The effects of a growth hormone-releasing hormone antagonist and a gastrin-releasing peptide antagonist on intimal hyperplasia of the carotid artery after balloon injury in a diabetic rat model☆
This research was partially presented as an abstract at the American College of Cardiology 2013 Scientific Sessions with publication as a supplement in the Journal of the American College of Cardiology: (J Am Coll Cardiol. 2013; 61 (10_S): doi:10.1016/S0735-1097(1361178)) however it has not been published in any other form and is being considered solely by Artery Research.
- DOI
- 10.1016/j.artres.2017.06.006How to use a DOI?
- Keywords
- Neointimal hyperplasia; Growth hormone-releasing hormone antagonist; Gastrin-releasing peptide antagonist; Arterial restenosis; Diabetes mellitus
- Abstract
Introduction: Arterial restenosis after angioplasty/stenting has hindered coronary artery disease treatment, especially in diabetics. We theorized that gastrin-releasing peptide (GRP) antagonists and growth hormone-releasing hormone (GHRH) antagonists might decrease neointimal hyperplasia and restenosis in diabetic rats after common carotid arterial balloon injury.
Methods: Two separate experiments were conducted to test the effects of a GRP antagonist (RC-3095) and a GHRH antagonist (MZ-4-71) on vascular smooth muscle (VSM) growth. In a preliminary in vitro experiment non-injured human aortic vascular smooth muscle (VSM) proliferation was compared between growth media and control. In a second in vivo experiment, intimal and medial area, intima/media ratio (IM) and percent stenosis were compared between injured carotid arteries in twelve Zucker type II obese rats treated with subcutaneously injected RC-3095, MZ-4-71, or control media.
Results: In the in vitro experiment, decreased VSM cell growth was observed in GRP antagonist (p < 0.05) and GHRH antagonist groups (p < 0.05) compared to the control group. In the in vivo experiment, the GRP antagonist group had a decreased IM ratio (1.63 ± 0.41, p < 0.05) and an increased area of stenosis (98.78% ± 1.48 p = NS) compared to control (2.38 ± 1.09) while the GHRH antagonist group had decreased IM ratio (1.33 ± 0.58 SD, p < 0.05) and percent area of stenosis (78.84% ± 24.97, p < 0.05) compared to control (2.38 ± 1.09).
Conclusions: The significant decrease in both IM ratio and percent area of stenosis in the GHRH antagonist group supports the hypothesis that this peptide may reduce neointimal hyperplasia and restenosis.
- Copyright
- © 2017 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Percutaneous cardiac procedures, whether with balloon angioplasty or stent, are commonplace in cardiology practice today. However, arterial restenosis following angioplasty or stent placement continues to prevent the long-term successful treatment of coronary artery disease, particularly for patients with diabetes.1,2 Diabetes has been demonstrated to be an independent risk factor for the development of such restenosis.3
The mechanisms of restenosis are complex and appear to result from interactions between vascular smooth muscle (VSM) cells and several growth factors resulting in VSM cell proliferation and neointimal hyperplasia.4–13 While the use of drug-eluting stents containing antiproliferative agents, such as paclitaxel and sirolimus, has reduced the incidence of restenosis, this complication still persists and results in considerable morbidity and mortality, especially in diabetics.14–16
A variety of new antiproliferative agents are actively being researched for their potential as cancer therapy. However, the potential role of these agents in treating cardiovascular disease has not yet been investigated. Two peptide classes that appear to have great potential for this application are antagonists of bombesin/gastrin-releasing peptide (GRP) and antagonists of growth hormone-releasing hormone (GHRH).
GRP, in addition to its role of inducing release of gastrin, is an autocrine growth factor that is expressed in a variety of neoplasms and promotes angiogenesis. GRP antagonists have demonstrated retardation of tumor growth and inhibition of tumor microcirculation, possibly through reduced expression of epidermal growth factor (EGF) receptors or direct blockage of GRP receptors.
Similarly, GHRH antagonists have shown the ability in nude mouse models to inhibit growth of renal cell carcinoma, prostate adenocarcinoma, osteosarcoma, and lung carcinoma. The antitumor effect of GHRH antagonists rests partially on their ability to reduce serum levels and tumor levels of insulin-like growth factor 1 (IGF-1). The blockade of GHRH receptors and resultant inhibition of autocrine tumoral GHRH appear to be responsible for the antiproliferative effects of these agents.17–46
Potentially, the therapeutic effects of GRP antagonists and GHRH antagonists may be extended beyond oncology. Both GRP antagonists and GHRH antagonists have demonstrated the ability to reduce the growth of benign prostatic hypertrophy.47,48 Thus, we hypothesized that both GRP antagonists and GHRH antagonists might decrease arterial restenosis by reducing VSM proliferation through mechanisms mediated by EGF, GRP, and IGF. Specifically, we were interested in determining whether GRP antagonist RC-3095 or GHRH antagonist MZ-4-71 might be effective in decreasing restenosis and neointimal hyperplasia in a diabetic rat model after experimental balloon injury to the arterial intimal layer.
