Early Initiation of ARV Therapy Among TB–HIV Patients in Indonesia Prolongs Survival Rates!
Prof. Dr. Sulianti Saroso Infectious Disease Hospital, Jakarta; Department of Public Health, University of Respati Indonesia, Jakarta, Indonesia
- DOI
- 10.2991/jegh.k.200102.002How to use a DOI?
- Keywords
- Antiretroviral; TB–HIV coinfection; Survival analysis
- Abstract
Background: The HIV epidemic remains a public health problem with rising tuberculosis (TB) numbers around the world. Antiretroviral (ARV) therapy (ART) is essential to increase the survival of patients with TB–HIV coinfection. The aim of this study is to investigate the effect of ARV treatment initiation within TB treatment duration for the survival of patients with TB–HIV coinfection.
Methods: This is a retrospective cohort study of patients with TB–HIV coinfection and who were ARV naive from Prof. Dr. Sulianti Saroso Infectious Disease Hospital between January 2011 and May 2014 (N = 275). The Kaplan–Meier method, bivariate with the log rank test, and multivariate with the Cox regression were applied in this study.
Results: Cumulative survival probability of the patients with TB–HIV coinfection receiving ARV in a year was 81.5%. The death rate in patients with TB–HIV coinfection who received late ART initiation during TB treatment is higher by 2.4 times [adjusted hazard ratio (aHR) = 2.4, 95% confidence interval: 1.3–4.5, p = 0.006] compared with the patients who were in early ART initiation and were thereafter adjusted by the location of Mycobacterium tuberculosis infection.
Conclusion:The effect of ART initiation is essential in the intensive phase (2–8 weeks) of anti-TB medication to increase the survival among TB–HIV coinfection group.
- Copyright
- © 2020 Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Tuberculosis (TB) remains to be a leading cause of death that has been projected to be 15 times higher among TB–HIV cases than non-TB–HIV cases [1,2]. TB cases among people living with HIV are almost 60% undiagnosed and untreated. Indonesia is one of the TB–HIV high-burden countries, with 360,565 cases of TB notified in all forms in 2016 including 14% with known HIV cases. Indonesia is listed among the eight countries with around 70% of all TB deaths among people living with HIV [1,2].
To ensure HIV programs integrate with regular TB screening and treatment, UNAIDS (The Joint United Nations Program on HIV/AIDS) collaborated with World Health Organization (WHO) has recommended countries to elaborate the programs that aim to respond immediately to TB active cases, beginning with antiretroviral (ARV) therapy (ART) immediately within 2–4 weeks after initiating TB therapy [2,3]. The Ministry of Health, Republic of Indonesia conducted guidelines to support concomitant treatment of the two diseases.
The initial of ART is often deferred until almost the completion of TB therapy due to concerns of potential side effects from the Immune Reconstitution Inflammatory Syndrome (IRIS), high pill burden, and drug interactions [4–6]. However, the controversial issue of timing of ART in TB patients plays a significant role [3].
The observational study in Sanglah Hospital showed that of the 60 patients with TB–HIV coinfection, only 20 patients (33.3%) initiated ART within 2 months during the TB therapy [6]. In fact, the risk of TB infection in people living with HIV is reduced with ARV by around 65% [2,7]. A trial showed a significant upsurge in survival with ART initiation within 2 weeks of the starting the TB therapy [6]. On the other hand, delay in ART initiation may result in AIDS-related illnesses and even death, as 63% of deaths occurred within the first 6 months of therapy [1,5].
The advantages of initiating TB–HIV co-treatment will have an impact on patients’ care and for receiving the optimal treatment outcomes. Therefore, the aim of this study is to examine the effect of ART initiation within the TB treatment duration for the survival of patients with TB–HIV coinfection.
2. MATERIALS AND METHODS
2.1. ARV Therapy for TB–HIV Program
The TB–HIV collaborative activities are being implemented at primary health care centers and hospitals in Indonesia. The activities are as follows: strengthening TB–HIV coordination at all levels of health facilities, TB–HIV surveillance, joint planning of TB–HIV, also the treatment monitoring and evaluation of TB–HIV program. The Ministry of Health regulations number 21 in 2013 mentioned that ART should be initiated regardless of CD4 count, and reduce the morbidity and mortality of TB–HIV patients [4].
