The implications of poor sleep quality on arterial health in persons with multiple sclerosis
- DOI
- 10.1016/j.artres.2017.06.005How to use a DOI?
- Keywords
- Multiple sclerosis; Sleep quality; Arterial function
- Abstract
Background: Multiple sclerosis (MS) is associated with increased risk of cardiovascular disease (CVD) and approximately 25–54% of patients report poor sleep quality. There is evidence from the general population of an association between poor sleep and increased CVD risk, but this is poorly understood in MS.
Purpose: This study examined the association between self-reported sleep quality and arterial health in persons with MS.
Methods: MS subjects (n = 31) and control subjects (n = 23) were recruited. Control subjects were age and body size matched. All subjects were administered the Pittsburgh Sleep Quality Index (PSQI) to assess self-reported sleep quality. Subjects with a global score >5 were classified as “poor sleepers”. Blood pressure, arterial stiffness, and the forearm blood flow responses following 5-min ischemic occlusion (endothelial function) were measured.
Results: Nineteen MS subjects and 5 control subjects were classified as “poor sleepers”. AIx was significantly higher in MS subjects who had poor sleep quality (32.5 ± 8.8 vs 22.0 ± 13.2; P < 0.05).
Conclusions: Markers of arterial dysfunction were significantly higher in MS subjects with poor sleep quality compared to those with good sleep quality. This study suggests novel evidence for the association of CVD risk and sleep quality in MS.
- Copyright
- © 2017 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Multiple Sclerosis (MS) is a chronic, autoimmune disease with an estimated prevalence of 2.5 million people worldwide.1 The disease is characterized by demyelination and transection of axons in the central nervous system.1,2 The pathophysiology directly and indirectly results in heterogeneous outcomes among those with MS. We are particularly interested in two of these outcomes, namely cardiovascular disease (CVD) and poor sleep quality.
Comorbidity is common in MS and there is an increased risk of cardiovascular disease (CVD) and early mortality3 in this population. Furthermore, patients with MS report sleep disturbances more frequently compared to the general population.1,4 Approximately 25%–54% of patients with MS report sleep related problems,1,2 with sleep quality being associated with disease severity.2 Shorter sleep duration and poor sleep quality have been associated with a higher risk of coronary heart disease, CVD, and overall mortality among healthy populations,5–8 but this association is poorly understood in MS.
The pathophysiology of CVD and poor sleep quality is unknown in MS, but the inflammatory nature of the disease could be associated with both outcomes.1 For example, reductions in sleep duration and sleep quality have been associated with a proinflammatory environment and disrupted immune function.9–11 Acute increases in systemic inflammation result in temporary increases in large artery stiffness12 and reduced endothelial function.13 Increased aortic stiffness is a strong predictor of future cardiovascular events14 and vascular dysfunction is a common comorbidity observed in MS patients.15 Recently, it has been reported that patients with MS demonstrate altered arterial function compared to matched controls.15 It is possible that the disruption of various regulatory systems associated with poor sleep could have detrimental effects on arterial function. However, the extent to which poor sleep habits in patients with MS affect arterial structure and function is unknown.
This study examined the relationship between sleep quality and arterial health among patients with MS and a matched control group. We hypothesized that sleep quality would be lower in individuals with MS and that poor sleep would be associated with worse arterial function.
Methods
The information presented is a secondary analysis on data previously published.15 The study protocol was approved by the Institutional Review Board at the University of Illinois and all participants signed an informed consent document.
Participants
Thirty-three persons with MS were recruited from central Illinois. Age, sex, and body size matched control group (n = 33) were recruited from the community. All subjects were between the ages of 18–64 and both groups had equal number of women (n = 27) and men (n = 6). The current study includes subjects with complete arterial function and sleep quality data (MS n = 31; controls n = 23). Eligibility criteria included being ambulatory with or without single-point assistance, having the visual ability to read 14-point font, and having abstained from smoking for a minimum of six months. Additionally, MS subjects were included if they had been relapse free for at least 30 days prior to participating in the study. Persons who did not meet those criteria were excluded. Twenty-five of the subjects with MS were taking disease-modifying medications. Details regarding subject medications and disease severity have been described elsewhere.15 All subjects abstained from caffeine and food for at least 4 h before the testing visit.