Methods
Our preliminary investigation consisted of an in vitro experiment using human aortic VSM. For the cell proliferation assay, human VSM cell proliferation was determined using the BrdU Proliferation Assay Kit® (Oncogene Research Products, Cat# HTS01). Human aortic smooth muscle cells (passage 7) were grown to 80% confluence in a 75 cm2 flask. Cells were detached using 2 mL 0.25% Trypsin/EDTA® (Gibco), which was washed out with 20 mL SMBM complete medium (Cambrex). This cell suspension was then used to seed a well plate. Each well received 100 μL of the cell suspension and 100 μL of SMBM complete medium. The plate was then incubated at 37 °C, in a 5% CO2 atmosphere overnight to allow cells to attach. The medium was then drained and 200 μL of D-MEM/F-12 (Gibco) without serum was added to each well. Cells were serum starved in this medium for 20 h in a 37 °C, 5% CO2 incubator. The medium was removed and replaced with 200 μL of treatment medium (either D-MEM/F-12 or SMBM complete) plus BrdU labeling material. Treatment medium contained either GRP antagonist RC-3095 or GHRH antagonist MZ-4-71 at a concentration of 1 pM. The doses reported in previous publications were used in order to determine the appropriate concentration of RC-309528,49–53 and MZ-4118,19,54–56 for our experiment. Control wells had media with either 0.1% DMSO (the solvent for all the peptides), or lacked BrdU label, or had no label and no cells, or had label and media with no cells in the well. The LH-RH antagonist Cetrorelix was used as a peptide control in some wells. Treatment and labeling required 24 h at 37 °C, 5% CO2. The plate was then read in a fluorometer with an excitation wavelength of 360/400 nm and an emission wavelength of 460/400 nm. Data is expressed as relative fluorescence units (RFU), relative signal after subtracting the reading from the appropriate blank. The ELISA assays were performed according to the manufacturer’s protocol.
For the experiment in vivo, Zucker obese rats (average weight of 400 g; Charles River Laboratories, Wilmington, MA) aged nine to twelve weeks were used as a model of Type II diabetes. In this study, there were three groups. Group 1 was the control group and included three rats. Group 1 animals as a control received the peptide, Cetrorelix, a luteinizing hormone-releasing hormone (LHRH) antagonist, not thought to be involved in restenosis, at a dose of 250 μg/kg/day S.C for seven days. Group 2 included five rats; they received the GRP antagonist (mixed with Cetrorelix carrier solution), RC-3095, S.C. at a dose of 250 μg/kg/day for seven days. Group 3 included four rats; they received the GHRH antagonist (mixed with Cetrorelix carrier solution), MZ-4-71, at a dose of 250 μg/kg/day S.C. for seven days. The original study protocol called for five animals to be included in each of the three groups. However, two rats from group 1 and one rat from group 3 did not survive the surgical procedure.
All procedures were performed under sterile conditions, and with the approval of the Institutional Animal Care and Use Committee and in compliance with procedures and methods outlined by the National Institutes of Health. Carotid arterial injury was induced by balloon de-endothelialization. The animals were anesthetized, and a midline cervical incision was made to expose the right carotid artery and its branches. This external carotid artery was permanently ligated and the internal carotid artery was then temporarily ligated. A 2 Fr Fogarty balloon catheter (Baxter Healthcare Corp) was introduced into the aortic arch, and the balloon was distended with saline until resistance was felt on slight traction. The balloon was then pulled back into the common carotid artery and rotated while advancing it through the common carotid artery to the junction with the aortic arch. The procedure was repeated two more times. The surgical wound was then closed according to standard procedure, the animals were observed for any signs of postoperative distress, and were treated with an appropriate analgesic agent as needed.
The animals were sacrificed after thirty days by means of intracardiac Nembutal (120 mg/kg). A midline abdominal incision exposed the distal abdominal aorta. An 18-gauge IV catheter was inserted at the aortic bifurcation, passed to the aortic arch, and the vessel was flushed with 50 cc of Ringer’s lactated solution at 120 mm Hg. In vivo fixation with 200 cc of 5% Histochoice (Amresco, Solon, OH) was performed with a 5-min infusion at 120 mm Hg. The entire right carotid arterial system was then harvested and stored in 5% Histochoice for at least 24 h before embedding.
