Viscoelastic mechanical measurement of the healthy and atherosclerotic human coronary arteries using DIC technique
- DOI
- 10.1016/j.artres.2017.02.004How to use a DOI?
- Keywords
- Coronary artery; Atherosclerosis; Mechanical properties; Relaxation test; Quasilinear viscoelastic model
- Abstract
Purpose: Atherosclerotic is a specific form of vascular disease showed to be in charge of the 30% of mortalities in the United States alone. Many studies so far have been reported on the linear and nonlinear mechanical properties of the human and animal coronary arteries. However, the Quasilinear Viscoelastic (QLV) mechanical behavior of the healthy and atherosclerotic human coronary arteries have not been well quantified in spite of the time-dependent mechanical behavior of the arterial walls. This study was aimed to set up a new relaxation viscoelastic tests to characterize the QLV parameters of the healthy and atherosclerotic human coronary arteries.
Methods: Ten healthy and atherosclerotic human coronary arteries were subjected to relaxation test and the QLV parameters were calculated by comparing the QLV model to that of stress-relaxation data.
Results: The findings showed the highest stress in the atherosclerotic coronary samples (292.02 ± 18.14 kPa) (Mean ± SD) which is found to be higher than that of the healthy ones (18.12 ± 2.88 kPa) (p < 0.05). In addition, the stress-relaxation diagrams showed that the healthy coronary arteries can reach to a balance in slightly a lower time (1400 ± 24.15 sec) compared to the atherosclerotic ones (1800 ± 38.12 sec) (p < 0.05).
Conclusions: These data might provide a deep understanding not only for the viscoelastic time dependent mechanical behavior of the healthy and atherosclerotic human coronary arteries but also for the biomechanical experts in different fields of research including, tissue engineering, intervention and bypass surgery and stenting.
- Copyright
- © 2017 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Atherosclerosis is a typical source of heart attacks, strokes, and peripheral vascular disease which is ranked as the first cause of death in the United States with more than 800,000 deaths in 2005.1 The main function of arteries is to carry the blood from the heart all the way through the body by help of a thin layer of cells, namely the endothelium. Endothelium is in charge of providing a smooth condition to pave the way for the blood to flow in the body.2,3 However, atherosclerosis can subject these cells into a substantial alteration by different side factors, such as high blood pressure, high cholesterol, and smoking, which finally by entering the Low-density lipoprotein (LDL) to the damaged endothelium will result in plaque formation inside an artery wall.4,5
The mechanical properties of the coronary artery as a result of plaque formation inside the arterial wall may alter. This has been confirmed by Karimi et al.6 via a comparative study on the uniaxial linear elastic mechanical properties of the healthy and atherosclerotic human coronary arteries. The results of that study revealed that the elastic modulus of the healthy arteries is 2.53 times higher than the atherosclerotic arteries. A biaxial tensile test that has been conducted by Kural et al.7 on the mechanical properties of the healthy and diseased porcine coronary arteries exhibited that the diseased coronary specimens are relatively stiffer than that of healthy ones not only in terms of the Young’s modulus but also in terms of the maximum stress in both the axial and circumferential directions. The alteration of the biomechanical factors based on the contractile responses to endothelin-1 between the healthy and atherosclerotic arteries was investigated under the circumferential loading.8 The results reveled the important of endothelin-1 in the mechanical properties of the atherosclerotic arteries. Furthermore, the alteration of mechanical properties of the human coronary arteries by considering the age and sex were experimentally quantified using inflation test under circumferential loading.9 However, they only used healthy samples for their mechanical measurements. Moreover, several nonlinear isotropic or anisotropic constitutive models have been employed to designate the mechanical properties of the human coronary arteries, including Neo-Hookean,10,11 Mooney-Rivlin,12 and Ogden.13 Nonetheless, so far the Quasilinear Viscoelastic (QLV) mechanical properties of the coronary arteries through the Prony series and ramp/hold model have not been determined. Since most research communities want to benefit from the time-dependent mechanical behavior of the arteries, the findings of the current research can provide a wide range of data for the medical communities to have a better outlook of the arterial mechanical behavior. Therefore, the results of this study would provide such suitable mechanical data for diversity of disciplines, such as tissue engineering, cardiac surgeries, and robotic surgeries. Holzapfel et al.14 proposed a two-layer structural model for the viscoelastic behavior of the arterial walls. Their proposed model enables to predict the unstimulated or passive time-dependent three-dimensional stress and deformation state of the healthy young arterial walls under various loading conditions. Fung’s QLV model which has a advantage of small number of samples as well as smooth testing condition has also been employed to define the response of many types of soft biological tissues and, indeed, its suitability was well confirmed.15–17
In this study, a combination of Prony series as well as ramp/hold model18,19 was used to capture the mechanical response of the healthy and atherosclerotic human coronary arteries as a function of time. The results would provide a set of comparative understanding on the mechanical properties of the healthy and atherosclerotic arteries under stress relaxation loading.