Sleep quality
Sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI). The PSQI evaluates subjective sleep quality during the preceding month through 19 self-rated questions and five questions rated by a bed partner or roommate. The last five questions are used for clinical information and do not influence the final scoring of the PSQI. The 19 questions are grouped into seven component scores: sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction. The seven component scores are summed up to a global PSQI score ranging on a scale from 0 to 21. Higher scores indicate worse sleep quality, with a PSQI more than five discriminating between poor sleep (PSQI >5) from good sleep (PSQI ≤5).16
Brachial blood pressure
Subjects rested in a supine position for 10 min before resting measurements were obtained. Systolic BP (SBP) and diastolic BP (DBP) were measured using an automated oscillometric cuff (HEM-907XL; Omron Corporation, Kyoto, Japan). All BP measurements were obtained twice and the average of the two values was recorded.
Augmentation index
Applanation tonometry was performed to attain radial artery pressure waveforms using a high fidelity pressure transducer (SphygmoCor; AtCor Medical, Sydney, Australia). A central aortic pressure waveform was reconstructed from the radial artery pressure waveform using a generalized validated transfer function.15 Augmentation index (AIx) was calculated as the difference between the early and late systolic peaks of the arterial waveform divided by the total pulse pressure. AIx was expressed as a percentage of the central pulse pressure. Because of the influence of HR, AIx was also normalized to a HR of 75 bpm. Central artery pressure waveforms were calibrated against brachial mean arterial and diastolic pressure.
Pulse wave velocity
To measure carotid-femoral pulse wave velocity (cf-PWV) we used a single high-fidelity pressure transducer to sequentially obtain pressure waveforms between the right common carotid and the right femoral artery, using a commercially available system (SphygmoCor, Atcor Medical, Australia). Consecutive waveforms were captured for a 10-s epoch. Simultaneous ECG gating was used as a timing marker and to obtain HR. The foot of the pressure wave was identified automatically, using an algorithm that detects the initial upstroke via a line tangent to the initial systolic upstroke point of the pressure tracing and an intersecting horizontal line through the minimum point. Distances from the carotid artery to the femoral artery sampling sites, and from the carotid artery to the suprasternal notch, were measured as straight lines with a tape measure. The distance from the carotid artery to the suprasternal notch was then subtracted from the carotid-femoral segment to correct for differences in propagation direction along the arterial path length.
Intima-media thickness
Images of the common carotid artery were obtained with ultrasound (Aloka SSD-5500; Tokyo, Japan) and a 7.5-MHz linear-array probe. The intima-media thickness (IMT) of the common carotid artery was defined as the distance between the leading edge of the lumen–intima interface and the leading edge of the media–adventitia interface of the far wall. All measurements were made at end diastole. The IMT of the common carotid artery was determined from an average of five measurements obtained 20 mm proximal to the carotid bifurcation.
Carotid artery stiffness
Carotid artery stiffness was assessed using ultrasound imaging (Aloka SSD-5500) of the carotid artery in combination with carotid artery pressure waveforms attained using applanation tonometry. Heart rate (HR) was recorded with a single-lead ECG. β-stiffness index was then calculated as a means of adjusting arterial compliance for changes in carotid distending pressure using the equation: (log P1/P0)/(D1−D0/D0); where P1 and P0 are the highest (systolic) and lowest (diastolic) carotid pressures and D1 and D0 are the maximum (systolic) and minimum (diastolic) diameters.
