinical evidence suggests that central aortic pressure (CAP) provides additional information for assessing CV risk than brachial blood pressure (BrBP). Central hemodynamics can now be non-invasively assessed with a number of devices, however, the methodology employed to measure CAP, in order to better identify the patients at higher CV risk in clinical practice, is still controversial. The purpose of this article is to review the technology behind the non-invasive measurement of CAP via the effects of different classes of antihypertensive drugs on CAP and the data supporting the predictive value of assessing CAP on clinical outcomes, and to foster the transfer of methodological knowledge from clinical trials into routine clinical practice.
- Copyright
- © 2016 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Brachial blood pressure (BrBP) is an accepted surrogate marker and major independent risk factor for cardiovascular (CV) disease and decreases in blood pressure have been demonstrated to correlate with reduced incidence of myocardial infarction and stroke.1 However, the BP profile varies along the arterial tree from its origin to the periphery with mean and diastolic BP being relatively constant while systolic BP is higher in the periphery than in the aorta and elastic central arteries.2 Thus, systolic BP (SBP) values are dependent on the site of measurement. Central aortic systolic pressure (CASP) appears to be a more relevant measurement than peripheral pressure, as the aortic pressure the target organ beds receives is proportional to the pressure developed by the left ventricle to propel blood against the arterial pressure. The arterial pressure waveform used to calculate CASP is composed of the forward pressure wave created by ventricular contraction and a reflected wave, originating from the primary wave hitting intersections between elastic and more muscular arteries: the overlap between the anterograde and retrograde reflected waves producing the amplification phenomenon of the arterial pressure wave observed in the aorta.2
BrBP is thus a composite measure of both the CAP and the degree of amplification of the central pressure. The relationship between CAP and BrBP is not fixed, as it depends on a number of factors including arterial wall distensibility and arterial pressure, and the ratio between the two BPs has been termed the amplification ratio. The relationship between CAP and BrBP, specifically the respective pulse pressures, is also strongly dependent upon heart rate. In some hypertensive patients a reduced amplification ratio may be an indicator of the stiffness of the arterial tree. BrBP is usually higher than CAP due to pressure wave amplification.2 Systolic pressure amplification is the ratio between brachial and central SBP and pulse pressure amplification (PPA) is the ratio of brachial to central PP. In healthy individuals PPA is approximately 1.5 and varies from 1.7 at <20 years of age to 1.2 at >80 years of age.3 PPA is variable between subjects but relatively constant for a given individual reflecting the degree of stiffness of the large arteries and the magnitude of wave reflections.4
A number of factors such as age, heart rate and height have differential effects on central and peripheral pressure. In addition, CV risk factors such as hypercholesterolemia, hypertension, smoking and metabolic syndromes, which accelerate aortic stiffening in the large arteries, may have greater effects on CAP.5 CAP increases with age in part because large arteries become stiffer with age, is reduced by low heart rate and shorter body height (reflecting reducing aortic length and volume), and is reduced with low diastolic pressure. Female gender on average is associated with a lower CAP in comparison with males, although PPA is generally lower in females indicative of a higher central relative to brachial pressure.6,7
CAP at the aortic root is regarded as an index of aortic stiffness and represents the true load imposed on heart, brain, kidney and large arteries.8–10 Recent studies have shown that CAP and CAPP are better predictors of CV events and mortality than BrBP.11,12 CAP has also demonstrated clinical value in predicting clinical outcomes in selected populations such as patients with end-stage renal disease (ESRD) and patients with coronary artery disease (CAD) undergoing percutaneous coronary intervention.8,9 CAP measurement is of clinical relevance since it predicts clinical outcomes in both general populations and in patients with CV risk factors.13 Previous studies have looked at the predictive power of central versus peripheral pressure measurements,8,12,14,15 and a meta-analysis by Vlachopoulos et al.11 has shown a trend for CAPP to be more predictive than BrPP (p = 0.057), while no difference for SBP was observed.
The 2003 ESC/ESH guidelines for the management of arterial hypertension recommend that the assessment of total CV risk includes an assessment of target organ damage. CAP is dependent on pulse wave velocity (PWV) and augmentation index (AIx) that are linked to the development of target organ damage in patients with hypertension.16 CAP varies between subjects, and antihypertensive agents (i.e. primarily beta-blockers and heart rate modulating agents) have shown differential effects on CAP despite similar effects on BrBP.17 A substantial overlap of central and brachial BP among categories of hypertension implies that based simply on the brachial cuff BP, but in reference to the effects of central aortic BP on end-organ damage, there are some individuals who should be treated and who are not and others who are on treatment and perhaps might not require it5,18: the paradigm shift that was suggested in the BP Guide study. Measuring CAP in addition to BrBP in patients with CV risk may provide additional information and further characterize blood pressure patterns to improve treatment decisions. The BP GUIDE (value of central Blood Pressure for GUIDing managEment of hypertension) study showed that central BP guidance for hypertension management resulted in a significant reduction in the quantity of antihypertensive medication (across all drug classes) needed to achieve BP control.19 A recent critical analysis between brachial and central systolic pressure showed that their standard deviations were nearly identical.20 This is because the population variation caused by PPA is counterbalanced by the larger measurement and model errors embedded in central SBP. The practical implication is that in a comparative study, the sample size needs to be roughly the same whether brachial or central SBP is used as the primary dependent variable.
Until recently, CAP could only be assessed by invasive measurement. Since 2002, several non-invasive techniques, primarily applanation tonometry have been developed to estimate CAP. Cuff BrBP has been commonly used to calibrate peripheral pulse waveforms, the basis for all CAP estimation methods, obtained by tonometry.21 The widespread use of CAP measurement is hindered by the availability of diverse non-invasive devices and standardization of the method; furthermore, an evidence gap still exists on the predictive value of CAP in prospective studies. The aim of this review is to highlight the clinical relevance of CAP, which can be non-invasively measured in multicenter clinical trials, to assess the effects of antihypertensive treatments on CAP in comparison to BrBP and to better understand their respective predictive value for outcomes.