2.2. Study Design and Setting
This study used a retrospective cohort study design to enroll all patients aged >18 years with TB–HIV coinfection receiving TB treatment and ARV naïve at Prof. Dr. Sulianti Saroso Infectious Disease Hospital between January 2011 and May 2014. We compiled the data from Medical Record Department, Directly Observed Treatments TB program, HIV–AIDS program of Infectious Disease Hospital Prof. Sulianti Saroso, Jakarta, Indonesia.
In this study, we included patients who were diagnosed as HIV positive and at least 15 years old, TB first and second category, having proper anti-TB treatment, registered in national ARV program, and the duration of TB–HIV treatment more than 8 weeks as exposed group. Non-exposed group was patients diagnosed as HIV positive and at least 15 years old, TB first and second category, having proper anti-TB treatment, registered in national ARV program, and the duration of TB–HIV treatment 2–8 weeks. This study excluded pregnant women and patients with uncompleted database of medical record (Figure 1).
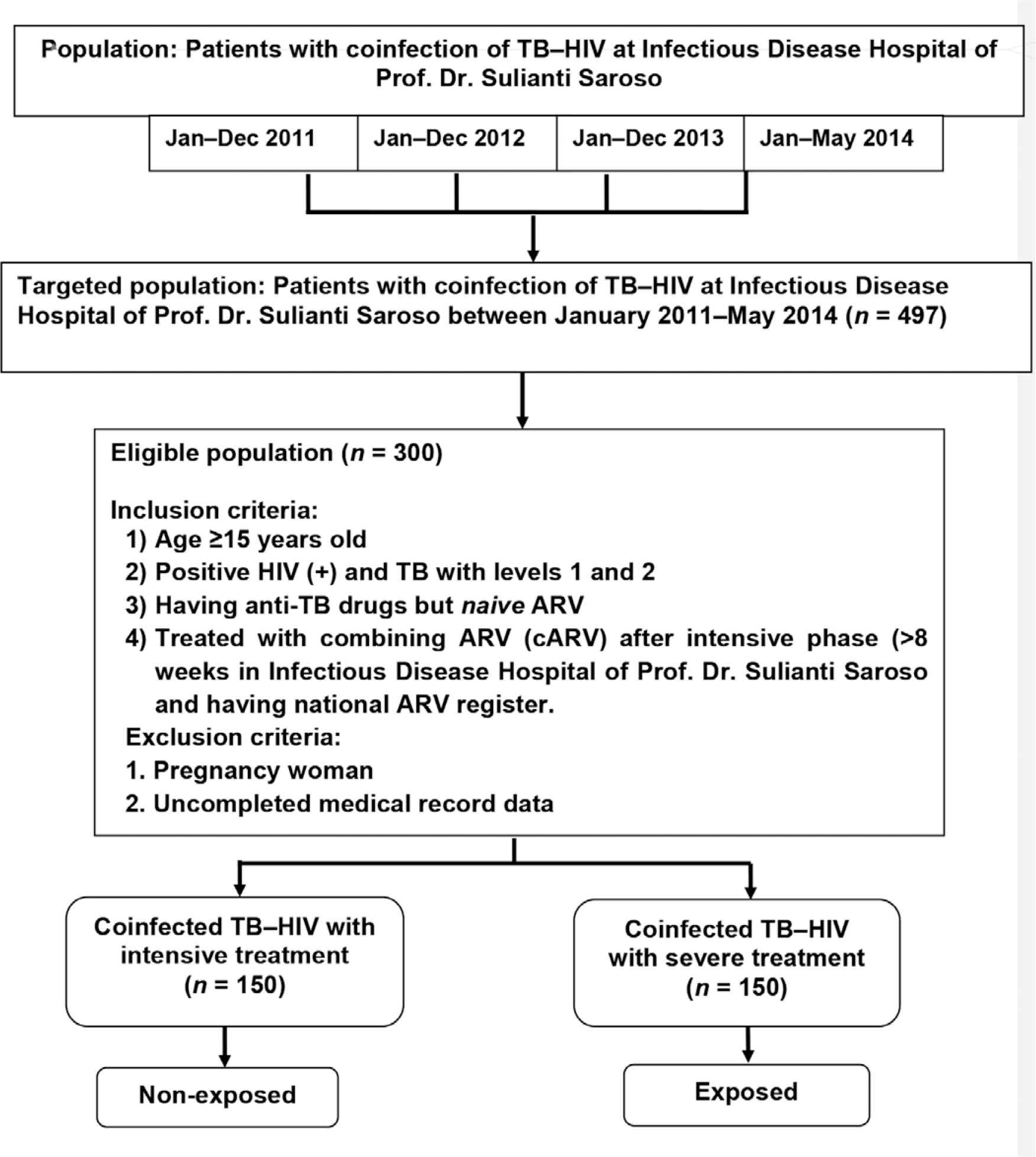
Sample procedure flowchart.