The injured common carotid arteries were then cut at five mm intervals from the junction with aortic arch to their bifurcation into external and internal carotid arteries. These arterial segments were then embedded in paraffin and sectioned. The sections were stained with either hematoxylin-eosin or trichrome (Fig. 1) for the control group, five segments were taken from each of the three rats; four sections were made from each segment. These were each stained with hematoxylin-eosin. For the GRP antagonist group, six segments were taken from five rats with four sections made from each segment. These slides were each stained with hematoxylin-eosin. For the GHRH antagonist group, six total segments were taken from each of the four animals. Four sections were stained with hematoxylin-eosin, and two sections were stained with trichrome.
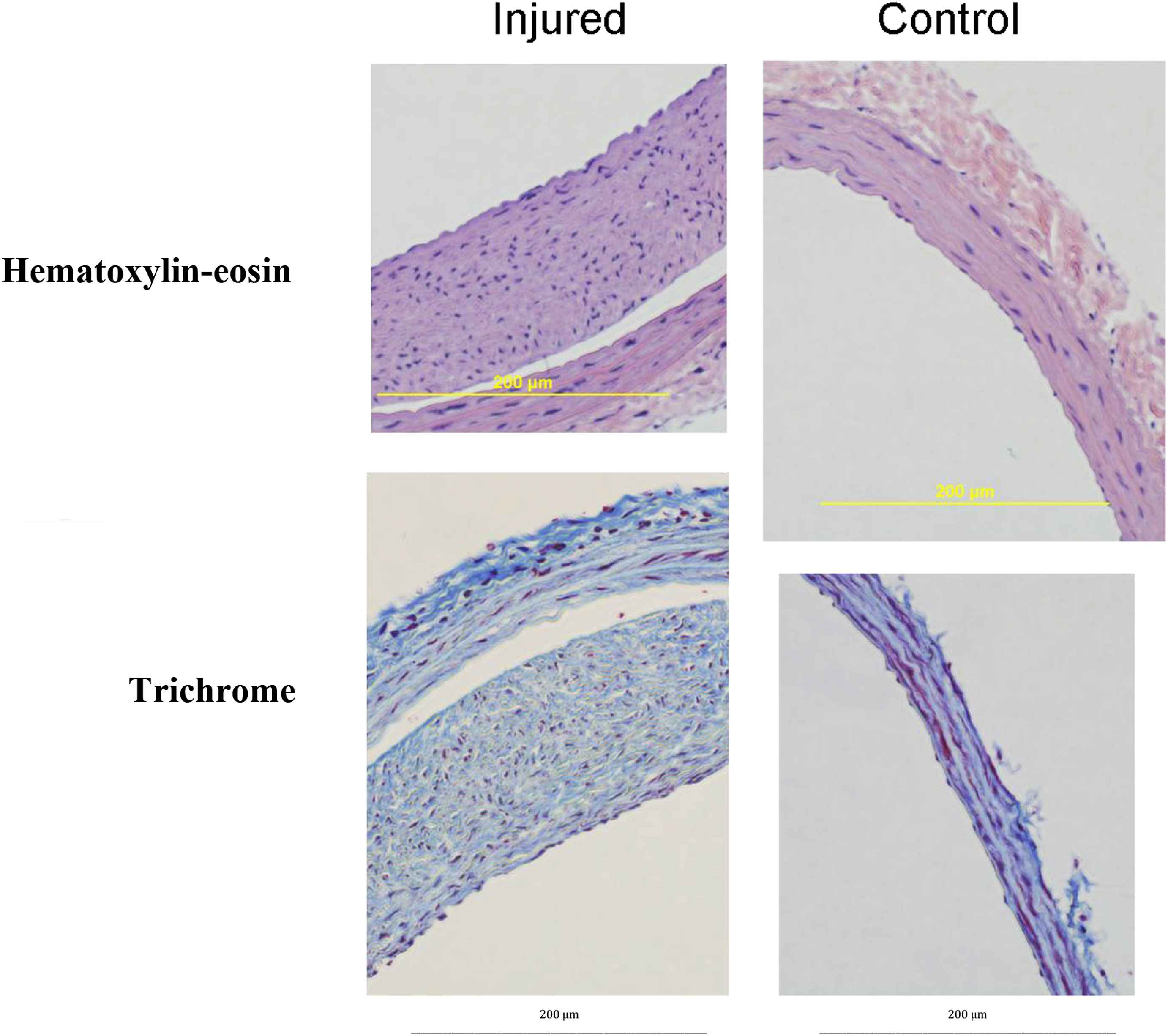
Carotid Arteries After Injury. Injured and non-injured common carotid arteries after being embedded in paraffin, sectioned and stained with either hematoxylin-eosin or trichrome. The images have been magnified for easier viewing but otherwise have not been manipulated or stretched.