Materials and methods
Specimen preparation, mechanical testing, Digital Image Correlation (DIC) technique
The process of preparation of the arterial tissues, testing procedure20–22 as well as data analysis23 were comprehensively discussed in the previous publications of the authors. Fleetingly, a group of ten (five healthy and five atherosclerotic) human coronary arteries were removed from the cadavers under permission from donators under the ethical rules of the TUMS according to the 2008 Declaration of Helsinki within five-hour postmortem to minimize the tissue degradation. The reason of the death for the healthy cadavers were all related to the accident or trauma, while the atherosclerosis cadavers were all died due to the stroke or heart-related diseases. At least 10 hearts were excised from the healthy and atherosclerotic individuals and their coronary arteries delicately removed for further study using surgical scalpel. The top (a) and side view (b) of the heart is shown in Fig. 1. The coronary artery was then carefully removed by a skilled surgeon for following mechanical measurements. In order to figure out whether the obtained tissues are healthy or atherosclerosis, picro Sirius red staining of the cap was done and the tissues were imaged using polarized light microscopy images (Olympus, Tokyo, Japan). In addition, since the samples used in the current study have also been used in the recent research of the authors.46 The process was that some of the samples were employed for this study and some other for our previous studies.24,25 A constant strain rate was applied to the arterial tissue samples using the universal testing machine. An arterial wall during the stress relaxation test is shown in Fig. 2a. An arterial wall was mounted on the testing machine and a constant strain rate was applied through a moving jaw. Displacement/strain of the samples, as in the previous section mentioned, were recorded using DIC method. Three cameras were set on each tissue and deformations of the samples were precisely recorded for further mechanical measurements. A pair of sand papers were also placed between the jaws of the machine to hinder slip boundary conditions. The arteries were, subsequently, carefully cleaned from the surrounding tissues and well-looked-after in a solution of 0.90% w/v of NaCl at 4–5 °C beforehand of the relaxation test. The dimensions of the samples were around 25 mm in length with inner and outer diameters of 3.98 ± 0.25 (Mean ± SD) and 5.00 ± 0.31 mm, respectively, for the healthy ones. The dimensions of the atherosclerotic arteries were 4.11 ± 0.18 and 5.09 ± 0.26 mm for the inner and outer diameters, respectively, and almost the same length as the healthy ones. All dimension measurements were carried out using a digimatic ruler with a resolution of 0.005 mm ± 0.05% (Insize, Vienna, Austria). It is actually quite difficult to measure the dimension of the soft tissues, especially arterial ones, as their thickness would be different at various locations. Therefore, at least fifteen random spots were defined on each sample and measured via an Insize digimatic ruler before the test. The stress relaxation test was conducted using a uniaxial tensile test apparatus which somehow modified for testing the soft biological tissues. The average value of those fifteen points were employed for further stress quantification.