Non-invasive measurement of central aortic pressure
To evaluate CAP, a variety of non-invasive devices have been developed and made available primarily to specialists and researchers. Techniques for measuring CAP, apart from direct intra-aortic CAP measurement by aortic catheterization, are dependent on the capture of an arterial waveform. There are two methods currently used to capture arterial waveforms: (1) applanation tonometry over the radial or carotid arteries, or ultrasound (2) oscillometric methods, using a standard BrBP cuff, to capture the brachial artery waveform at or near diastolic pressure.19 Conventional BrBP measurements are required to calibrate the arterial waveforms acquired by tonometry or oscillometry to yield a radial artery pressure waveform. For radial tonometry, BrBP is measured using a conventional cuff-based device before proceeding with tonometry while the patient is seated or supine, and a minimal amplification between brachial and radial arteries is assumed. For carotid tonometry, carotid waveforms are calibrated to mean and diastolic pressure based on the principle that mean and diastolic pressure remain relatively constant across the larger conduit arteries of the circulation. In the case of the cuff-based oscillometric method, the BrBP is measured contemporaneously with brachial arterial waveform acquisition, thereby overcoming concerns regarding brachial–radial amplification.3,13,21 Currently there are three main approaches to non-invasively derive CAP from predominantly radial or brachial arterial pressure waveforms: 1) the use of a generalized transfer function or mathematical modeling to process waveforms acquired using volume plethsymography or applanation tonometry21; 2) identification of an inflection point on the descending slope of the systolic pressure wave, called the secondary systolic wave (SBP2) method; and 3) the N-point moving average (NPMA) method.3,13
Recently published reviews on various non-invasive central BP devices have discussed in great detail waveform recording and calibration techniques, clinical applicability, validation, and the respective strengths and limitations of different devices.21–26 Tonometry over the carotid artery or reconstruction of the CAP wave form using a radial-to-aortic transfer function (SphygmoCor) are currently considered to be the gold standard methods for non-invasive CAP measurement (Fig. 1).23 The BPro wrist device (HealthStats, Singapore) derives CAP from non-invasively acquired radial artery pressure waveforms using an N-point moving average method which acts as a simplified transfer function.27
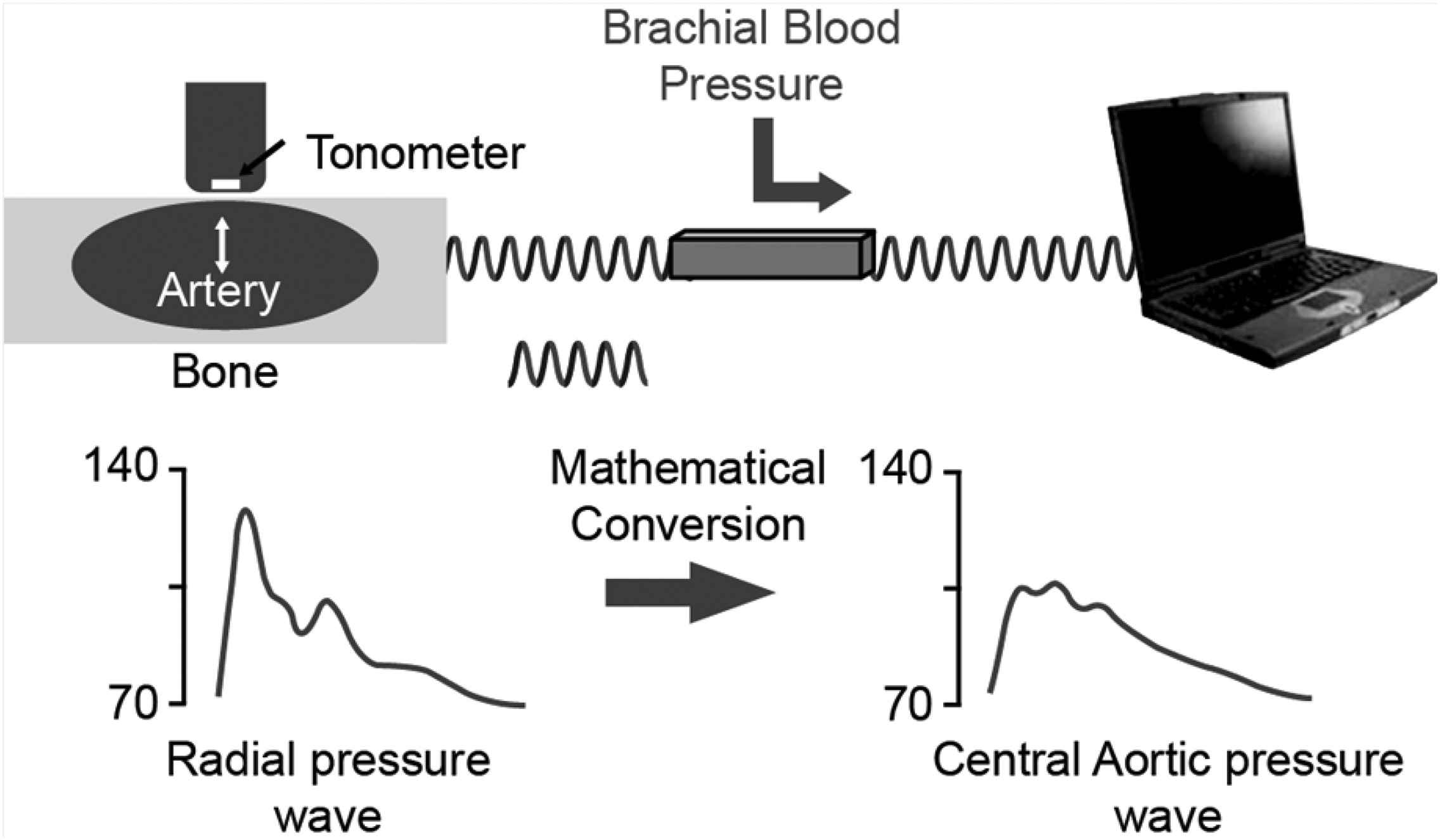
Deriving CAP from the radial pulse wave.
Brachial oscillometric devices use brachial cuff oscillometric waveform measurement and a specific brachial pulse volume waveform transfer function to derive CAP (e.g. Mobil-O-Graph, SphygmoCor XCEL, WatchBP Office, Vicorder, CardioMon). The Pulsecor device (Arteriograph) uses another method consisting of analyzing the oscillometric waveform and using the amplitude of the late second systolic shoulder to derive CAP. The oscillometric method with a generalized transfer function and the NPMA method allow for 24-h central systolic pressure monitoring. Compared to the radial approach, using the brachial cuff-based approach allows results to be free of the brachial to radial amplification phenomena. However, these devices cannot overcome the inherent inaccuracy of cuff-based techniques.21
Other parameters to estimate central hemodynamics and stiffness include AIx, PWV, cardio-ankle vascular Index (CAVI) and ambulatory arterial stiffness index (AASI):
CAP-related indices
- ○
Augmentation Pressure (AP): The height of the late systolic peak above the inflection point defines the augmentation pressure. Simply, AP is the pressure difference (mmHg) between the second and first systolic peaks of the aortic waveform. The first peak (or inflection point) is thought to correspond to the peak of the outgoing pressure wave, whilst the second peak is attributed to the effects of pressure wave reflections augmenting the outgoing pressure wave in late systole (Fig. 2).
- ○
Augmentation Index (AIx) is the augmentation pressure expressed as a percentage of the waveform pulse pressure. AIx is one way to assess global wave reflections.26
- ○
Pulse pressure amplification (PPA) is defined as the ratio of peripheral to central pulse pressure.