A technician, who was blinded to the study groups, performed morphometric analyses of the arterial segment by using computerized digital microscope planimetric software (Image-Pro Plus). The segment with the most severe degree of luminal narrowing in each injured arterial segment was assessed as the “lesion” point, and each section of the “lesion” point segment was used for quantification and analysis.
Measurements were made of external elastic lamina (EEL area), the internal elastic lamina (IEL area), and the luminal area. Other areas were calculated as follows:
- 1.
Media area = EEL area − IEL area.
- 2.
Intima area = IEL area − luminal area.
- 3.
Intima/media ratio (I/M) = intima area/media area.
- 4.
% area of stenosis = intima area/IEL area × 100.
Statistical analysis
All data relating to study aim specifics were summarized using descriptive statistics such as mean, standard deviation, and standard error of means. Frequency tables were drawn up for nominal and ordinal data. Where meaningful, the results have been presented graphically. Statistical analyses that compare mean values were performed by using the analysis of variance method. Dunnett’s test was used for multiple comparisons in ANOVA to observe differences between the mean of each group (GRP and GHRH) and the mean of a control group for post hoc analysis. Fisher exact test was used for comparison of percentages as shown in Fig. 3. A two-sided 5% significance level was applied throughout the analyses. All data analyses, summaries and listing were performed using SAS software (version 9.4) in a Windows environment.
Results
The analyses of the results of BrdU labeling showed that in the in vitro cell-proliferation assay, the growth of human aortic vascular smooth muscle (VSM) was decreased in the presence of both 1 pM and 1 nM concentrations of GRP antagonist, RC-3095 (p < 0.05 by post hoc Dunnett’s test) or GHRH antagonist, MZ-4-71 (p < 0.05 by post hoc Dunnett’s test) incubation media (Fig. 4). LHRH antagonist Cetrorelix, which was used as peptide control, had no effect. All proliferation was compared to that in control media containing 0.1% DMSO.
In the in vivo experiment of obese diabetic Zucker rats, the peptide control group treated with LHRH antagonist Cetrorelix (250 μg/day for 1 week) showed a mean intima/media (I/M) ratio of 2.38 ± 1.09 (Fig. 2), and a percentage of stenosis area of 93.09% ± 10.83 (Fig. 3). Subcutaneous therapy with 250 μg/day of GHRH antagonist, MZ-4-71 for 1 week reduced the intima/media (I/M) ratio for the injured carotid arteries to 1.33 ± 0.48 (Fig. 2) and diminished the percentage of area stenosis to 78.84% ± 24.97 (Fig. 3). Both are found to be statistically significant (p < 0.05) compared with control using post-hoc multiple comparisons by Dunnett’s test. This finding suggests that GHRH antagonists may induce beneficial effects in diabetic rats after common carotid arterial balloon injury, by reducing restenosis and decreasing hyperplasia. The group treated with 250 μg/day of GRP antagonist, RC-3095 for 7 days also showed a decreased Intima/Media (I/M) ratio of 1.63 ± 0.41 and was found to be statistically significant using Dunnett’s test (p < 0.05, compared to control) (Fig. 2). However, the percentage area of stenosis was actually increased to 98.78% ± 1.48 compared to control but not significant at 5% level of significance by Dunnett’s test (Fig. 3). This result is not thought to be due to any problems in morphometric methodology. The increased percentage of stenosis might be explained by enlargement of the medial area and a difference in the effects of GHRH antagonists, which tend to decrease IGF-1 levels from GRP antagonists, which influence EGF.