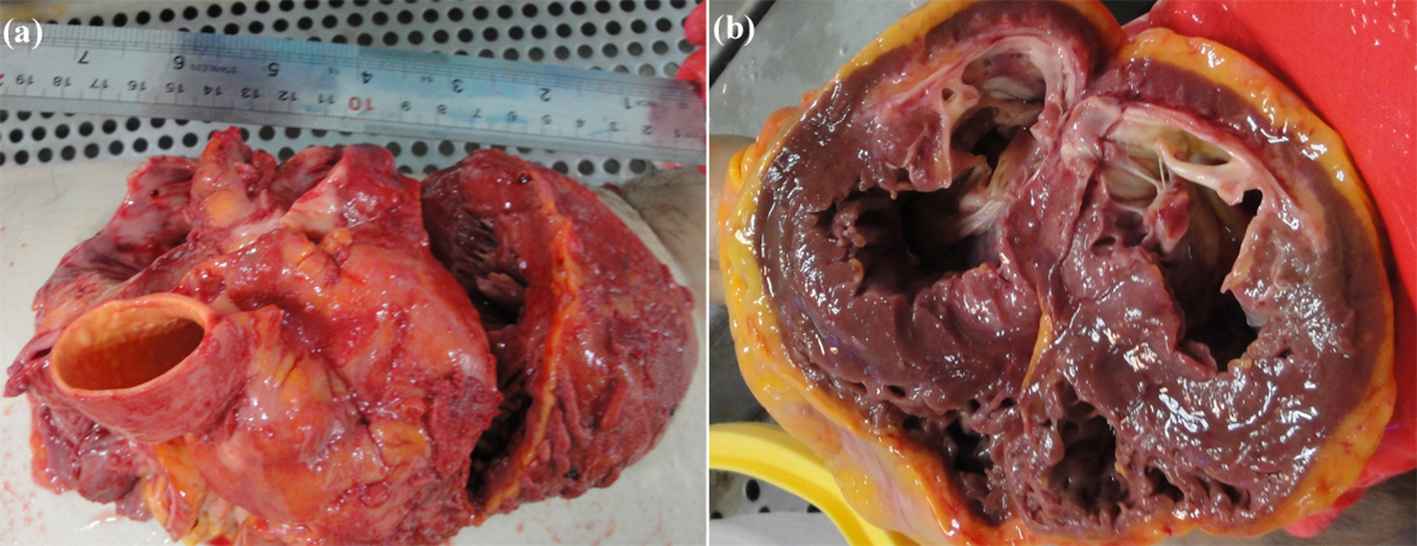
The (a) side and (b) top view of a heart.
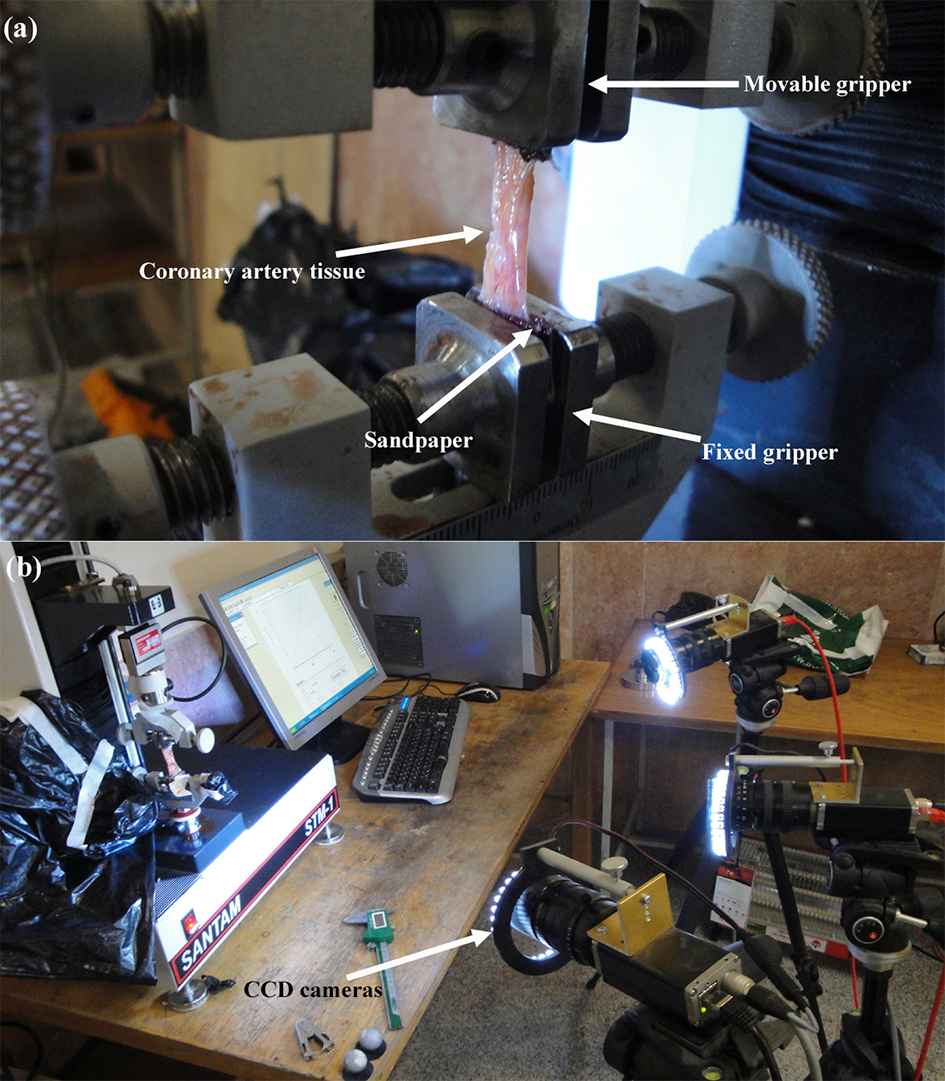
The coronary artery tissue sample under uniaxial loading. The (a) sample was mounted on machine in a way that its lower side was fixed while the upper side was moved upward. The (b) displacement/strain was also graphically measured using DIC method.