- ○
Wave separation analysis (WSA) uses (aortic) pressure and flow to separate pressure waveforms into their forward and backward (reflected) waves.
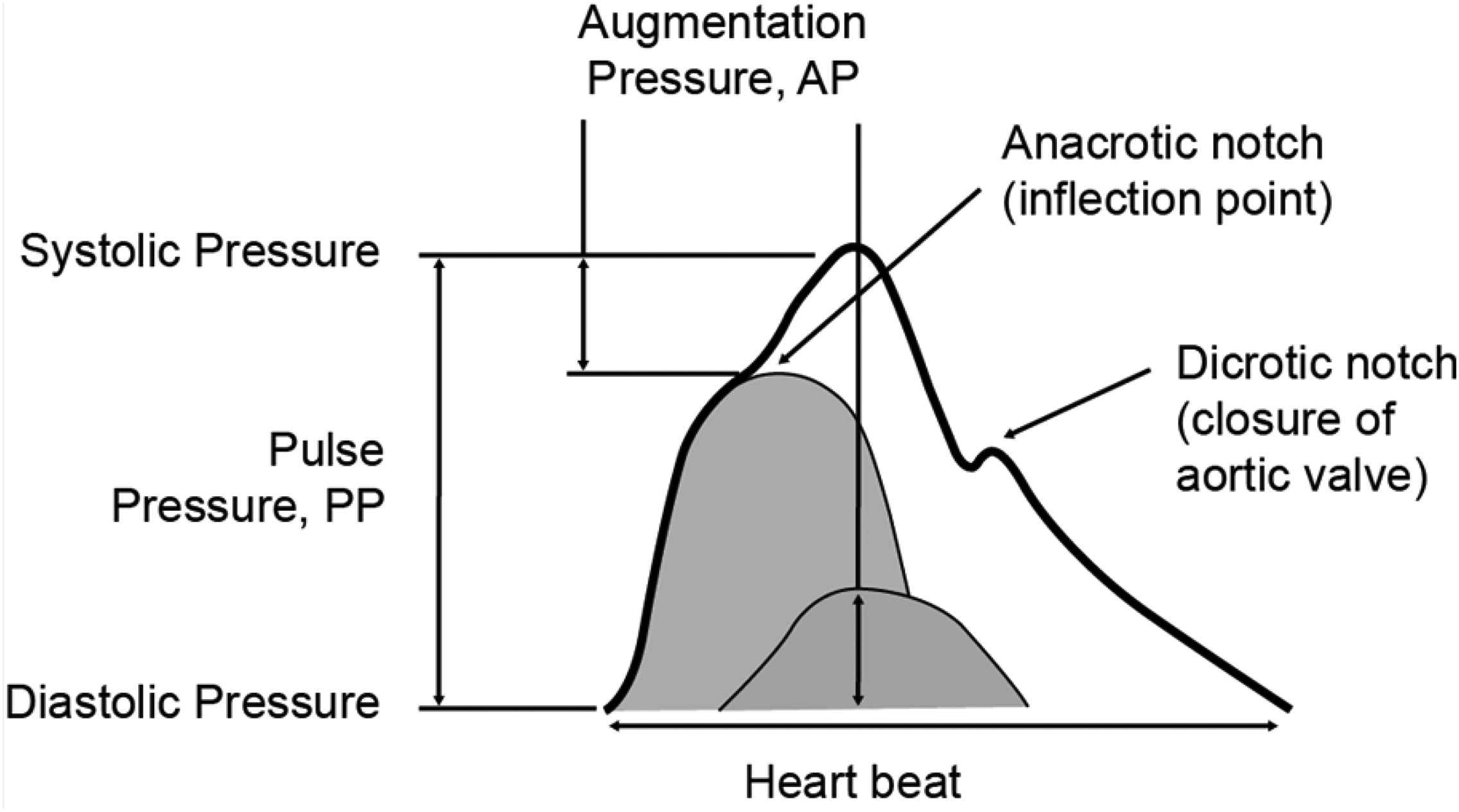
Central pressure wave form.
Aortic stiffness-related indices
- ○
Arterial stiffness is defined as stiffening of large vessels and is measured using PWV: velocity of the pulse wave along a length of artery, measured as a distance/time (m/s). Carotid to femoral PWV is the preferred method to assess arterial stiffness non-invasively.28
- ○
Ambulatory arterial stiffness index (AASI) is derived from individual ambulatory blood pressure monitoring (ABPM) recordings and evaluates the relationship between diastolic and systolic BP over a 24 h period. The AASI index is calculated according the formula: 1-s (the regression slope of diastolic pressure on systolic BP). In theory the stiffer the arterial tree the greater decline in slope or an increase in the AASI.
- ○
Cardio-Ankle Vascular Index (CAVI) is an index reflecting whole body stiffness of the arterial system from the heart to ankles. CAVI is related to the established arterial stiffness parameter β and is obtained by recording the distance from the level of the aortic valve (ie, brachial level) to the measuring point (ie, the ankle) and the time delay between the closing of the aortic valve to the detected change in arterial pressure wave at the set point. One of the advantages of CAVI assessment of arterial stiffness is that it is not affected by changes in BP, unlike cfPWV.
Key practical aspects of implementing CAP measurement based on our clinical experience are listed below. These are based on extensive experience with the SphygmoCor and BPro devices from the aliskiren development programme. More recently, additional experience with the SphygmoCor Excel device has been gained in the PARAMETER study, a study in hypertensive patients using the new drug sacubitril/valsartan (LCZ696).
Key practical aspects of implementing CAP measurement
Selection of the device
Selection of the device should be driven based on what needs to be measured and also the accuracy and validation aspects
Training
- •
Training of the investigators at the investigator meetings which is critical not only to get quality data but also to minimize the intra-and inter-operator variability
Quality control (Qc) aspects
- •
The Qc aspects are critical to increase the quality and limit the variability of the measurements at a given visit and between visits;
- •
Qc is part of the algorithm but should be done by independent cardiologists who should not only review the data but also the individual pulse wave forms and regular feed-back should be given to the centre to improve quality etc.