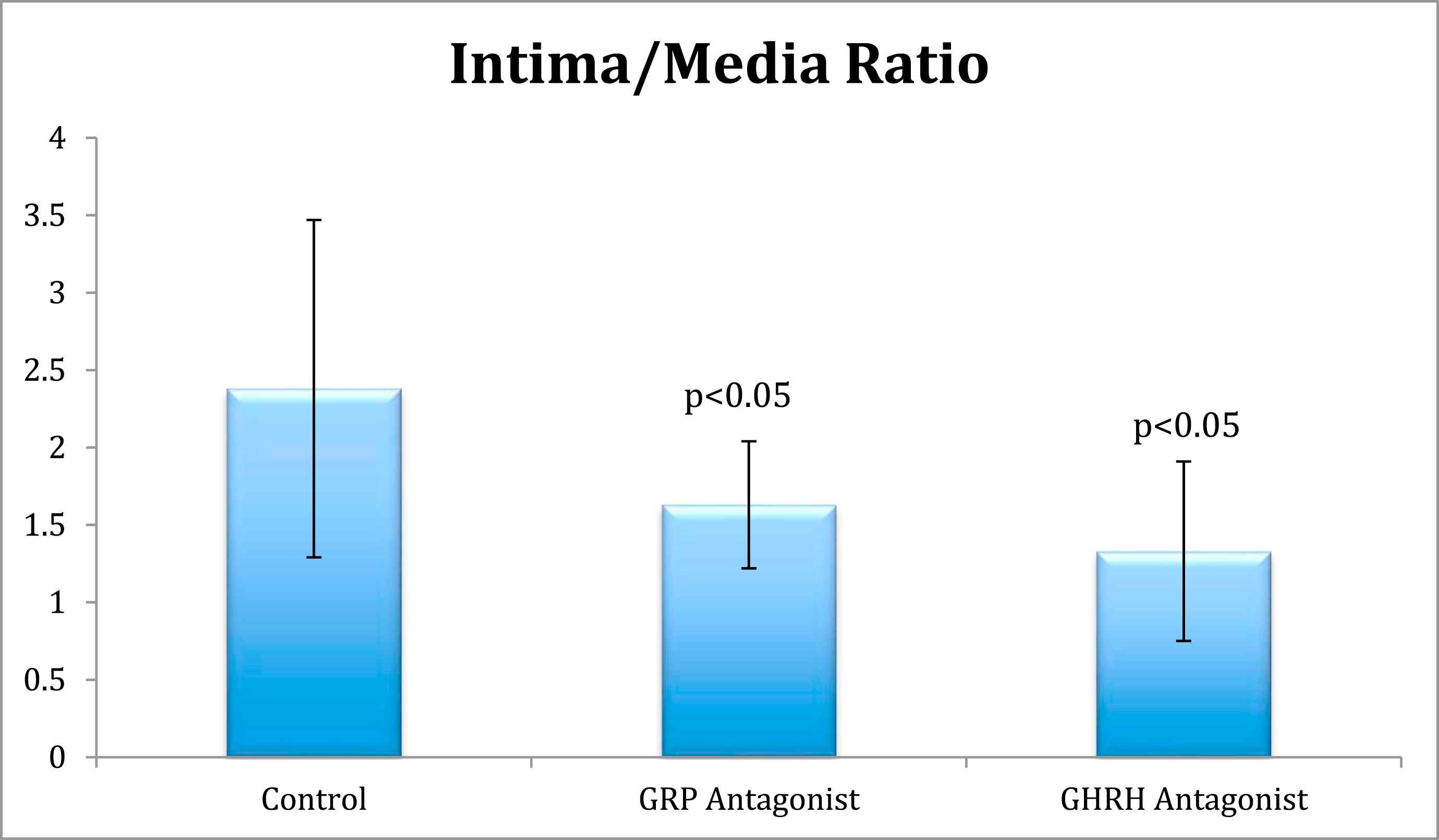
Intima/Media Ratios. Comparison of intima/media ratios between control, GRP antagonist, and GHRH antagonist groups.