Forearm blood flow and reactive hyperemia
Microvascular function was evaluated using strain gauge plethysmography (EC-4; Hokanson, Inc., Bellevue, WA) and described in detail elsewhere.15 Briefly, a rapidly inflating venous occlusion cuff was placed around the upper arm with a second BP cuff placed over the venous occlusion cuff. A pediatric blood pressure cuff was placed around the wrist to occlude hand circulation. A mercury-in-silastic strain gauge strain gauge was placed around the forearm at the greatest circumference. Resting forearm blood flow (FBF) was determined by occluding circulation to the hand and inflating the venous occlusion cuff to 50 mmHg for 7-s followed by 8-s of deflation. Reactive hyperemia (RH) of the forearm vessels was measured immediately after resting FBF. The second BP cuff around the upper arm was inflated to 250 mmHg to occlude arm circulation for 5-min. Circulation to the hand was occluded 1 min prior to upper arm cuff release. Changes in forearm volume were measured in 15-s cycles as described above, for 3-min after release. The highest reading observed was recorded as the peak blood flow. FBF was expressed as milliliters per minute per 100 mL of forearm tissue.
Physical activity
Physical activity (PA) was measured by ActiGraph single-axis accelerometer (model 7164 version; Manufacturing Technology, Inc., Fort Walton Beach, FL). All subjects wore the accelerometer for a 7-day period. Minute-by-minute counts were summed for each individual days and then averaged to total daily movement counts for the entire 7-day period.
Statistical analysis
Statistical analysis was conducted using IMB SPSS statistics version 22. The Shapiro–Wilk test was conducted to test normal distribution of data. Variables that failed the test for normality were AIx, AIx@75, cf-PWV, and IMT when assessing the effect of good vs poor sleep in all subjects. Variables that failed the test for normality were AIx, AIx@75, and IMT when assessing the effect of good vs poor sleep in the MS group. These data were log transformed, however, raw data values are presented in tables.
To compare group differences between the MS and control group for study variables on descriptive and vascular outcomes, we used multivariate ANOVA (MANOVA) analyses, followed by univariate F-test comparisons if the overall MANOVA was significant. The MANOVAs was conducted using the descriptive variables (age, height, weight BMI) in one analysis, and the cardiovascular variables (except FBF) in a separate analysis, as dependent variables. To compare the effect of good vs poor sleepers we also used MANOVA analyses, followed by univariate F-test comparisons if the overall MANOVA was significant. Similarly, the MANOVA was conducted using the descriptive variables (age, height, weight BMI) in one analysis, and the cardiovascular variables (except FBF) in a separate analysis, as dependent variables.
We also performed ANOVAs with repeated measures evaluating the forearm blood flow at rest and the peak forearm blood flow during reactive hyperemia (2 × 2; group (MS vs control, or poor sleepers vs good sleepers) at rest and peak). We then repeated these ANOVAs with repeated measures using the global sleep quality scores as a covariate.
We also used a multivariate analysis of variance with a covariate (MANCOVA), comparing group differences between MS and controls using the cardiovascular variables (other than FBF) as the dependent variables, and the sleep quality scores as a covariate. To test for differences between poor and good sleepers within each group (controls and MS) we used a MANOVA with arterial function measures as the dependent variables and good vs poor sleepers as the group variable. Pearson correlations were performed to determine if variables were linearly related. We also conducted a multivariate blocked regression analysis, to evaluate which cardiovascular variables would contribute to sleep quality, while controlling for age, sex, BMI and mean arterial pressure (MAP). Age, sex, BMI and MAP were entered in the first block, followed by cf-PWV, AIx@75, change in forearm blood flow from rest to peak and HR entered in the second block.
Data are presented as mean ± SD, or as mean ± SE when adjusted values are presented. Statistical significance was considered a value of P < 0.05.
Results
To investigate specific variables of interest in this secondary analysis, the previously published data set15 was condensed to eliminate missing data points. This resulted in a final n = 31 in the MS group and n = 23 in the control group. Subject characteristics are presented in Table 1. There were no significant differences between the MS group and control group with regards to age, height, weight, BMI, or blood pressure (P > 0.05). Resting HR was higher (P < 0.05) and both resting and peak FBF was lower (P < 0.05) in the subjects with MS compared to the control group (Fig. 1). Furthermore, there was a significant group by time interaction (P < 0.05) showing the increase from rest to peak FBF was lower in subjects with MS compared to controls (Fig. 1). There was a considerable difference in PA between the two groups, with the MS group being less active (P < 0.001). This supports what has been previously published.15 We also used a multivariate analysis of variance comparing group differences between MS and controls on the cardiovascular variables using the sleep quality scores as a covariate (MANCOVA). When controlling for sleep quality, there were no differences between subjects with MS and controls for any of the cardiovascular variables (Table 2). The effect of sleep quality on FBF was evaluated through a 2 × 2 (group by time (rest vs peak FBF)) ANOVA with repeated measures using sleep quality as a covariate. This analysis showed there was still a significant group effect (P < 0.05) with a lower FBF (rest and peak combined) for subjects with MS, but the interaction was no longer significant (P = 0.099).