We used the sample size for hypothesis testing for two population proportions at 95% confidence interval with a power of 80% [8]. We estimated that the minimum sample size to be 248 patients and they were divided into two groups: early initiation of ART during TB treatment group (124 patients) and late initiation of ART group (124 patients) (Figure 2).
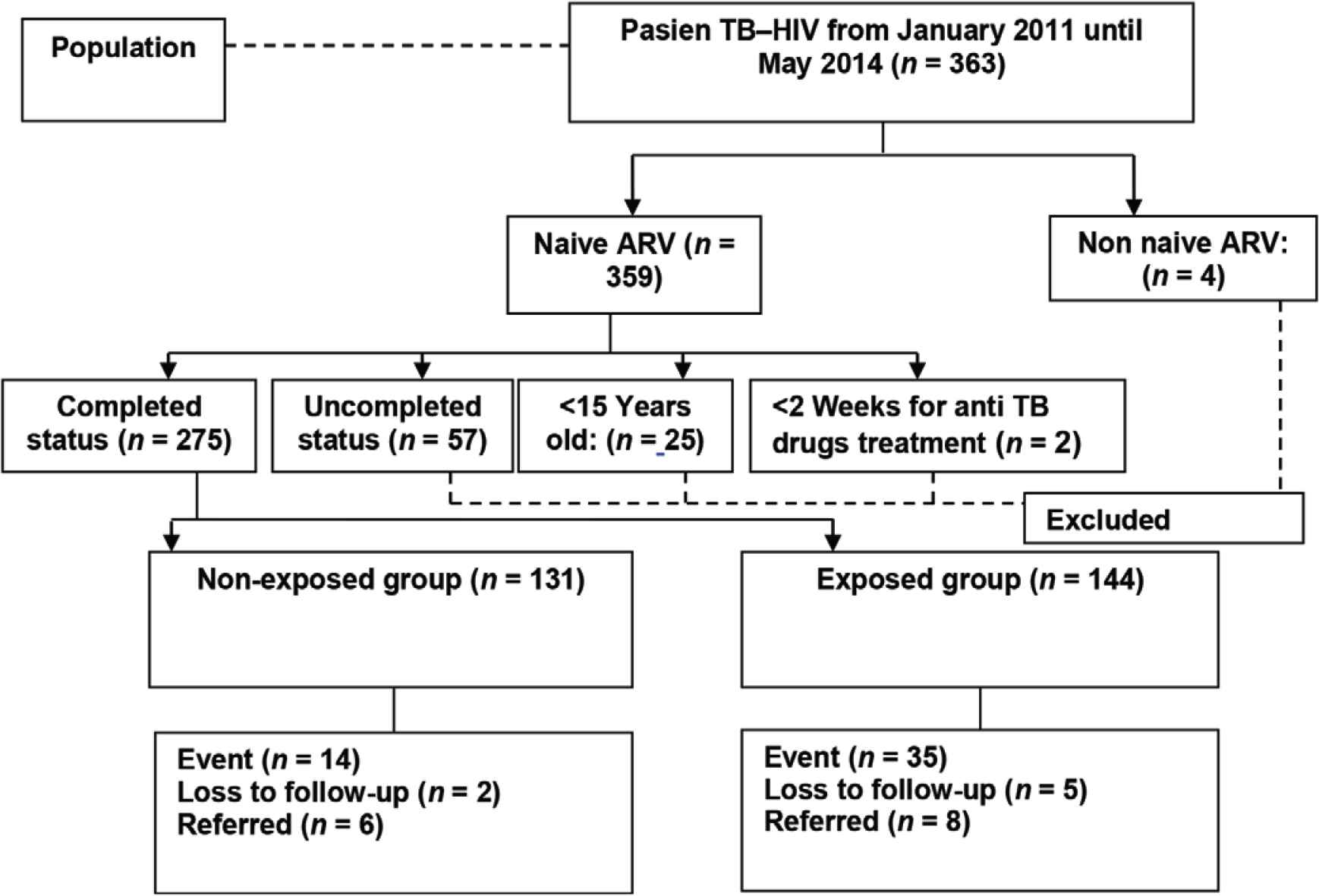
Sample collection flowchart.