Before the stress relaxation test starts, a group of primary testing, including tissue preconditioning assessment, the recovery time evaluation, and relaxation delay analyses, were performed to find the most suitable process of testing for tissue specimens. In summary, these analyses clarified that the conditioning of the tissue is going to be realized in about 8 cycles.26 All testing, i.e., stress failure and stress relaxation, were carried out at the strain rate 5 mm/min, as a lower strain rate would more deeply reflect the mechano-biological mechanical behavior of the arterial wall.27–30 In addition, at a low strain rate the nature of the strain history can be better clarified by a linear ramp trailed by holding at a continuous strain.31–33 A very firm and no-slip boundary were also provided by a steel made gripper plus two coarse sandpapers glued to the jaws of the machine.
Since the viscoelastic time-dependent mechanical response of the arterial wall has been well approved by previous studies, stress relaxation test was carried out according to the protocol proposed by the authors for polyvinyl alcohol sponge.34,35 The protocol starts by measurement of the thickness, preconditioning, preloading up to physiological range, ramp up to relaxation point, recovery for a minute, ramp up to long term behavior of the tissue.36 In detail, the sample was located between the jaws of the machine as then a strain rate of 5 mm/min was applied to the samples and the load in the tissue was recorded by a 50 kgf load cell. Thereafter, eight cycles of preconditioning up to 30% strain were applied up to relaxation point and was kept for at least 1 min. This also can be considered as a physiological preload. Although samples were cut at a suitable angle to avoid or at least minimize residual stress, it is the authors’ belief that the imposed preload and cyclic precondition load can also help the tissue to dissipate the residual stress for further analysis. Lastly, arterial wall let be freely release their energy of stress for about 33 min of relaxation and their stress-time diagrams were recorded for the rest of the study.
In this study in order to measure the strain/displacement of the tissues at each position, the video cameras with the capture of 280 frame/second with the resolution of 2048 × 1088 pixels were used. The Simi Motion® 2D/3D (Simi Reality Motion Systems GmbH, Max-Planck-Straße, Unterschleiβheim, Germany) video camera software was also helped us to lively measure the deformation of each marker in respect to the other one.37
Quasi–linear Viscoelastic (QLV) model
Viscoelastic materials are the type of materials that show different stress–strain paths in cyclic tests. QLV materials is a bit progressed as they contain not only the elastic recoverable region but also the viscous nonrecoverable region. In the other words, QLV can capture the ramp and hold sections of a viscoelastic material in a better way compared to the usual Maxwell, Kelvin-Voigt, and Prony series models. The QLV model actually is a well approved model that firstly proposed by Fung38,39 and later on developed by other researchers in order to be enabled to capture the nonlinear mechanical properties of the soft tissues at the same time.40–42
In a typical Fung’s model, the background of the tissue is added to the equations by a relaxation function, σ(λ,t). This function also has a specific part as normalized function of time, called reduced relaxation function, G(t), and the stretch, λ(=εeng+1), that is to say the elastic retort, T(e).