| MS (n = 31) | Control (n = 23) | |
|---|---|---|
| Age (yr.) | 47 ± 10 | 49 ± 10 |
| Height (cm.) | 166.1 ± 10.3 | 168.0 ± 9.2 |
| Weight (kg.) | 72.6 ± 14.2 | 72.9 ± 16.8 |
| BMI (kg m−2) | 26.4 ± 5.3 | 25.8 ± 6.1 |
| HR (bpm)* | 63 ± 8 | 56 ± 9 |
| SBP (mmHg) | 120 ± 15 | 120 ± 11 |
| DBP (mmHg) | 74 ± 10 | 74 ± 8 |
| AIx (%) | 28.4 ± 11.8 | 25.2 ± 14.3 |
| AIx@75 (%) | 22.7 ± 11.5 | 16.6 ± 14.0 |
| cPWV (m s−1) | 6.96 ± 1.38 | 6.36 ± 0.95 |
| IMT (mm) | 0.49 ± 0.11 | 0.45 ± 0.09 |
| PA (counts day−1)* | 1,86,448.35 ± 1,09,101.24 | 3,33,576.22 ± 1,48,402.48 |
BMI: body mass index; HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; AIx: Augmentation Index; AIx@75: Augmentation Index at a heart rate of 75 bpm; cPWV: central pulse wave velocity; IMT: Intima-Media thickness; PA: physical activity.
P < 0.05.
Values are mean ± SD.
Subject characteristics.a
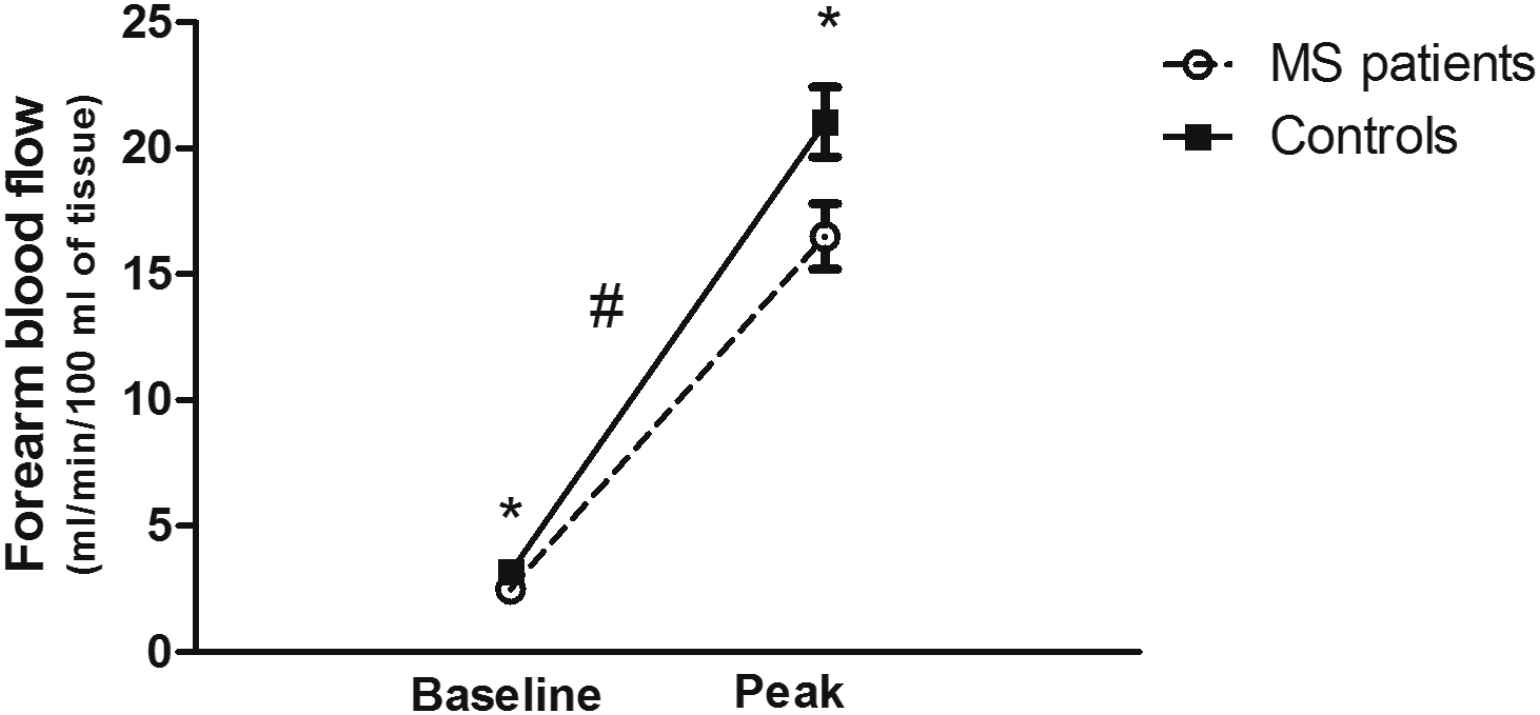
Forearm blood flow at baseline (rest) and at peak hyperemic flow following reactive hyperemia testing in persons with MS and controls. Both baseline and peak values were lower in persons with MS (P < 0.05) and the change from baseline to peak was also significantly lower in persons with MS. * denotes significant differences between groups (P < 0.05). # denotes a significant interaction (group by baseline vs peak; P < 0.05).
| MS (n = 31) | Controls (n = 23) | |
|---|---|---|
| HR (bpm) | 62 ± 1.6 | 58 ± 1.9 |
