Emerging Exotic Fruits: New Functional Foods in the European Market
- DOI
- 10.2991/efood.k.200406.001How to use a DOI?
- Keywords
- Superfruits; functional food; dragon fruit; cherimoya; finger lime; star fruit
- Abstract
The consumption of exotic fruits is rapidly increasing in European countries. Some of these products have attracted much interest due to their alleged properties of preventing malnutrition, over-nutrition, and disease, maintaining a healthy body. Scientific studies on these fruits are multiplying, including chemical characterizations and biological investigations on in vitro and in vivo experimental models. This review concerns four edible fruits: Hylocereus undatus (dragon fruit), Annona cherimola (cherimoya), Citrus australasica (finger lime), and Averrhoa carambola (carambola or star fruit). By screening biomedical databases, viz. Scopus, WOS, and PubMed, a total of 131 papers have been selected. Data reveals a wide series of biological effects that confirm traditional medicinal uses or suggest new therapeutic applications. Most studies concern problems related to nutrition, such as body redox balance, metabolic syndrome, and hepatoprotective effects, but other properties have been highlighted, including anticancer, antimicrobial, anti-inflammatory, and neuroprotective effects, as well as cardiovascular and skin protection. Pharmacological investigations have also been focused on specific compounds, assuming a potential role in drug discovery. In summary, food products, byproducts, and single compounds derived from these plants could be exploited in the prevention of disease or for specific treatments of health problems.
- Copyright
- © 2020 International Association of Dietetic Nutrition and Safety. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
The consumption of exotic fruits is rapidly increasing in many European countries. Some of these products are appreciated since long by consumers, such as pineapple, mango and avocado. However, less known exotic fruits like tamarind, feijoa, lychee, guava, rambutan, and many others, are spreading in European markets, where the interest for new flavors and varieties is expanding. Following this positive trend, producers and retailers are introducing in the market an increasing number of exotic fruits with high nutritional power, also known as “superfruits” [1]. This term is generally used to indicate the superior health benefits of these fruits, being rich in fibers, vitamins, minerals, and antioxidant components, such as phenolic acids, flavonoids, and anthocyanins. Superfruits include species native to different countries and continents, and belonging to many botanical families, viz. açaí (Euterpe oleracea Mart., Arecacae), acerola (Malpighia emarginata DC., Malpighiaceae), and pitanga (Eugenia uniflora L., Myrtaceae) from South America; goji (Lycium barbarum L., Solanaceae) from China; durian (Durio zibethinus L., Malvaceae) from Southeast Asia; moringa (Moringa oleifera Lam., Moringaceae) from northwestern India, etc. The success on the market of these products is linked to the belief that fruits and vegetable prevent malnutrition, over-nutrition, and disease, maintaining a healthy body [2]. Consequently, many researches are underway on the phytochemical and pharmacological properties of these fruits.
The aim of this review is to provide an overview of the functional, biological, and physiological properties of four different emerging exotic fruits coming from different countries, which have recently appeared on the European market: namely dragon fruit or pitahaya [Hylocereus undatus (Haw.) Britton & Rose], native to Mexico and Central America; cherimoya or chirimoya (Annona cherimola Mill.), also known as custard apple, native to Colombia, Ecuador, Peru, Bolivia and Chile; finger lime (Citrus australasica F. Muell.), native to Australia, and carambola or star fruit (Averrhoa carambola L.) native to tropical Southeast Asia.
2. MATERIALS AND METHODS
A survey of scientific literature on the selected species was conducted on Scopus (https://www.scopus.com), Web of Science (https://apps.webofknowledge.com), and PubMed (https://pubmed.ncbi.nlm.nih.gov) databases, from inception to 2019. We used scientific and common English names of H. undatus, A. cherimola, C. australasica, and A. carambola as keywords in either title or title and abstract, finding totals of 336, 256, 70, and 329 papers, respectively. Thereafter, subsets of 24, 29, 17, and 61 papers were selected for H. undatus, A. cherimola, C. australasica, and A. carambola, respectively, concerning traditional uses, phytochemistry, nutritional value, medicinal properties, ethnobotanical uses, experimental studies, and clinical trials.