Overall, measurement of central BP was successful in many multi-centre trials with aliskiren and change from baseline in central SBP closely tracked the change from baseline in brachial SBP (difference ∼1.5 mmHg). Thus, changes in central BP closely track changes in brachial SBP as shown in Table 1.
| CASP studies | Alis Dose (mg) | Comparator Dose (mg) | Duration (wks) | Peripheral SBP Change (mmHg) | Diff (mmHg) | Central SBP Change (mmHg) | Diff (mmHg) | + Central BP (mmHg) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alis | Comparator | Alis | Comparator | ||||||||
| SPP234471 | Alis/HCTZ/Aml vs Ram | 300 ± (12.5–25) ± (5–10) | 10 ± (12.5-25) ± (5–10) | 36 | −22.9 | −20.6 | −2.3 | −20.9 | −18.3 | −2.6 | 0.3 |
| SPPUS0772 | Alis/HCTZ vs Ram | 300/25 | 10 | 8 | −27.9 | −16.4 | −11.5 | −21.7 | −13.3 | −8.4 | −3.1 |
| SPP240973 | Alis/HCTZ vs Aml | 300/25 | 10 | 8 | −28.8 | −26.2 | −2.6 | −27.7 | −25 | −2.7 | 0.1 |
| SPPUS0374 | Alis/HCTZ vs Aml | 300/25 | 10 | 8 | −28.6 | −28.2 | −0.4 | −30.1 | −21.2 | −8.9 | 8.5 |
| SPAUS0175 | Alis/Aml vs Aml | 300/10 | 10 | 8 | −34.1 | −28.9 | −5.2 | −29.8 | −24.2 | −5.6 | 0.4 |
| Average | −28.5 | −24.1 | −4.4 | −26.0 | −20.4 | −5.6 | 1.2 | ||||
Alis: aliskiren; Aml: amlodipine; CASP: central aortic systolic pressure; HCTZ: hydrochlorothiazide; Ram: Ramipril; SBP: systolic blood pressure. BP change represents least square mean difference evaluated using an ANCOVA model.
Bold values show the difference between Aliskiren and comparator for BP change (brachial and central). Bold also shows where the difference (Aliskiren minus comparator) favours the change in brachial over central BP (last column).
Data from Aliskiren CASP studies.
Central aortic pressure as a predictor of clinical outcome
There is growing evidence indicating that CAP is more closely associated with traditional CV risks and arteriosclerotic target organ damage than BrBP. Moreover, aortic stiffness, a major determinant of CAPP, has been shown to be predictive of CV events beyond BrBP. Several prospective observational studies showed the predictive value of CAP parameters for CV events29 in a general population,10,30patients with kidney disease,8,31–33 CAD,14,34,35 or elderly.15 The results from these studies are summarized in Table 2.
| Source | Population | N | Central BP parameter | End point |
|---|---|---|---|---|
| London et al. 200133 | ESRD | 180 | Carotid AIx | All-cause mortality and CV mortality |
| Safar et al. 20028 | ESRD | 180 | Carotid PP, PP amplification | All-cause mortality and CV mortality |
| Briet et al. 201132 | CKD | 180 | Carotid PP | Hemodialysis |
| Weber et al. 200476 | Suspected CAD | 465 | Aortic AP, AIx | Incident CAD |
| Weber et al. 20059 | CAD –PCI | 262 | Radial AIx@Hr75 | CV events |
| Weber et al. 201035 | CAD-coronary angiography | 520 | Radial AIx | CV events |
| Chirinos et al. 200534 | CAD | 297 | Aortic AP, AIx | CV mortality and events |
| Jankowski et al. 200414 | CAD | 1109 | Aortic PP | CV events |
| Roman et al. 200077 | General | 266 | Carotid systolic BP | Relative LV wall thickness |
| Roman et al. 200712 | General | 2403 | Aortic PP | Carotid IMT and mass |
| Roman et al. 200936 | General | 2405 | Aortic PP | CV events |
| Pini et al. 200815 | General | 398 | Carotid PP | CV events |
| Wang et al. 200930 | General | 1272 | Carotid PP | CV mortality |
| Chirinos et al. 201237 | General | 5960 | AIx, RM or PPA | CVE and CHF |
| Chirinos et al. 201538 | General | 6124 | L/ESPTI | HF |
| Williams et al. 200617 | Hypertensive | 2073 | Aortic PP | CVE and procedures, plus renal impairment |
AP: augmented pressure; AIx: augmentation index; AIx@Hr75: AIx adjusted for heart rate of 75 bpm; BP: blood pressure; CAD: coronary artery disease; CKD: chronic kidney disease; CV: cardiovascular; CVE: cardiovascular event; CHF: congestive heart failure; ESRD: end-stage renal disease; HF: heart failure; L/ESPTI: late/early systolic pressure-time integral ratio; LV: left ventricular PP: pulse pressure; PPA: pulse pressure amplification; PCI: percutaneous coronary intervention; RM: reflection magnitude = [Reflected/Forward wave amplitude]× 100.
Studies on predictive value of central BP parameters for CV events.
General population
In the Strong Heart Study, measurement of CAP in 2403 CVD-free American Indians who were followed for 4.8 years, showed that CAPP was a better predictor of fatal and nonfatal CV events than brachial PP, independent of conventional risk factors.12 A subsequent study of the same population with a longer follow-up (5.6 years), reconfirmed the predictive superiority of central over brachial pressure.36 Similar results were observed in a cohort of normotensive and untreated hypertensive elderly individuals.15 A study of the prognostic significance of CAP evaluated in an Asian population showed that central systolic and pulse pressure measurements, but not brachial pressure measurements, predicted 10-year CV mortality.30 A Multiethnic Study of Atherosclerosis (MESA) in an ethnically diverse population (White, African American, Hispanic or Chinese) free of clinically apparent CV disease, showed that reflection magnitude was independently associated with incident CVE and strongly associated with incident CHF. Arterial wave reflections represent an important novel risk factor for CHF and a potential therapeutic target for primary CHF prevention.37 In a subsequent study in the same cohort, late systolic hypertension was shown to be strongly associated with incident HF.38
Vlachopoulos (2010) published a meta-analysis of eleven longitudinal studies in 5648 subjects with hypertension, ESRD, CAD, and in the general population. The results of the analysis showed a 9% increase in the risk of a CV event for every 10 mmHg increase in CAP. Likewise a 10 mmHg increase in CAPP was associated with a 14% increase in CV event risk and an increase in AIx of 10% was associated with a 32% increase in the CV event risk and a 38% increased risk of death from all causes.11 The Dublin outcome study showed that central hemodynamic indices such as AASI derived from ambulatory blood pressure predicted CV events in patients with CV risk and offers new insights into arterial stiffness.39 In a multicenter study, aortic PP was significantly correlated with the presence and extent of CAD in patients without antihypertensive therapy.40 In hypertensive and normotensive subjects, 24-h ambulatory central BP may be a better predictor of CV risk than office BrBP.41
Epidemiological/observational evidence
Patients with renal disease
Patients with chronic kidney disease (CKD) are at greatly increased risk for CV disease. Hypertension is both a cause and effect of chronic kidney disease (CKD). Hypertension causes functional and structural changes in the kidney and is a major risk factor for CV complications. A longitudinal study by London et al. was the first study to demonstrate an adverse impact of increased arterial wave reflection on CV prognosis in patients with end-stage renal disease (ESRD).33 In the same ESRD cohort, Safar et al. demonstrated that an increase in carotid PP was associated with 40% increase in all-cause mortality.8 In patients with mild to moderate CKD, CAPP predicted progression to ESRD significantly and independently of other risk factors.32 A prospective study in patients with CKD stages 2–4, showed that CASP derived from brachial mean and diastolic pressure calibration provides independent, and on top of brachial systolic pressure, prognostic value in the prediction of all-cause mortality.42 In summary, currently available data indicate that elevated CAPP resulting from increased peripheral wave reflection predisposes to subsequent major CV and renal events in patients with various stages of CKD.