| SBP (mmHG) | 121 ± 2.6 | 123 ± 2.8 |
| DBP (mmHG) | 74 ± 1.7 | 76 ± 1.9 |
| AIx (%) | 27.2 ± 2.3 | 26.8 ± 2.7 |
| AIx@75 (%) | 21.2 ± 2.2 | 21.2 ± 2.4 |
| cf-PWV (m s−1) | 6.9 ± 0.2 | 6.6 ± 0.3 |
| IMT (mm) | 0.48 ± 0.02 | 0.46 ± 0.02 |
| Beta stiffness | 7.9 ± 0.39 | 7.2 ± 0.4 |
HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; AIx@75: augmentation index normalized to a heart rate of 75 beats per minute; cf-PWV: carotid-femoral pulse wave velocity; IMT: intima-media thickness; FBF: forearm blood flow.
Values are mean ± SE. There were no significant group differences.
Comparison of arterial and hemodynamic variables between subjects with MS and controls, using the global sleep quality score as a covariate.
PSQI scores were analyzed to classify good or poor sleep quality in all subjects, MS and controls. The MS group had higher PSQI scores when compared to controls (7.03 ± 4.34 vs 4.13 ± 2.47; P < 0.05), indicating poorer sleep quality. The effect of sleep quality on vascular measures was analyzed in all subjects. Data are presented in Table 3. The poor sleep quality group consisted on 19 MS subjects and 5 control subjects. Subjects with poor sleep quality had higher HR (P < 0.05) and AIx@75 (P < 0.05) compared to subjects with good sleep quality. HR and AIx@75 remained significant after controlling for PA. There were no significant differences between good and poor sleepers for cf-PWV, carotid beta stiffness or IMT.