2.3. Statistical Methods
Demographic characteristics, timing of TB and HIV diagnosis, time of TB treatment and ART initiation, number of deaths, and medical characteristics were obtained from the hospital registration book, the ARV monitoring book, and medical records. The Kaplan–Meier method, the log rank test and the Cox regression model were applied to analyze the data statistically. The data analysis used STATA 11 (Stata Corporation, College Station, TX, USA).
3. RESULTS
Of 275 patients who participated in this study, 131 patients (47.64%) received ART within 2–8 weeks (early ART initiation during TB treatment group) and 144 (52.36%) patients received ART >8 weeks after TB treatment (late ART initiation group). By the end of the first year observation, 205 (74.5%) patients were still alive, 49 (17.8%) patients had died, and 21 patients (7.6%) were lost to follow-up. There were 49 death cases reported during the follow-up, they consisted of 14 patients (10.7%) who were in the early ART initiation group and 35 patients (24.3%) who were in the late ART initiation group (Table 1).
| Variables | Late (N = 144), N (%) | Early (N = 131), N (%) |
|---|---|---|
| Sex | ||
| Male | 107 (74.30) | 92 (70.23) |
| Female | 37 (25.70) | 39 (29.77) |
| Age (median) | ||
| ≥41 years | 69 (47.92) | 66 (50.38) |
| <41 years | 75 (52.08) | 65 (49.62) |
| Education | ||
| ≤High school | 18 (12.50) | 20 (15.27) |
| >High school | 126 (87.50) | 111 (84.73) |
| Adherence | ||
| No | 30 (20.83) | 33 (25.19) |
| Yes | 114 (79.17) | 98 (74.81) |
| CD4 count before ART | ||
| ≤101 cells/mm3 | 115 (79.86) | 108 (82.44) |
| >101 cells/mm3 | 29 (20.14) | 23 (17.56) |
| Location of M. tuberculosis | ||
| Extra/combination pulmonary | 34 (23.61) | 26 (19.85) |
| Pulmonary | 110 (76.39) | 105 (80.15) |
| Stage of HIV/AIDS | ||
| IV | 54 (37.50) | 37 (28.24) |
| III | 90 (62.50) | 94 (71.76) |
The demographic and clinical characteristics of patients who received ART within 2–8 weeks (Early ART initiation during TB treatment group) and patients who received ART >8 weeks after TB treatment (Late ART initiation group)
The proportion sample of male patients was 92 (46.2%) in the early ART initiation group and 107 (53.8%) in the late ART initiation group. The proportion of female patients accounted for 39 (51.3%) in the early ART initiation group and 37 (48.7%) in the late ART initiation group.
The Kaplan–Meier method estimates that the cumulative proportion for those surviving over the first year in early ART initiation during TB treatment was 81.5% (Figure 3), whereas the survival rates of patients in the early and late ART initiation groups were 89.1% and 74.5%, respectively. The result from the log rank test showed a significance between two group (p = 0.003) (see Figure 4). The bivariate Cox regression analysis revealed that late therapy increased mortality rate [crude hazard ratio (cHR) = 2.5; 95% confidence interval (CI): 1.3–5.1; p = 0.001]. The patients who received late ART initiation had 2.4 times higher risk of death [adjusted hazard ratio (aHR) = 2.4; 95% CI: 1.3–4.5; p = 0.006] compared with those who were in the early ART initiation. Other covariates, for example, the location of Mycobacterium tuberculosis, were also associated with mortality (aHR = 1.9; 95% CI: 1.0–3.4; p = 0.039) (see Table 2).