Patients with coronary heart disease
Left ventricular afterload and coronary circulation are determined by CAP. Weber (2010) showed that adjusted AIx was significantly predictive for the primary endpoint of death, myocardial infarction and restenosis in patients with CAD undergoing PCI.35 In a prospective study in 297 CAD patients undergoing coronary angiography, Chirinos (2005) found that the aortic augmentation pressure as well as AIx predicted major adverse CV events in male CAD subjects undergoing coronary angiography.34 This finding was further strengthened by Jankowski (2008) who showed that CAPP was a powerful predictor of CV events in CAD patients.14 This evidence clearly indicates that increased and/or premature wave reflection and the resultant widened CAPP, are associated with worse long-term prognosis in CHD. Further, the Japan morning surge 1 study showed that CAPP measurement may be more important to assess cardiac load than BrPP in patients with hypertension.43
Clinical trial evidence
Large, randomized controlled trials such as ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) and the LIFE (The Losartan Intervention For Endpoint reduction) study suggested that drugs like angiotensin converting enzyme inhibitors (ACEIs) and angiotensin-receptor blockers (ARBs) may have effects on arterial stiffness that are beyond the effect on BP.44,45 Further, the clinical value of central BP was demonstrated in ASCOT-CAFE (Conduit Artery Function Evaluation). The main ASCOT trial reported a more favorable CV outcome in hypertensive patients treated with an amlodipine-based treatment regimen relative to an atenolol-based treatment regimen. In the CAFE sub study, amlodipine-based treatment had a significantly greater effect than atenolol-based treatment in reducing CAP whilst BrBP did not differ between treatments. CAPP was also shown to be a predictor of cardiovascular events in this study.46 The REASON study showed that the remodeling effect of a combination regimen with perindopril/indapamide on arterial properties (reflection coefficient) was still active even after 9 months of treatment, but without further reduction of peripheral blood pressure at the same time.47
Guidelines on the measurement of CAP in the context of hypertension management
Great emphasis has been placed on the role of arterial stiffness in the development of CV disease. According to ESC guidelines, evidence of arterial stiffness indicates subclinical organ damage and puts the patient into a higher risk category. For these reasons, arterial stiffness measurement is increasingly used in the clinical assessment of hypertensive patients. The majority of guidelines stress the importance of PP measurement specifically, including the key American, European, Latin American and Japanese guidelines,28,48–50 however they do not provide treatment recommendations for CAP. ESC/ESH 2013, AHA 2015 and JSH 2014 make a strong recommendation for the measurement of PWV, as shown in Table 3. Guidelines have shown that arterial stiffness, assessed by carotid-femoral PWV or indirectly by PP is an independent predictor of CV disease in adults.
| Guideline | PP | PWV |
|---|---|---|
| AHA Scientific statement (2015)28 | NA |
|
| JSH (2014)50 |
|
|
| ESC/ESH 201349 |
|
|
| LASH (2013)48 |
|
NA |
| KDIGO (BP in CKD) (2012)78 |
|
|
| ACCF/AHA (elderly) (2011)79 |
|
NA |
| NKF-KDOQI (CVD in dialysis patients) 200580 |
|
|
ACEI: angiotensin converting enzyme inhibitor; ACCF: American College of Cardiology Foundation; AHA: American Heart Association; ARB: angiotensin receptor blocker; ba: brachial/ankle; CAVI: cardio-ankle vascular index; CAD: coronary artery disease; Cf: carotid/femoral; CKD: chronic kidney disease; CVD: Cardiovascular disease; DBP: diastolic blood pressure; ESC/ESH: European Society of Cardiology/European Society of Hypertension; JSH: Japanese Society of Hypertension; KDIGO: Kidney Disease: Improving Global Outcomes; LASH: Latin American Society of Hypertension; NKF-KDOQI: National Kidney Foundation- Kidney Disease Outcomes Quality Initiative; PP: pulse pressure; PWV: pulse wave velocity; RCTs: randomized clinical trials; SBP: systolic blood pressure.
Existing guidelines on CAP.
Differential effect of antihypertensive treatments on central aortic pressure
Antihypertensive drugs such as renin–angiotensin blockers and/or calcium antagonists are more potent than beta-blockers (BBs) and/or diuretics for lowering CASP even after adjusting for brachial systolic blood pressure (BrSBP).51,52 Beyond the importance of predicting CV disease, knowledge of central pressure may provide guidance in the choice of antihypertensive therapy.53 Treatment with different classes of antihypertensive drugs might have similar effects on BrBP and differential effects on CAP.54 This is evident for BBs, mainly atenolol, which are less effective than other classes of antihypertensives at lowering CAP for any given change in BrBP. This lack of effectiveness at reducing CAP is largely due to the heart rate-lowering effect of BBs, which reduces PPA.17 The EXPLOR study showed that CASP decreased significantly more in the amlodipine-valsartan group than in the amlodipine-atenolol group, despite similar changes in BrSBP.55 RAS inhibitor-based combination therapy with CCB or with diuretics based on the 24-h ambulatory BP profile and arterial properties, may achieve more individualized and ideal 24-h BP control in high-risk hypertensive patients.56
A review article by Protogerou (2009) summarized the effects of different classes of antihypertensive drugs on CAP beyond peripheral blood pressure. This review reported that newer antihypertensive drugs (ACEIs, ARBs and CCBs) as well as nitrates have a more beneficial effect on pressure amplification than older drugs (diuretics and BBs). It also reported compelling evidence regarding the relative detrimental effect of BBs (mainly atenolol) on CAP and convincing evidence that ACEIs increase pressure amplification.57,58 A meta-analysis of the comparative effects of different classes of antihypertensive drugs showed that reduction of BrSBP was larger than CASP with each class of drug. In placebo-adjusted drug vs. drug comparison, treatment with BB, omapatrilat (although treatment with omapatrilat did produce the biggest fall in PPA seen to date) and thiazide diuretics lowered CASP significantly less than BrSBP (i.e. central to brachial amplification decreased), whereas other monotherapies lowered CASP and BrSBP to similar extents.59
Table 4 summarizes the results from the studies evaluating the effects of different classes of antihypertensive drugs as monotherapy or in combination on CAP. Recent studies with newer, more selective vasodilating BB agents, such as nebivolol, carvedilol, and celiprolol, demonstrated these agents to have a greater capacity to reduce CASP than traditional BBs because of their additional vasodilatory property that reduces the influence of wave reflections. The reduction in wave reflections seen with vasodilating BBs appears to offset the adverse heart rate-dependent increases in AIx seen with traditional BBs, such as atenolol. Moreover, the reduction in heart rate itself tends to be less with these vasodilating agents compared to atenolol.