| MS (n = 31) | Controls (n = 23) | |
|---|---|---|
| HR (bpm) | 62 ± 1.6 | 58 ± 1.9 |
| SBP (mmHG) | 121 ± 2.6 | 123 ± 2.8 |
| DBP (mmHG) | 74 ± 1.7 | 76 ± 1.9 |
| AIx (%) | 27.2 ± 2.3 | 26.8 ± 2.7 |
| AIx@75 (%) | 21.2 ± 2.2 | 21.2 ± 2.4 |
| cf-PWV (m s−1) | 6.9 ± 0.2 | 6.6 ± 0.3 |
| IMT (mm) | 0.48 ± 0.02 | 0.46 ± 0.02 |
| Beta stiffness | 7.9 ± 0.39 | 7.2 ± 0.4 |
HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; AIx@75: augmentation index normalized to a heart rate of 75 beats per minute; cf-PWV: carotid-femoral pulse wave velocity; IMT: intima-media thickness; FBF: forearm blood flow.
Values are mean ± SE. There were no significant group differences.
Comparison of arterial and hemodynamic variables between subjects with MS and controls, using the global sleep quality score as a covariate.
The effect of sleep quality on vascular measures was analyzed within both the MS and control group. There were no significant differences in PA, pulse wave velocity, carotid beta stiffness or IMT between MS subjects who had good sleep quality vs poor sleep quality. In subjects with MS who had poor sleep quality, only AIx@75 was higher (26.7 ± 2.5 vs 15.7 ± 3.0 P < 0.05; errors are SE) compared to MS subjects who had good sleep quality. In controls, 5 subjects exhibit poor sleep quality and 18 were classified as having good sleep quality. Only the change in FBF from rest to peak was significantly different between poor and good sleepers in the control group (11.3 ± 3.5 vs 19.4 ± 1.8 ml/min/100 mL of tissue, P < 0.05; errors are SE).
The block regression indicated that age, BMI and mean BP, which were entered in block one, did not significantly contribute to sleep quality (R = 0.24, P > 0.05). The variables in block two (HR, cf-PWV, AIx@75, change in FBF form rest to peak) significantly increased R2, contributing to a significant overall R = 0.56 (P < 0.05). However, only HR reach significance as an individual contributor (standardized beta = 0.307, P < 0.05).
Discussion
The novel finding from this analysis is that poor sleep quality is associated with increased arterial dysfunction in both persons with MS and controls. Furthermore, among the arterial function variables, only FBF significantly differed between subjects with MS and controls. Subjects with MS had lower values, consistent with prior findings.15 However, sleep quality did not affect the difference in FBF between the MS group and controls.
MS subjects who had poor sleep quality had significantly higher AIx and AIx@75 compared to those who had good sleep quality. These findings indicate that wave reflections were increased in poor sleepers, thus the reflected wave contributed more to the overall central pressure.17,18 With increasing arterial stiffness, this reflected wave returns prematurely during systole, augmenting the pressure in late systole and increasing AIx.17,18 This may also be a function of peripheral factors such as resistance artery function (peripheral resistance) or central factors such as the impact of incident wave size which also affects AIx.19 Considering the reduction in forearm resistance artery function in persons with MS, one might suggest that peripheral factors related to resistance artery function might contribute to the higher AIX. However, there was no difference between controls and individuals with MS in AIx@75 while persons with MS exhibited lower resistance artery function. Also, poor sleepers with MS had similar resistance artery function as good sleepers with MS. Consequently, the potential reason for higher AIx in poor sleepers with MS needs further study.
Traditionally, AIx and cf-PWV are considered indicators of systemic arterial stiffness.19 In this sample, cf-PWV appeared to be higher in poor sleepers among the MS group and the MS/control group combined, although this difference was not statistically significant. Furthermore, carotid beta stiffness was not different between groups supporting a lack of effect of sleep quality on arterial stiffness. Nonetheless, higher AIx is associated with cardiovascular and coronary artery disease risk.17,18 Considering persons with MS are at an increased risk for CVD, these results could have significant health implications in this population.