3. DRAGON FRUIT
Hylocereus undatus (Haw.) Britton & Rose (Cactaceae).
3.1. Features
The plant is commonly known as dragon fruit or white-fleshed pitahaya. It is a climbing or recumbent epiphytic cactus, with a triangular green stem, 10–12 cm in diameter, and up to 6–10 m long. The stem is covered with areolas bearing small whitish thorns and gives origin to surface and aerial roots. Flowers are up to 30 cm long, with a crown of acuminated bracts and several green, yellow, or white petals. Blossoming is ephemeral, generally occurring during the night, while pollinators are nocturnal bats and moths. The fruit is oblong ovate (Figure 1A and 1B), weighs 150–600 g, is covered by large red bracts with a greenish apex, and contains an edible whitish pulp with numerous black seeds [3,4].
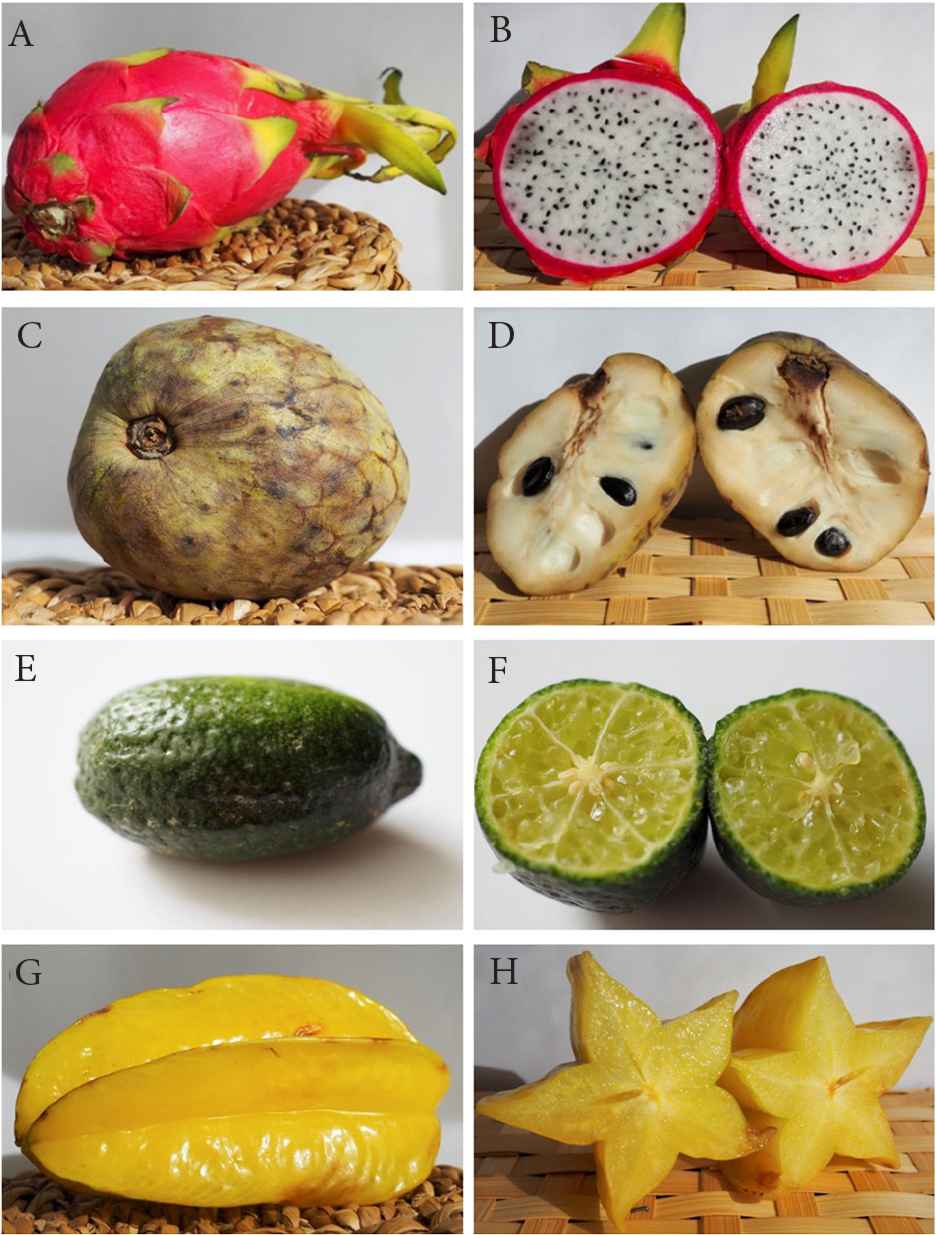
Pictures of whole fruits and their half sections. (A and B) Dragon fruit (Hylocereus undatus). (C and D) Cherimoya (Annona cherimola). (E and F) Finger lime (Citrus australasica). (G and H) Carambola (Averrhoa carambola).