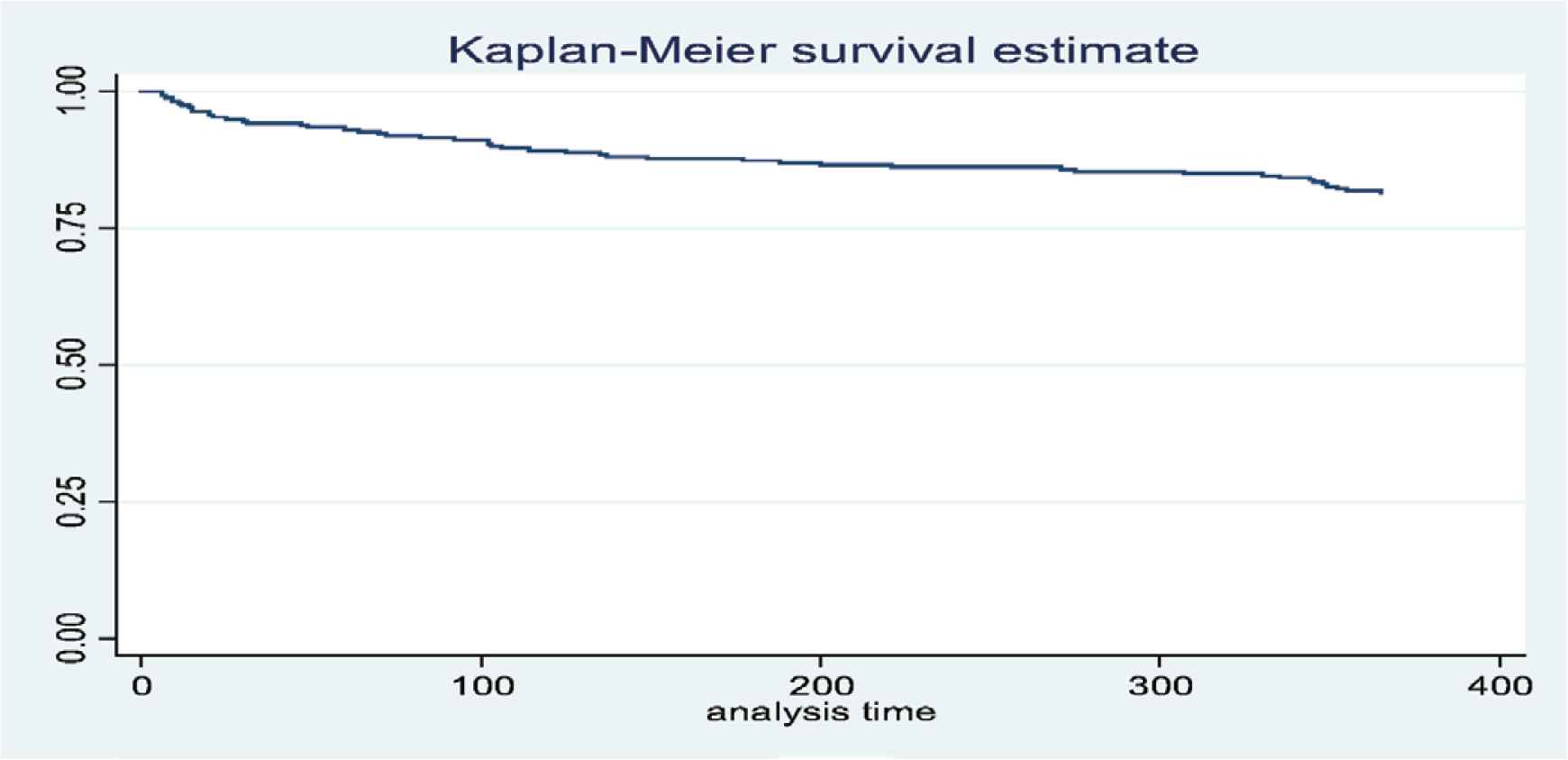
The Kaplan–Meier estimates of cumulative survival among 275 TB–HIV coinfected patients [X = time (days); Y = cumulative probability].