Accumulating evidence supports an association between sleep quantity/quality and cardiovascular mortality, occurrence of CVD, and CVD risk factors.7,20–22 Consistent with these findings, the change in forearm blood flow from rest to peak hyperemia, a measure of resistance artery endothelial function,15 was reduced in poor sleepers in the control group. We have also provided novel evidence for the association between sleep and CVD in subjects with MS. Although the exact mechanisms are unknown, this increased risk may be mediated by sleep induced changes in immune, endocrine, and metabolic functioning.20,23 Sleep deprivation has been found to increase circulating levels of pro-inflammatory cytokines,24,25 which can result in temporary increases in arterial stiffness.12 A recent study by Sunbul et al. demonstrated that one night of sleep deprivation was associated with a significant increase in pulse wave velocity in 42 healthy subjects.20 In the present study, pulse wave velocity did not reach significance among poor sleepers; however, this may be a function of a lack of power. The mean difference in cf-PWV between good and poor sleepers was 0.5 m/s, which is often considered clinically significant.
The link between sleep and CVD may also be influenced by a decline in endothelial function, which is thought to precede the clinical development of atherosclerotic disease.22,26,27 Evidence supports a decline in flow-mediated brachial artery vasodilation with impaired sleep in healthy subjects.22,26,28 We assessed endothelial function via strain gauge plethysmography and showed significant reductions in both resting and peak hyperemic FBF among poor sleepers. This finding suggests that sleep quality may have an influence on vasomotor dysfunction in the smaller resistance arteries as well. However, this was not the case in subjects with MS. Within the MS group, there was no difference in endothelial function between good and poor sleepers, although the MS group had significantly lower peak FBF when compared to controls. Nevertheless, poor sleepers within the control group had lower endothelial function, evaluated as the change in FBF from rest to maximal hyperemia (11.3 ± 3.5 vs 19.4 ± 1.8 ml/min/100 mL of tissue, P < 0.05), which was statistically significant even though only five control subjects had poor sleep quality. This might suggest that poor sleep decreases endothelial function in apparently healthy individuals without MS; however, in individuals with MS, the disease manifestation is associated with low endothelial function which was not further affected by poor sleep. Thus, our data suggest that poor sleep quality affects arterial function differently in people with MS vs controls, but this needs to be further evaluated in future studies, given the small number of subjects in our groups.
Patients with MS experience an increased number of nocturnal awakenings, a higher total arousal index, and a lower sleep efficiency index compared to controls.2,29 Studies have reported significant associations between these sleep problems and depression,30 fatigue,29,31 and disease severity2,31; however, there are currently no treatments or interventions implemented to treat the sleep related aspect of the disease.32 In the current study, more than half of the MS subjects were classified as poor sleepers, which is consistent with previous findings.1,2 Given the high percentage of MS patients who report sleep disturbances and the strong link between sleep and CVD, more research is needed to establish a clearly understanding of the relationship between sleep and arterial health in this population. There is a critical need for interventions that improve sleep in MS.
A few limitations need to be addressed. First, this is a secondary analysis and thus the sample sizes in each group are not equal in number. In the MS group, there was a relatively small sample size when subjects were classified as good and poor sleepers, which limited the statistical power. This was especially the case when comparing good vs poor sleepers in the control group. Finally, the cross-sectional design of this study provides no evidence of causality.
In conclusion, markers of arterial stiffness and wave reflection were significantly higher in MS patients with poor sleep quality. This finding has substantial clinical implications given the strong relationship between arterial stiffness and CVD development. Additionally, significant reductions in the change in FBF during reactive hyperemia were observed in control subjects with poor sleep quality; however, this finding may have been influenced by the small number of subjects in the group with reduced endothelial function.
Conflict of interest statement
None of the authors report a conflict of interest.
Acknowledgements
This study was funded in part by a grant from the Research Board,
References
Cite this article
TY - JOUR AU - Brooke M. Shafer AU - Sushant M. Ranadive AU - Tracy Baynard AU - Robert W. Motl AU - Bo Fernhall PY - 2017 DA - 2017/07/10 TI - The implications of poor sleep quality on arterial health in persons with multiple sclerosis JO - Artery Research SP - 49 EP - 55 VL - 19 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2017.06.005 DO - 10.1016/j.artres.2017.06.005 ID - Shafer2017 ER -