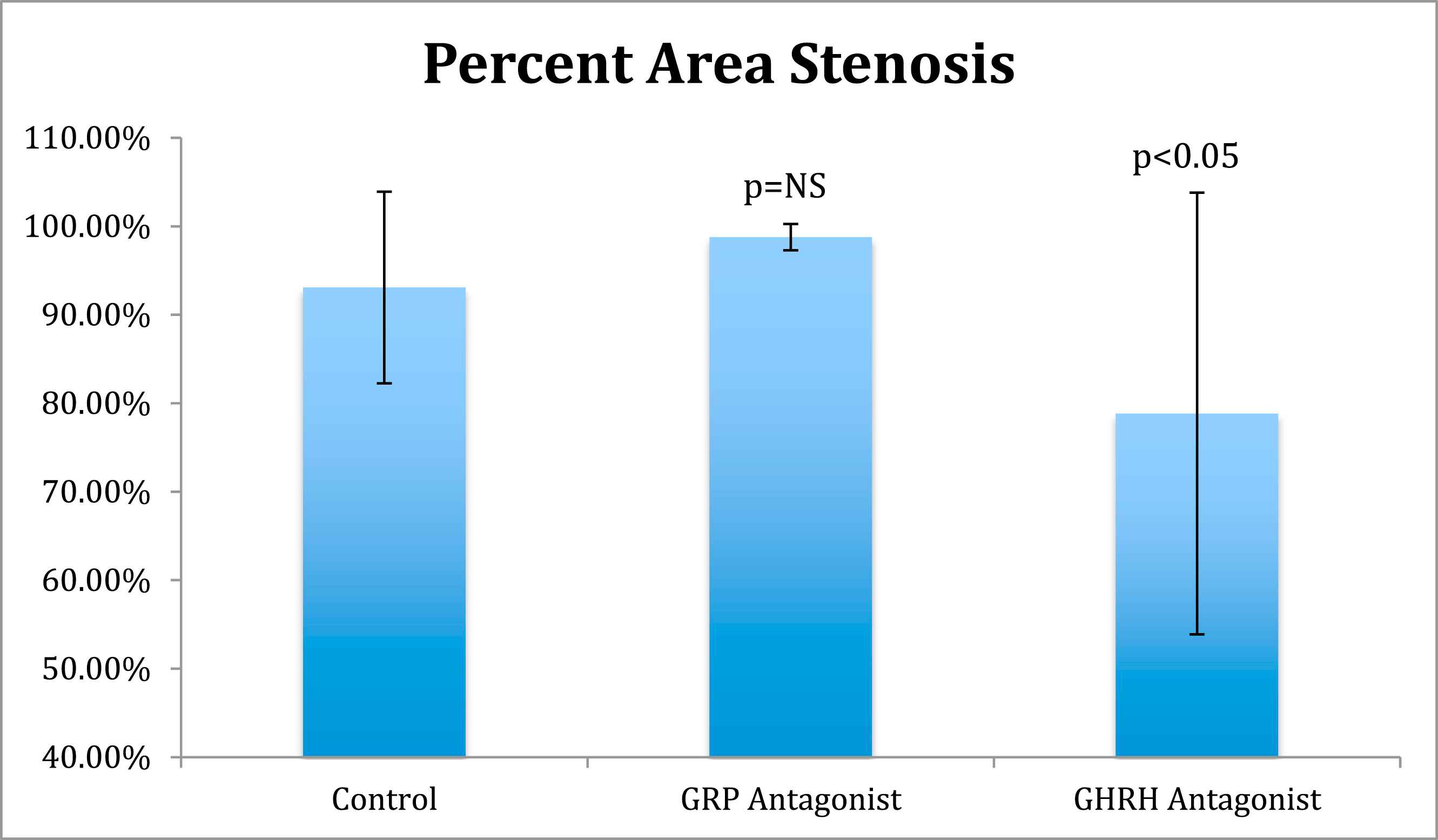
Percent Area Stenosis. Comparison of percent area stenosis between control, GRP antagonist and GHRH antagonist groups.
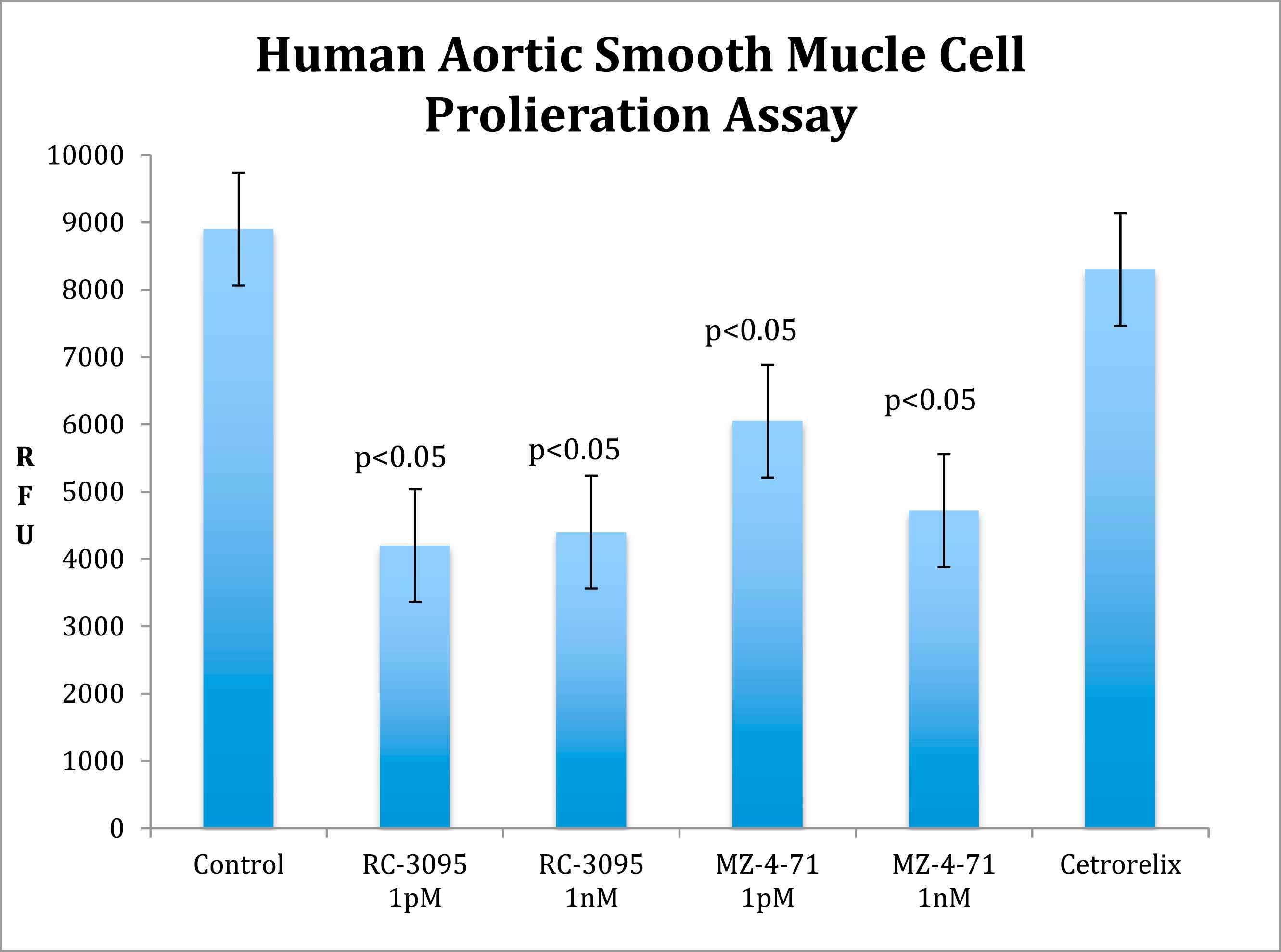
Smooth Muscle Cell Proliferation. In vitro cell proliferation analysis demonstrating decreased smooth muscle cell growth in 1 pM and 1 nM concentrations of RC-3095 and MZ-4-71 incubation medium compared to Cetrorelix and control medium. RFU: relative fluorescence units; nM: nanometer; pM: picometer.
Discussion
The mechanisms leading to development of restenosis are complex, involving multiple processes that include vascular remodeling and neointimal hyperplasia.4,5 Neointimal hyperplasia after percutaneous coronary interventions occurs in response to growth factors that promote the migration and proliferation of vascular smooth muscle cells at the site of injury.6–13 While stent placement reduces vessel recoil and lessens negative vascular remodeling, neointimal hyperplasia with in-stent restenosis frequently results.57 Over the last decade the use of antiproliferative agents, such as paclitaxel and sirolimus, has led to significant decreases in restenosis after stent placement.58,59 The incidence of restenosis is still quite significant however in specific populations, such as diabetics.60
Diabetics are at great risk of death due to coronary artery disease, with 80% likely to die of a cardiovascular event.58,59 In additional, diabetics have higher rates of myocardial infarction, need for repeat revascularization, and mortality compared to those without diabetes.60–62 The presence of diabetes mellitus itself appears to be an independent risk factor for the development of in-stent restenosis; patients with diabetes are reported to have higher rates of restenosis and mortality after percutaneous coronary intervention (PCI) than non-diabetics.63
Several growth factors seem to stimulate the proliferation of VSM cells.9–13 Recent animal studies have noted the involvement of growth factors, such as IGF-I and II, in the development of neointimal hyperplasia and restenosis after PCI.64,65 Experimental evidence indicates that GHRH antagonists can reduce both systemic levels of IGF-I as well as local levels of autocrine and paracrine IGF-I and IGF-II.18,44,45,64,66 IGF-I and II have the ability to promote migration and proliferation of VSM cells, leading to the development of neointimal hyperplasia.67,68 Interestingly, while other growth factors such as platelet-derived growth factor and angiotensin II also stimulate proliferation of cells, the action of IGF-I at the G1/S phase of the cell cycle is required for these other factors to be effective.69 The influence of IGF-I and II in promoting proliferation of cells is thus not surprising, considering evidence that GHRH antagonists were shown to be beneficial in experimental studies as therapy for a variety of cancers, including non-Hodgkin’s lymphoma, renal cell carcinoma, and osteosarcoma as well as prostate, lung, endometrial, ovarian, breast, pancreatic, and colon cancers.17–46