| Source | Drug | Sample size | Result | Central parameters result |
|---|---|---|---|---|
| ACEI | ||||
| London G et al., 1994 | Perindopril | 14, ESRD patients with LVH | Positive | Decrease in central BP, aortic and arterial PWV |
| Chen et al., 1995 | Fosinopril | 41, Hypertension | Positive | Lowered 24-hr ambulatory BP and normalized the elevated AIx |
| London G et al., 1996 | Quinapril | 12, Hypertension and ESRD | Positive | Pronounced and sustained decrease in carotid SBP and PP |
| Mitchell GF et al., 2002 (CHOIR study) | Enalapril | 87, Hypertension | Neutral | Reduced significantly and to the same degree both peripheral and central SBP and PP |
| Deary AJ et al., 2002 (ADLIB study) | Lisinopril | 30, Hypertension | Neutral/negative | Reduced significantly and equally both brachial and aortic SBP in men. No reduction in AIx, BNP and arterial stiffness |
| Stokes GS et al., 2003 | Captopril | 11, Hypertension | Neutral | Decrease in aortic and brachial BP was similar with no reduction in AIx |
| Morgan T et al., 2004 | Enalapril or Perindopril | 32, Hypertension | Positive | Higher effect on central SBP and PP. Reduction in pressure wave reflections and augmentation pressure |
| Hirata K et al., 2005 | Ramipril | 30, Patients with coronary risk factors | Positive | Reduction in central BP, pressure wave reflections (AIx) and arterial stiffness (PWV) |
| Dart AM et al., 2007 (ANBP2, substudy) | Enalapril | 258, Hypertension | Positive | Reduction in central BP |
| Aznaouridis K et al., 2007 | Captopril, Quinapril | 25 per group, Hypertension | Positive | Decreased central BP and reduced AIx |
| Jiang XG et al., 2007 | Enalapril | 46, Hypertension | Positive | Reduced aortic systolic, PP and AIx. |
| Mackenzie et al., 2009 | Perindopril | 15, ISH | Positive | Reduced central PP and AIx |
| ARB | ||||
| Mahmud A, Feely J. 2000 | Valsartan | 18, Hypertension | Positive | Reduced central BP and AIx |
| Asmar 2001 | Telmisartan | 23, Hypertension and T2DM | Positive | Reduced carotid/femoral PWV, central BP and PP |
| Stokes GS et al., 2003 | Eposartan | 11, Hypertension | Neutral | Decrease in aortic and brachial BP was similar with no reduction in AIx |
| Dhakam Z et al., 2006 | Eposartan | 21, Hypertension | Positive | Reduced pressure wave reflections (AIx), aortic stiffness (cf-PWV) |
| Aznaouridis K et al., 2007 | Telmisartan | 25, Hypertension | Neutral | No significant effect on central BP |
| Schneider et al., 2008 | Irbesartan | 75, Hypertension | Positive | Preserved PP amplification and decreased AIx |
| Kim et al. 201481 | Losartan | 88, Hypertension | Positive | Reduced central BP, cf-PWV and AIx |
| Shimizu et al. 201582 (J-TOP) | Candesartan | 180, Hypertension | Positive | Reduction in central SBP |
| CCB | ||||
| London G et al., 1994 | Nitredipine | 10, ESRD patients with LVH | Positive | Decrease in central BP, aortic and arterial PWV |
| Deary AJ et al., 2002 (ADLIB study) | Amlodipine | 30, Hypertension | Neutral | No change in pressure wave reflections, AIx was reduced in men, but not in women. |
| Morgan T et al., 2004 | Amlodipine or Felodipine | 32, Hypertension | Positive | Reduced AIx |
| Mackenzie et al., 2009 | Lercanidipine | 14, ISH | Positive | Reduced central PP and AIx |
| Williams et al., 2006 (CAFE study) | Amlodipine | 1042, Hypertension | Positive | Reduction in CAP, CAPP and AIx |
| Diuretics | ||||
| Deary AJ et al., 2002 (ADLIB Study) | Bendrofluazide | 30, Hypertension | Negative | Aortic SBP was not reduced, although brachial SBP was significantly reduced |
| Morgan T et al., 2004 | Hydrochlorothiazide | 32 | Neutral | Reduced AIx |
| Mahmud A, Feely J. 2005 | Bendroflumetazide | 24 | Neutral | Reductions in PWV and AIx were not significant |
| Dart AM et al., 2007 (ANBP2 substudy) | Diuretics | 221 | Positive | Reduction in central BP |
| Jiang XG et al., 2007 | Indapamide | 55, Hypertension | Neutral | No change in AIx |
| Mackenzie et al., 2009 | Bendrofluazide | 13, ISH | Positive | Reduced central PP |
| Beta blockers | ||||
| Chen et al., 1995 | Atenolol | 38, Hypertension | Negative | Lowered 24-hr ambulatory BP and lowered the elevated AIx |
| Asmar RG et al., 2001 (REASON Study) | Atenolol | 202, Hypertension | Negative | Increased AIx |
| Deary AJ et l, 2002 (ADLIB study) | Bisoprolol | 30, Hypertension | Negative | Reduced central BP and Increase AIx |
| Morgan T et al., 2004 | Atenolol | 32, Hypertension | Negative | Greater rise in augmentation pressure and a larger AIx |
| Hirata K et al., J 2005 | Atenolol | 30, Patients with coronary risk factors | Negative | Little change in either aortic or brachial systolic pressure and no significant reduction in PWV |
| Dhakam Z et al., 2006 | Atenolol | 21, Hypertension | Negative | Reduced PP amplification and pressure wave reflections (AIx) |
| Schneider et al., 2008 | Atenolol | 81, Hypertension | Negative | Increased AIx and attenuated PP amplification |
| Dhakam Z et al., 2008 | Nebivolol | 16, ISH | Neutral | Reduction in central PP and less pronounced increase in AIx |
| Dhakam Z et al., 2008 | Atenolol | 16, ISH | Negative | Increased central PP and significant increase in AIx |
| Mahmud A, Feely J. 2008 | Nebivolol | 20, Hypertension | Positive | Increased PP amplification and reduction in augmentation pressure and AIx |
| Mahmud A, Feely J. 2008 | Atenolol | 20, Hypertension | Negative | Decreased PP amplification and AIx |
| Mackenzie et al., 2009 | Atenolol | 17, ISH | Negative | No effect on central PP and AIx increased |
| Kampus et al. 201183 | Nebivolol | 30, Hypertension | Positive | Reduction in central BP and PP, AIx and PWV |
| Kampus et al. 201183 | Metoprolol | 33, Hypertension | Neutral | No significant change in AIx and PWV |
| Soanker et al. 201284 | Nebivolol | 13, Hypertension | Positive | Reduced CAP, AIx and cf-PWV |
| Studinger et a. 201385 | Carvedilol, Nebivolol, or Metoprolol | 21,21,18, Hypertension | Neutral | Similar decreases in central BP with no significant alterations in AIx and PWV |