The species is native from tropical and subtropical America, where it grows spontaneous or cultivated for fruits. Cultivation has been extended to tropical areas of Asia and Oceania, while the fruit market has reached Europe. The fruit can be eaten fresh or used for juices, desserts, and other food. The plant has an important role in Mexican heritage as food, medicine, and ornament [5].
3.2. Chemical Composition
Data on the chemical composition of the plant are limited. The fruit is especially rich in glucose, fructose, proline, and ascorbic acid. Major minerals include potassium, magnesium, and calcium. The fruit is also rich in mucilage, due to the abundant presence of mucopolysaccharides that arrive to make up about 1% of the mesocarp pulp, and consist mainly of hemicellulose, cellulose, and pectin [6].
Carotenoids are abundant and include β-carotene, lycopene and tocopherols [7]. Major flavonoids are kaempferol, quercetin, isorhamnetin, and their glycosylated derivatives [8]. A study has reported the identification of two triterpenes from a leaf chloroform extract, taraxast-20-ene-3α-ol and taraxast-12,20(30)-dien-3α-ol [9].
The fruit peel has been found to contain betalain indole pigments, a complex consisting of red-purple betacyanins and yellow betaxanthins [10]. A fixed oil extracted from seeds has been found to consist mainly of linoleic, oleic, palmitic, stearic, cis-vaccenic, and linolenic acids, and to contain in addition tocopherols, and sterols [11].
3.3. Traditional Uses
The plant has been traditionally used since ancient times in the Mexican medicine. Leaves and flowers were used by Maya as hypoglycemizing, diuretic, and wound healing remedies [12].
3.4. Nutritional Value
The fruit is considered a health-preserving nutrient owing to its antioxidant properties [13]. Comparison with other well-known antioxidant fruits has revealed high radical scavenging activity, possibly thanks to the presence of betalains [10,14]. In addition, nutritional value is conferred by high contents in vitamin C, thiamine, niacin, riboflavin, omega-3 and -6 fatty acids, calcium, phosphorous, and iron [15]. The fruit oligosaccharide has been found to act as prebiotic in mice gut, and to modulate microbiota and stimulate gut immune responses in rats [16,17].
3.5. Therapeutic Properties
3.5.1. Antidiabetic effect
Dietary consumption of the fruit is thought to impact positively on carbohydrate metabolism, and more specifically on type 2 diabetes [15]. Addition of dried dragon fruit to the diet has determined a lowering of blood glucose levels [14]. Antidiabetic properties of the fruit have been confirmed by a study on insulin-resistant rats [18]. Moreover, administration of the fruit juice to mice fed with a high-fat diet has limited the occurrence of insulin resistance, hepatic steatosis, and hypertrophy of the adipose tissue [19].