Here T(e) exhibits the Cauchy/true stress in an initial ramp section of the stress relaxation test. At time t = 0, when the stress shows an action to the change of stress or strain, the Boltzmann superposition principle can be used to calculate the stress.
If G(t) is supposed to be interminably differentiable, the mien can also be articulated as:
And because in the current study the reduced relaxation function was approximated by the Prony series, the following equation can be expected:
Here G∞ called as a long term relaxation coefficient (G∞=limt→∞ G(t)) and the Gi parameters present the relaxation strength conforming to the β decay constant.41,43
The prompt elastic stress and its derivative are signified by the following nonlinear equations:
It is known that the behavior of the soft biological tissue under stresses are not linear, hence, a more complicate ramp history model should be adopted to be able to address this behavior and also can be implemented into Eq. (1) whether implicitly or explicitly by numerical integration. As a result, in order to make this integration easier, the differential operator was crossed out from the input strain history thru integration by parts:
A specific QLV stress relaxation curve fit algorithm developed by Abramowitch and Woo32 was employed and the parameters of the model were, at that juncture, premeditated by MATLAB v. R2015a (The MathWorks, Inc., Natick, MA, United States). The mean number of a determination (R2) was designated between the model and experimental results for each tissue data.
Statistical analysis
Data were first analyzed by analysis of variance (ANOVA); when statistical differences were detected, student’s t-test for comparisons between groups was performed using SPSS software version 16.0 (SPSS Inc., Chicago, IL, United States). Data are reported as mean ± std at a significance level of p < 0.05.
Results
The stress-relaxation curves of the healthy and atherosclerotic human coronary arteries are plotted in Fig. 3a and b separately. Therefore, the results are reported in terms of the stress versus the relaxation time. In addition, the stress–strain diagrams of the tissues during the stress-relaxation tests were also recorded and presented in Fig. 3c and d. The results in this regard were reported as the applied stress versus the relaxed strain which refers to the value that a tissue experienced up to release the experienced stresses. The curves were all calculated at the same strain rate as well as the same testing condition.
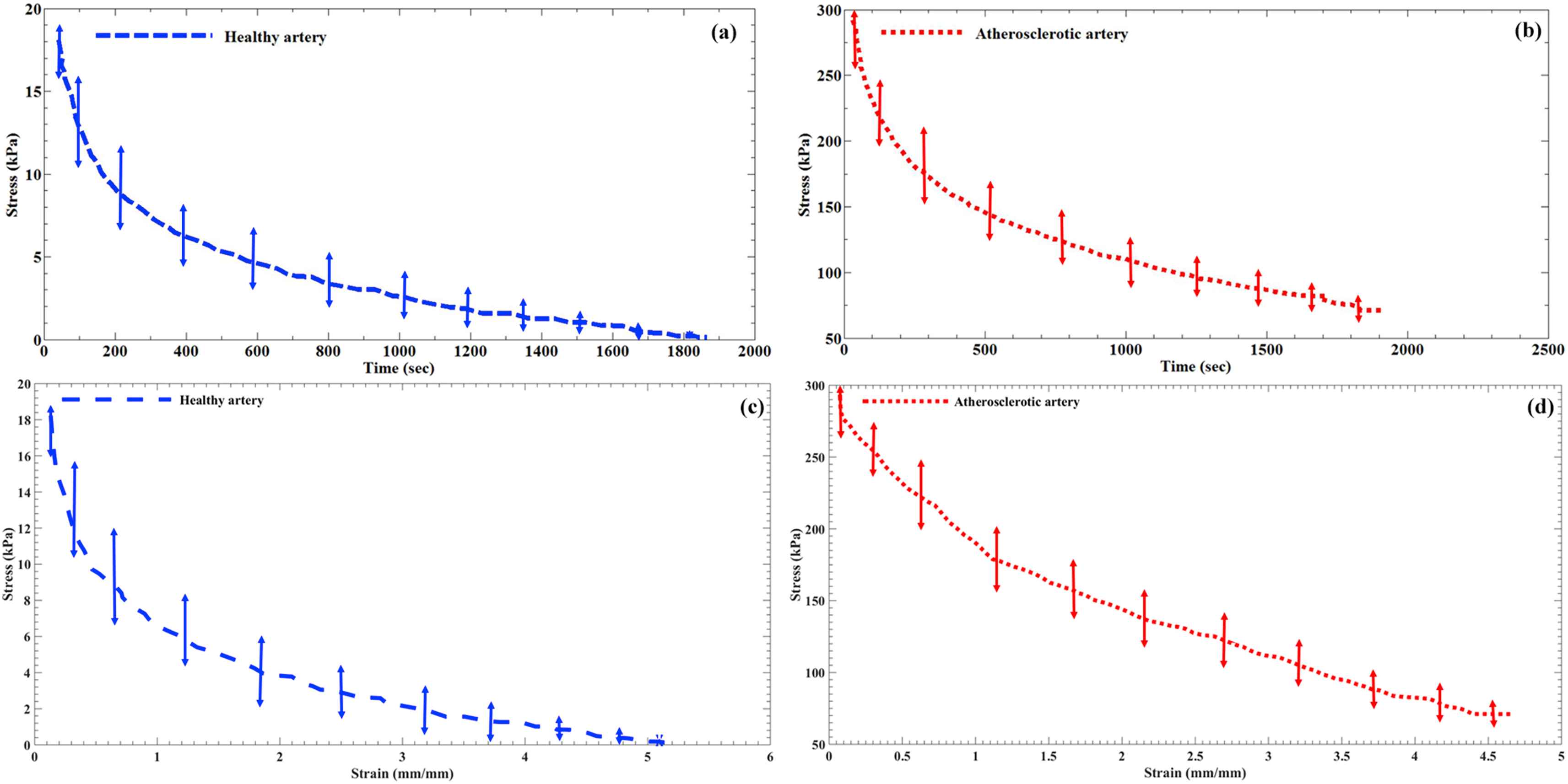