Gastrin-releasing peptide is a mammalian equivalent of amphibian peptide bombesin, an autocrine growth factor for neoplasms, and GRP antagonists have been demonstrated to inhibit the growth of experimental tumors in nude mouse models.49,66,70–73 GRP antagonists appear to exert some of their antiproliferative effects through the down regulation of EGF receptors along with decreased expression of c-jun and c-fos oncogenes both of which appear to be involved in the cellular signaling cascades that promote restenosis.74,75 Furthermore, animal models have supported the role of EGF in the pathogenesis of neointimal hyperplasia and vascular remodeling after PCI.76,77 It seems that GRP antagonists also have the potential to limit angiogenesis in tumors.50,78
The goal of this experiment was to examine whether GHRH antagonists or GRP antagonists could reduce the degree of restenosis within the carotid artery following balloon injury. In the in vitro non-injured human aortic experiment, we observed significant reductions in VSM growth with both GRP and GHRH antagonists. In the in vivo injured Zucker obese rats carotid artery experiment, we observed significant reductions in both the carotid arterial intima/media ratio as well as the percentage of stenosis area with GHRH antagonist infusions. In the latter experiment, while the GRP antagonist group revealed significant reductions in intima to media ratio, the percentage of stenosis area was actually increased, although not statistically significant based on ANOVA and post hoc testing. This finding in the GRP antagonist treated group might be explained by having a decreased intimal area but also an increased medial area. The effects of GRP antagonism on intracellular signaling in VSM cells may be different from that of tumor cells and could have resulted in reductions to the intima/media ratio while also leading to a greater degree of stenosis.