| Zhou et al. 201386 | Bisoprolol | 54, Hypertension | Positive | Reduced aortic PP and increased AIx |
| Zhou et al. 201386 | Atenolol | 55, Hypertension | Negative | Increase in Aortic PP, and augmentation pressure and decrease in PP amplification |
| Redon et al. 201487 | Nebivolol | 69, Hypertension | Neutral | Reduction in CAP and less pronounced impact on AIx |
| Kim et al. 201481 | Carvedilol | 94, Hypertension | Positive | Reduced central BP, cf-PWV and AIx |
| Williams et al., 2006 (CAFE study) | Atenolol | 1031, Hypertension | Negative | Increase in central pressure and AIx |
| DRI | ||||
| Kanaoka et al., 201288 | Aliskiren-based therapy | 21, Hypertension | Positive | Decrease in central BP and ba-PWV |
| Williams et al., 2013 (AmCAP)89 | Aliskiren | 87, Hypertension | Positive | Decreased central BP and PP amplification |
| Baschiera et al., 2014 (AGELESS sub-study)71 | Aliskiren-based therapy | 78, Hypertension | Positive | Reduction in CASP and PP amplification |
| Bonadei et al. 201490 | Aliskiren | 30, Hypertension | Positive | Decreased cf-PWV |
| Lacy et al. 201491 | Aliskiren | 16, Hypertension | Positive | Reduced central BP during exercise |
| Lacy et al., 2015 (ASSERTIVE-CASP)92 | Aliskiren | 157, Hypertension | Positive | Sustained control of central SBP |
| Source | Combination | Drug | Sample size | Result | Central parameters result |
|---|---|---|---|---|---|
| Matsui et al., 2009 (J-CORE) | ARB + CCB | Olmesartan + Azelnidipine | 103, Hypertension | Positive | Reduction in central SBP, aortic PWV and AIx. Increase in PP amplification |
| Doi et al., 2010 | ARB + CCB | ARB + Azelnidipine | 18, Hypertension | Positive | Reduction in AIx |
| Boutouyrie 2010 (EXPLOR) | CCB + ARB | Amlodipine + Valsartan | 193, Hypertension | Positive | Reduction in central SBP and AIx |
| Matsui et al., 2009 (J-CORE) | ARB + Diuretic | Olmesartan + Hydrochlorothiazide | 104, Hypertension | Positive | Decrease in central BP and PP |
| Doi et al., 2010 | ARB + Diuretic | ARB + Trichlormethiazide | 19, Hypertension | Neutral/Positive | Reduction in AIx |
| Kwon et al. 201393 | Diuretic + ARB | Chlorthalidone + Candesartan | 13, Hypertension | Positive | Reduction in central BP, PWV and AIx. |
| Asmar 2001 (REASON) | ACEI + Diuretic | Perindopril + Indapamide | 204, Hypertension | Positive | Reduction in carotid and aortic AIx; increase in PP amplification |
| Ferdinand et al., 2011 (ATLAAST) | DRI + Diuretic | Aliskiren + Hydrochlorothiazide | 166, Hypertension | Positive | Reduction is central SBP |
| Whaley-Connell et al., 2011 (ATTAIN)72 | DRI + Diuretic | Aliskiren + Hydrochlorothiazide | 193, Hypertension | Positive | Reduction in central BP |
| Townsend et al. 201173 | DRI + Diuretic | Aliskiren + Hydrochlorothiazide | 85, Systolic hypertension and T2DM | Positive | Reduction in central SBP and AIx |
| Black et al. 201175 | DRI + CCB | Aliskiren + Amlodipine | 72, Hypertension | Positive | Reduction in central BP |
| Williams 2006 (CAFE study) | BB + Diuretic | Atenolol + Bendroflumethiazide | 1031, Hypertension | Negative | Increase in central pressure and AIx |
| Williams 2006 (CAFE study) | CCB + ACEI | Amlodipine + Perindopril | 1042, Hypertension | Positive | Reduction in CAP, CAPP and AIx |
| Boutouyrie 2010 (EXPLOR) | CCB + BB | Amlodipine + Atenolol | 200, Hypertension | Negative | Increase in AIx |
| Izzo et al. 201294 | ACEI + ARB | Lisinopril + Valsartan | 30, Hypertension | Positive | Lowered central systolic pressure and augmentation pressure |
| Izzo et al. 201294 | ACEI + BB | Lisinopril + Carvedilol | 30, Hypertension | Neutral | No reduction in augmentation pressure |
Data modified from Protogerou et al., 2009 and Manisty et al., 2013 (References are cited for studies which are additional to those cited in Protogerou et al., 2009 and Manisty et al., 2013).
The studies are classified according to the antihypertensive class and effect (positive/negative/neutral) on central blood pressure, studies using monotherapy or combination treatment are included.
ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; AIx: augmentation index; BP: blood pressure; BB: beta blocker; BNP: brain natriuretic peptide; ba-PWV: brachial/ankle pulse wave velocity; CAP: central aortic pressure; cf-PWV: carotid/femoral pulse wave velocity; DRI: direct renin inhibitor; ESRD: end stage renal disease; LVH: left ventricular hypertrophy; PP: pulse pressure; T2DM: type 2 diabetes mellitus.
Note: Data from the CAFE study is presented under both mono and combination therapy as treatment in ASCOT was initiated as monotherapy and many participants were up-titrated to combination therapy.
Studies evaluating the effects of different classes of antihypertensive drugs (monotherapy or combination) on central aortic pressure.
Discussion
There is growing evidence to support the importance of CAP as a marker of risk and treatment efficacy. Individuals stratified by BrBP experienced a considerable overlap in CASP. Over 70% of individuals categorized as having ‘high-normal’ BrSBP had similar CASP to those with stage 1 hypertension.22 An individual patient data meta-analysis of prospective observational data from 22,433 subjects showed CASP to be a better predictor for future stroke than BrSBP, but not MI.60 Assessment of CASP may improve stroke prediction particularly in younger individuals. Hence measuring CAP in addition to BrBP may bring additional information and further characterize blood pressure patterns to improve treatment decisions. There is a need for large, well-designed trials to investigate whether lowering CASP will produce more benefit in terms of reduction in CV events than reduction in BrBP. Such trials should involve use of an agent that reduces CASP differentially to BrBP. Potential agents would include a nitrate or a nitrate/hydralazine combination.