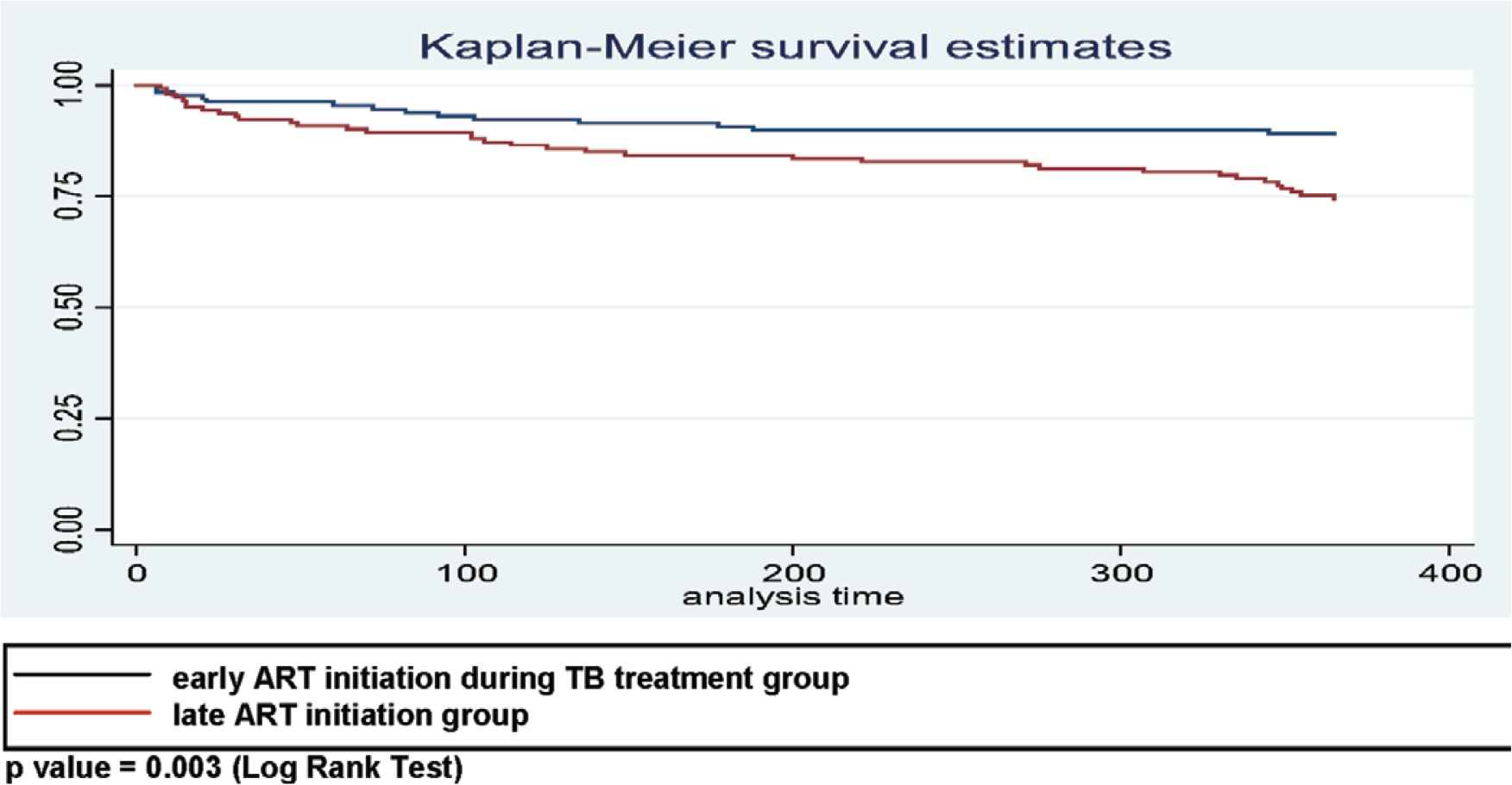
The Kaplan–Meier estimates of cumulative survival among 131 TB–HIV patients in early ART and 144 TB–HIV patients in late ART during TB treatment (p = 0.003; log rank test).
| Variables | Crude hazard rate (95% CI) | p-value | Adjusted hazard rate (95% CI) | p-value |
|---|---|---|---|---|
| Late ART initiation | 2.512 (1.317–5.053) | 0.002 | 2.398 (1.289–4.461) | 0.006 |
| Extra/Combination pulmonary | 2.012 (1.034–3.761) | 0.028 | 1.876 (1.032–3.411) | 0.039 |
p-value < 0.05.
Cox regression, bivariate and multivariate analysis
4. DISCUSSION
This study analyzed survival outcomes over the first year among patients with TB–HIV coinfection receiving early and late ART during TB treatment at Prof. Dr. Sulianti Saroso Hospital for the first time. The survival time for the patients who received early ART initiation was better than those who received the late ART initiation. This result is in line with a study that the people after the follow-up are less frequently to reporting as death cases than in the deferred time (p = 0.02) [9].