3.5.2. Vascular protection
The use of an increased skin vascular permeability model in the rabbit, consisting in the measurement of the leakage of Evans blue dye, has shown protective effects on microcirculation of an aqueous extract. The same experimental model has been used to reveal protective effects on microcirculation of the triterpenes taraxast-20-ene-3α-ol and taraxast-12,20(30)-dien-3α-ol [20,21].
3.5.3. Anticancer effect
Anticancer properties have been ascribed to polyphenols, flavonoids, and betalains present in the fruit. Accordingly, an ethanolic extract of the peel has contrasted the growth of human hepatocellular carcinoma cells [15], while a methanolic extract of the fruit, containing polyphenols and flavonoids, has been tested against the AGS human gastric cancer, HeLa human cervical cancer, and MCF-7 human breast cancer cell lines and patented as anti-cancer composition [22].
3.5.4. Wound healing effect
Aqueous extracts from different plant portions were applied to wounds in streptozotocin diabetic rats, finding amelioration of different physiological parameters of wound healing, including increases in total proteins, collagen content, hydroxyproline, tensile strength, as well as improved epithelialization [9].
3.5.5. Skin protection
The fruit aqueous extract is deemed to possess skin antiaging, firming, and humectant properties due to the presence of mucilage, while a depigmenting formulation based on a pulp extract has been patented [23]. A few patents have been developed containing extracts for different skin care applications, e.g. [24].
4. CHERIMOYA (CHIRIMOYA)
Annona cherimola Mill. (Annonaceae).
4.1. Features
Semi-evergreen shrub or small tree, with single and alternate leaves, ovate to elliptic, blunt pointed at the apex, dark green on the upper surface and velvety on the underside. Flowers have greenish, fleshy sepals and pinkish petals, and are borne solitary or in groups of 2 or 3. The fruit is a syncarp formed by pistil aggregation, is heart-shaped, conical, or oval, measures 10–20 cm in length and about 10 cm in width, and has a white, juicy flesh, containing numerous seeds (Figure 1C and 1D). The species is typical of Guatemala, Peru and Ecuador, and has spread in tropical and subtropical areas distributed worldwide, both wild and cultivated, with various varieties and cultivars [25].
The fruit is edible and can be used fresh or in the preparation of cream, milk shakes, sorbets, yoghurt, flans, fruit juice, and wine. The plant has been used since ancient times as a medicinal remedy for a number of diseases [26].
4.2. Chemical Composition
Different bioactive compounds have been characterized in the plant, classified as sugars, amino acids, organic acids, carbohydrates, choline, phenolic acids and derivatives, flavonoids, phenylpropanoids, alkaloids, saponins, tannins, phenols, and phytosterols [27–29].
The fruit contains a high amount of carbohydrates and low content of acids, and is rich mainly in carotenoids, namely lutein, β-cryptoxanthin, and β-carotene, vitamin C and B6, thiamine, riboflavin, and folate [30]. The fruit peel and pulp have been found to contain oleic, palmitic, linoleic, α-linolenic, cis-vaccenic, and stearic acids, while the seed linoleic, oleic, linolenic, γ-linolenic, and heptadecenoic acids. Other fatty acids of peel, pulp, and seeds include lauric, myristic, pentadecanoic, palmitoleic, margaric, arachidic, gadoleic, eicosadienoic, behenic, docosadienoic, and lignoceric acids. The seeds have been found to contain α-tocopherol and δ-tocopherol, while peel and pulp only α-tocopherol. Fruit phytosterols include ergosterol, campesterol, stigmasterol, stigmastanol, γ-sitosterol, β-sitosterol, lanosterol, and 24-methylencycloartanol. Main phospholipids are phosphatidylethanolamine, phosphatidylinositol, and phosphatidylcholine [31]. The fruit is a good source of potassium, phosphorous, calcium, magnesium, copper, and manganese [26]. Among phenolic compounds, procyanidins are the main class identified in the fruit pulp and peel, including procyanidin dimer type A and procyanidin tetramer type B, together with catechin, epicatechin, and quercetin, while higher quantities of organic acids, e.g. citric acid, and flavonoids have been found in seeds [32]. The seeds have been also found to contain the acetogenin alkaloids annomolin and annocherimolin, and the cyclopeptides cherimolacyclopeptide A and B [33,34].