The hold section of the stress-time diagram of the (a) healthy and (b) atherosclerotic human coronary arteries. In addition, the stress–strain diagram of the tissue samples during the hold section for the (c) healthy and (d) atherosclerotic arteries.
By looking at the obtained results in the current study, the starting/initial region of the stress-time or stress–strain diagrams illustrated the stresses of 18.12 ± 2.88 kPa and 292.02 ± 18.14 kPa for the healthy and atherosclerotic coronary arteries, respectively (Fig. 3). The arterial walls demonstrated a transient stress-relaxation behavior to the functional/step displacement and is highly viscoelastic with a percentage of ∼74% and ∼98% for the healthy and atherosclerotic tissues, respectively. These values are defined according to the highest and lowest amount of stress between the initial and final regions of the stress-time diagrams of the samples in a way that, for example, the healthy arterial wall had the initial stress of 18.12 kPa and by the passage of time it reached to 0.12 kPa which invokes the viscoelasticity of 98%. The stress is reached in balance in about 1400 ± 24.15 s for the healthy arterial wall while it took 1800 ± 38.12 s for the atherosclerotic ones. Furthermore, to assess the stress relaxation time for both type of the arterial tissues, the time at which the stress reaches the 50% of its peak value was quantified. The results revealed that the stress relaxation time of the healthy artery was significantly lower than that of the atherosclerotic ones. This shows a difference between the stress-relaxation behaviors during tension for both arterial tissues.
In order to have a comparative outlook about the behavior of the arterial tissues under the applied relaxation loading, the normalized reduced relaxation function versus the time for both the healthy and atherosclerotic tissues is plotted and presented in Fig. 4. These curves helped us to be able to calculate the QLV coefficients of the arterial walls by using the suitable mathematical function. The results in this regard are reported in Table 1. The results were investigated not only in terms of decay constant values (β) but also other parameters among the healthy and atherosclerotic tissues. The decay constant showed the variance of 12.75%, among the healthy and diseased tissues which is not significant. According to the stress relaxation diagrams it is observed that healthy and diseased arteries grasp to a stress balance in dissimilar times (Fig. 3). Long time shear modulus (G∞) showed a significant alteration for the healthy (0.0009 ± 0.0001 kPa) and atherosclerotic (0.0111 ± 0.0009 kPa) tissues.