Our findings should be considered in the light of several methodological considerations. First, the number of surviving rats included in the final experimental analysis is inconsistent amongst the different groups. Two rats from group 1 and one rat from group 3 were unfortunately lost while performing the surgical procedure and therefore could not be included in the analysis. While we are concerned that this may have affected the final results, we believe the data observed in this experiment are notable and should be reported. Our hope in the future is to improve upon this preliminary data and perform a more appropriately powered and consistent experiment to determine if these results can be duplicated. A second consideration involves our control group, which received the peptide Cetrorelix. While our thought was to compare GRP and GHRH antagonists to a peptide not involved in restenosis, we also could have considered the left-sided uninjured carotid arteries as a control, comparing the effect of GRP and GHRH antagonism on both injured and uninjured vessels. This could be completed in a future study. Another consideration is that the carotid injury mechanism for developing stenosis may be different from the mechanism of restenosis following human angioplasty, where it has been theorized that adventitial remodeling alone is responsible for restenosis. Therefore, our experimental model may not necessarily reflect comparison to human pathology. Finally, the person who performed the measurements of each histological specimen was not blinded to the specimen groups, which should be taken into account when examining the final results.
Conclusion
Despites the limitations described above, we believe that our findings are interesting and may support a potential role for GHRH antagonism in the reduction of neointimal hyperplasia and restenosis. However, overall interpretation of these results is difficult. Further research is necessary to elucidate any mechanisms by which these peptides might influence the degree of restenosis after arterial injury.
Funding
This material is based upon work supported by the
The work in Dr. A.V. Schally’s laboratory at the Southeast Louisiana Veterans Medical Center on the development of antagonists of GHRH and GRP was supported by the medical research service of the
Conflicts of interest
The authors have no other conflicts of interest (including financial, consultant or institutional) or other sources of support to acknowledge.
References
Cite this article
TY - JOUR AU - John C. Moscona AU - Matthew N. Peters AU - Andrew V. Schally AU - Sudesh Srivastav AU - Patrice Delafontaine AU - Anand Irimpen PY - 2017 DA - 2017/07/13 TI - The effects of a growth hormone-releasing hormone antagonist and a gastrin-releasing peptide antagonist on intimal hyperplasia of the carotid artery after balloon injury in a diabetic rat model☆ JO - Artery Research SP - 56 EP - 64 VL - 19 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2017.06.006 DO - 10.1016/j.artres.2017.06.006 ID - Moscona2017 ER -