Survival analysis of this study found that the risk of death for the TB–HIV cases that received late ART was higher compared with the patients who received early ART. The patients who engaged in ART for 8 weeks of TB treatment had 1.89 times the rate of death (aHR = 1.89, 95% CI: 1.05–3.40, p = 0.03) than the cases who never or late engaged ART >8 weeks of TB treatment [9].
Havlir et al. demonstrated that ART can be safely administered early in the onset of TB treatment [10]. The urgency of starting ART during the course of TB therapy is dependent on the immune status of the patient. The patients who started ART 2 weeks after the start of TB treatment compared with those who started over 8 weeks had reduced mortality and AIDS-related illnesses (26.6% to 15.5%) [11]. On the other hand, mortality in the CAMELIA study (median entry CD4+ lymphocyte counts of 25 cells/mm3) (interquartile range, 10.56) and death or AIDS-defining illnesses in the low CD4+ strata (<50 cells/mm3) and in the starting antiretroviral therapy at three points in tuberculosis (SAPIT) study were all significantly reduced among patients starting immediate vs early ART [9,11]. However, especially for patients with CD4 counts ≥50 cells/mm3, the decision of early initiation must require clinical judgment to confirm that the patient has the capacity to manage IRIS and toxicities [7,11]. The recommendation of early initiation in TB–HIV coinfected patients with a better IRIS management practice was important to maintain the benefits with minimal TB IRIS risk in health care facilities [3].
In this study, the overall baseline mean of CD4 cell count was 66 cells/mm3 and the proportion of extra pulmonary TB was 60 patients (21.82%). The data showed that the patients in this study defined advance HIV clinical stadium. Patients who delay ART initiation more likely increase the risk of new opportunistic diseases and death, especially in patients with advanced HIV disease [12].
HIV infection causes a rapid decline of the immune responses, resulting in the multiplication of the mycobacterium within the granuloma and leading to the reactivation of the infection. It was also theorized that there can be reported increasing replication of HIV infection by the multiplying within the activated CD4+ T cells and the macrophages accumulating at the site of granuloma also causing the reactivation of the infection, thus resulting in a lower ability to control M. tuberculosis and survival [13].
We also found that the first year’s cumulative proportion of surviving patients were higher than the cumulative survival time from other hospitals in Indonesia, Dharmais Cancer Hospital (66.4%); Fatmawati Hospital (79.4%); Drug Dependence Hospital (54.46%). The reason for these differences is frequently to be a result of the fact that the study population in the three hospitals did not consider the status of initiation of ART, early or late initiation of ART.
There are a few limitations in this study. First, the exact dates and the loss of patients not following up were not collected (2.54%). Second, misclassification is likely to occur in a grouping of adherence categories. In conclusion, TB–HIV coinfection accelerates the disease progression when the patients received late or never started the ART initiation. The effect of ART initiation is essential in the intensive phase (2–8 weeks) of anti-TB medication to increase the survival of patients with TB–HIV coinfection.
5. CONCLUSION
We conclude that it is crucial to start ART in the intensive phase (2–8 weeks) of anti-TB medication to increase the survival rate among the TB–HIV coinfection cases. Elaborate ART in TB program might play significant role in TB–HIV coinfection program in hospital or health care services.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
SM and NM contributed in study conceptualization and writing (review and editing) the manuscript, data curation, formal analysis, and writing (original draft). NM contributed in study conceptualization and writing (review and editing) the manuscript, and supervised the project. AR supervised the project and wrote the manuscript. RM contributed in study conceptualization and supervised the project. TBP contributed in writing (review and editing) the manuscript.
ACKNOWLEDGMENTS
We would like to thank the staff of POKJA MELATI of Prof. Dr. Sulianti Saroso Infectious Disease Hospital for making this research possible.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Siti Maemun AU - Nina Mariana AU - Adria Rusli AU - Renti Mahkota AU - Tri Bayu Purnama PY - 2020 DA - 2020/01/13 TI - Early Initiation of ARV Therapy Among TB–HIV Patients in Indonesia Prolongs Survival Rates! JO - Journal of Epidemiology and Global Health SP - 164 EP - 167 VL - 10 IS - 2 SN - 2210-6014 UR - https://doi.org/10.2991/jegh.k.200102.002 DO - 10.2991/jegh.k.200102.002 ID - Maemun2020 ER -