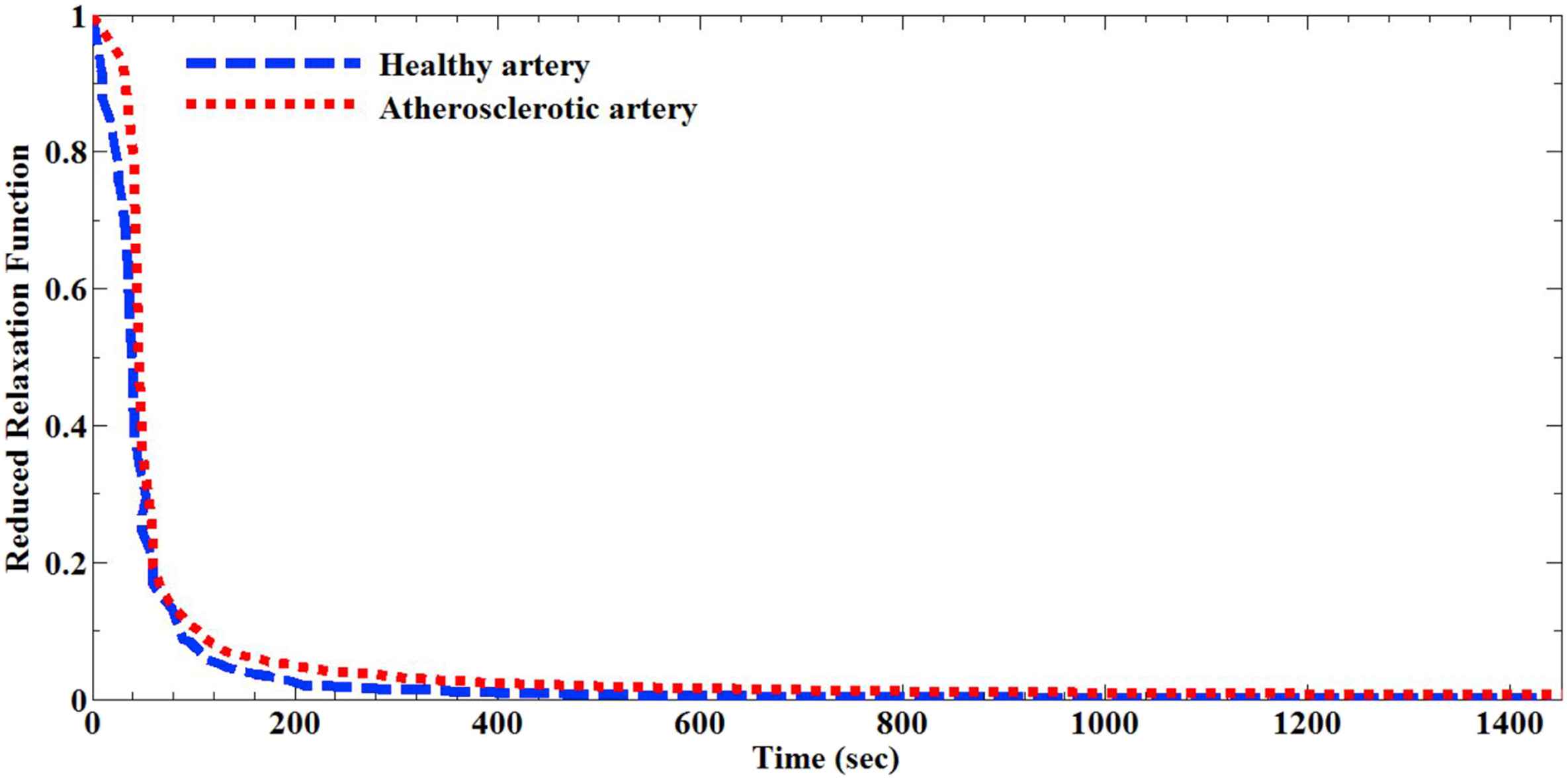
Normalized reduced relaxation function versus time for the healthy and atherosclerotic human coronary arteries.
| Material constants | Healthy | Atherosclerotic | |
|---|---|---|---|
| G∞ | 0.0009 ± 0.0001 | 0.0111 ± 0.0009 | |
| G1 | 0.4437 ± 0.0010 | 0.333 ± 0.0007 | |
| G2 | 0.2414 ± 0.0012 | 0.0816 ± 0.0009 | |
| G3 | 0.5044 ± 0.0010 | 0.7213 ± 0.0010 | |
| β | 0.0243 ± 0.0008 | 0.0212 ± 0.0011 | |
| R2 | 0.9598 | 0.9124 | |
| A (kPa) | 19,260 ± 155 | 249,600 ± 198 | |
| B | 0.0078 ± 0.0006 | 0.0196 ± 0.0014 | |
| R2 | 0.9117 | 0.9326 | |
Quasi linear viscoelastic parameters of the healthy and atherosclerotic human coronary arteries.