Each of the non-invasive devices has its own limitations regarding methodological procedures, reproducibility and validity. Below are the practicalities of implementing CAP measurement in clinical trials.19,22,24,61
- ➢
The currently available non-invasive techniques provide only an estimation of CAP and not a direct measurement. Thus new devices probably have to demonstrate good agreement with invasively measured CASP as the “gold standard”.
- ➢
Errors can occur by using inaccurate upper arm cuff BP values to calibrate the pressure waveforms.
- ➢
Calibration errors of pressure waveforms with or without the use of transfer function.
- ➢
Perception of error being introduced into CAP estimations by using a transfer function.
- ➢
A small degree of amplification between the carotid artery and aorta might lead to over-estimation of CAP.
- ➢
Applanation tonometry is highly operator-dependent, requires skill and experience and might be difficult to use routinely in a non-specialist setting.
- ➢
Quality control issues on validity and inter-device variability.
- ➢
Lack of consensus on standardization of CAP assessment methods. A consensus document for protocols to validate central pressure measurement devices is currently being written by a group from The Society for the Assessment of aRTErial structuRe and physiology (ARTERY).62
There are several studies such as ONTARGET (Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial), TRANS (TRanscend Arterial stiffNess Substudy), LAAS (Losartan Anti-Atherosclerosis Study), ADVANCED-J and PARAMETER (The Prospective comparison of Angiotensin Receptor neprilysin inhibitor with Angiotensin receptor blocker MEasuring arterial sTiffness in the eldERly) evaluating the effects of BP lowering treatment on arterial stiffness. Two large studies TRANS63 and ONTARGET64 are still ongoing to evaluate the effects of telmisartan alone or in combination with ramipril. TRANS study would assess the beneficial effects of telmisartan on CV outcome and its relation to improvement in arterial stiffness. ONTARGET showed that telmisartan was equally effective to ramipril in patients with vascular disease or high-risk diabetes and was associated with less angioedema. The ADVANCED-J study is a prospective clinical study that was designed to compare the increase in dose of ARB and the combined use of amlodipine in terms of BP control in patients with diabetes and hypertension and whose BP levels are inadequately controlled by single treatment with an ARB.65 LAAS is a randomized, open-label, comparative, non-inferiority, multicenter study comparing the efficacy of losartan and carvedilol on arterial stiffness in patients with essential hypertension (ClinicalTrials.gov Identifier: NCT00496834).
PARAMETER is the first prospective randomized study showing that sacubitril/valsartan (LCZ696), the first ARNI, reduces CAP and CAPP more effectively than an ARB in high-risk, older patients with systolic hypertension and an increased PP. These results suggest that LCZ696 provides beneficial effects on central aortic hemodynamics and function that could provide a therapeutic advantage beyond those observed with RAS blockade alone.66
Measurement of CAP from clinical trials to clinical practice
Measurement of CAP in clinical practice is likely to have important implications for the future management of hypertension. The study by Chen (2013) represents an important step towards the application of the CAP concept to assess clinical risk for CVD. It showed that a CAP of 130/90 mm Hg was the threshold for the diagnosis of hypertension and was characterized by a greater discriminatory power for CV mortality.67 A further study by Herbert (2014) provided the distributions of CAP values and amplification of BP in a normal population without CV risk factors and in a reference population with CV risk factors according to age and sex.68 Additionally, the recent approval of non-invasive CAP assessment by the Renal Physicians Association (US), to move CAP waveform analysis from Category III (tracking) to Category I (fully eligible for reimbursement) has been a significant step in the recognition of the value of CAP measurement. This latest development is expected to further accelerate the clinical adoption of CAP measurement and should contribute to improve BP management in the United States (AMA CPT Report). Future recognition, by regulatory bodies such as the FDA, of CASP as a surrogate end-point in hypertension trials should also help to accelerate this process.69
Future directions for CAP research
Robust data from large clinical trials/meta-analysis is required to show the value of CAP over BrBP in predicting CV outcomes, particularly outcomes relating directly to hemodynamic stress. Further data is also required to show that CAP provides added value for risk stratification to complement information provided by conventional BrBP. Such data may be acquired in younger people where amplification is particularly variable.
Data from clinical trials looking at CAP as a therapeutic target are warranted to further characterize the value of CAP as a new therapeutic target and to further distinguish antihypertensive drugs that may differentially influence CAP and BrBP. Large scale application of CAP measurement is now possible due to the ease of use of new devices making CASP measurement available to paramedic personnel in routine practice. This might allow potential assessment of the real life impact of clinical practice change. Development of self-measurement of CAP would also enable patients to measure CAP at home without the need for a specialist operator. Comparison of home CAP data against the newly arising technique of 24-h CAP measurement will also provide important data. Further data is also required from large cohort studies to establish firm threshold values for central pressure in the diagnosis of increased CAP across different ranges of BrBP where there is considerable overlap in CAP values. Information may also be collected to investigate whether such potential thresholds might be influenced by factors such as age or gender, known to influence pressure amplification. Moreover, the beneficial effects of lowering CAP to target treatment and the value of such treatment targets needs to be further established. A further logical development would be measurement of CAP with non-cuff based systems. Indeed, such systems are currently in development.70 CAP measurement may improve efficacy and safety in drug development. FDA considered the possibility of including CAP as a surrogate in anti-hypertensive drug trials, and makes the point that some non-cardiovascular drugs may increase CAP whilst having little effect on brachial pressure, antiretroviral drugs being a good example.69
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
Dr Patrick Brunel, Dr Fabio Baschiera and Dr Dion Zappe were involved in the writing of this review; they are employees of Novartis and are therefore eligible for Novartis stock and stock options.
Acknowledgements
All authors participated in the development and writing of the paper, and approved the final manuscript for publication. The authors take full responsibility for the content of the paper and thank medical writer Madhavi Dokku (Novartis) for medical writing support, editorial assistance, collation and incorporation of comments from all authors.
References
Cite this article
TY - JOUR AU - Bryan Williams AU - Patrick Brunel AU - Peter S. Lacy AU - Fabio Baschiera AU - Dion H. Zappe AU - Kazuomi Kario AU - John Cockcroft PY - 2016 DA - 2016/12/05 TI - Application of non-invasive central aortic pressure assessment in clinical trials: Clinical experience and value JO - Artery Research SP - 1 EP - 15 VL - 17 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2016.10.154 DO - 10.1016/j.artres.2016.10.154 ID - Williams2016 ER -