Discussion
Although up to now many linear elastic or nonlinear hyperelastic material models have been employed to capture the mechanical properties of the healthy and atherosclerotic human coronary arteries, there is a lack of knowledge on the viscoelastic time-dependent mechanical response of these arteries. On the one hand, since arteries experience a large displacements and/or strains under any type of loading condition, the application of linear elastic models might not be operative. On the other hand, hyperelastic material models unable to take the time-dependent mechanical properties of the arterial wall into account.15 Hence, it is obvious that there should be a set of study to investigate the quasilinear viscoelastic mechanical behavior of the arterial tissue under stress-relaxation loading.
Many reports have been proposed to show that usual exponential or logarithmic mechanical models would be enough for characterizing the mechanical properties of soft biological tissues, especially arterial wall. They believed that these type of material models are more advantageous compared to the intricate ones, such as visco-hyperelastic.46 However, it is obvious that these number of parameters would pave the way for the models to address the biomechanical complexities of the tissues.47,48 In addition, the viscoelastic mechanical behavior of the arteries cannot be explained using linear or nonlinear mechanical models since the load bearing behavior of the tissues at each time point would be so crucial for tissue analysis.
The results in here well explained that the atherosclerotic arterial walls are stiffer than that of the healthy ones (Fig. 3). According to our data, when the load up to the holding limit applied to the arterial tissues, on a basis of their conditions, i.e., healthy or atherosclerosis they took different time to release their stored energy. Our results revealed that the healthy arterial tissues need less time to release their stored energy while the atherosclerotic ones need more time regardless to their first stored stresses. The results also exhibited a severe dropping of peak stress thru the experimental measurement in the atherosclerotic arteries (Fig. 3b and d as well as Fig. 4). This might be related to the breakage of the arterial wall collagens due to overload as showed by Vogel.49 The results also showed that the atherosclerotic artery still keep some of the applied stress on that up to the final relaxation stress while the healthy arterial wall was well released this stress energy. It suggests that the healthy arterial walls are being able to well tolerate a suitable amount of energy and then release it up to their recovery point whereas the atherosclerotic ones may not be able to release that energy suitably and it may lead to an improper performance in them. This is also mentioned by Munster et al.50 as the collagen fibers have substantial role in bearing the applied load by their contribution through their orientations. This is why in the healthy arterial walls the applied load well absorbed by the tissue and then easily released. However, since the atherosclerotic arterial collagen fibers lose their natural elasticity they could not release the absorbed load and, as a result, such trend happened in the curves.
Conclusions
The quasilinear viscoelastic mechanical behavior of the human arterial tissue in the healthy and atherosclerotic conditions under tensile loading was deeply investigated in the current study. Five healthy and five atherosclerotic tissues were removed from the cadavers and subjected to a succession of stress-relaxation tests. The QLV parameters were all calculated and reported. The findings indicated that the maximum stress in the atherosclerotic arteries is larger than that of healthy ones. Furthermore, the stress balance of the arterial tissues fulfilled at dissimilar times.
Conflicts of interest
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical statement
The use of experimental on the human body was approved by the committee of the Legal Medicine Organization (LMO) with the letter ID of 65987/253. This study was also entirely adhered to the declaration of the Helsinki in 2008.
References
Cite this article
TY - JOUR AU - Alireza Karimi AU - Ahmad Shojaei AU - Reza Razaghi PY - 2017 DA - 2017/03/08 TI - Viscoelastic mechanical measurement of the healthy and atherosclerotic human coronary arteries using DIC technique JO - Artery Research SP - 14 EP - 21 VL - 18 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2017.02.004 DO - 10.1016/j.artres.2017.02.004 ID - Karimi2017 ER -