Volatile constituents of cherimoya bark have been identified by gas chromatography and mass spectrometry, reporting the presence of methyl butanoate, butyl butanoate, 3-methylbutyl butanoate, 3-methylbutyl 3-methylbutanoate, and 5-hydroxymethyl-2-furfural as major compounds [35].
4.3. Traditional Uses
Crushed seeds have been popularly used as insecticide against lice and skin parasites, due to the presence of annonaceous acetogenins with antimicrobial and insecticidal power [36]. The fruit is popular as food for its exceptional taste but is also used in traditional medicine and as antimicrobial and insecticide, and for digestive disorders [37]. The immature fruit also finds culinary uses in curry and as cooked vegetable. Various parts of the plant are used as decoctions, the bark as a tonic and a remedy for diarrhea, the root to treat fever, and the leaves against parasite worms [29].
4.4. Therapeutic Properties
4.4.1. Antioxidant activity
Methanol, ethanol, and dimethyl formamide fruit extracts have exhibited antioxidant, radical scavenging, and metal chelating properties in different tests. The dimethyl formammide extract has exhibited the highest scavenging activity of DPPH, ABTS+, FRAP, and superoxide radical. The ethanol extract has shown best protection against the effect of tert-butyl hydroperoxide on lipid peroxidation. In addition, all extracts have enhanced cell survival and decreased lactate dehydrogenase release in human lymphocytes exposed to tert-butyl hydroperoxide [38]. In another study, the activities of radical scavenging of peel and pulp ethanolic extracts have been evaluated by the DPPH and ABTS tests, while the antioxidant capacity has been measured by the β-carotene bleaching test. The peel extract has shown higher free radical scavenging and antioxidant activity and has been found to contain a higher content of phenols and flavonoids [39].
4.4.2. Antimicrobial and antiparasitic effects
Strong antimicrobial activity on fungi and bacteria has been exerted by the essential oil [40]. The seed cyclic peptide cherimolacyclopeptide E has exerted antimicrobial activity against Pseudomonas aeruginosa, Escherichia coli, and Candida albicans, and in addition anthelmintic activity against the earthworms Megascolex konkanensis, Pontoscolex corethrusus, and Eudrilus sp. [41]. In addition, leaf methanolic extracts have shown antiviral activity against herpes simplex type 2 virus [42].
Traditional uses of the plant for gastrointestinal disorders have been accounted for by the identification of kaempferol as a major antiprotozoal agent in an in vitro bioassay-guided fractionation of a leaf ethanol extract [43].
4.4.3. Antidiabetic and antihyperlipidemic effects
A leaf ethanol extract has reduced blood glucose levels in streptozotocin-induced diabetic rats, possibly by stimulating insulin release, while a leaf methanol extract has been able to produce a hypoglycemic effect in normal rats [29]. High-performance Thin-layer Chromatography (HPTLC) coupled to diode array detector mass spectrometry has allowed to identify α-glucosidase inhibitors in the fruit, identified as the phenolamides N-trans-feruloyl tyramine, N-trans-p-coumaroyl tyramine, and N-trans-feruloyl phenethylamine [44].
The traditional anti-hypercholesterolemic use of the leaf decoction has been experimentally confirmed in vitro, by evaluating the inhibition of cholesterol uptake and of the cholesterol biosynthesis enzyme 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase in the human colorectal adenocarcinoma Caco-2 cells [45].
4.4.4. Neuroprotective effect
The plant is popularly known as an antidepressant and such a property has been verified in a study on mice subjected to forced swimming test by using an alkaloid-rich leaf extract fraction containing a mixture of the oxo-aporphine liriodenine, and the aporphines 1,2-dimethoxy-5,6,6a,7-tetrahydro-4H-dibenzoquinoline-3,8,9,10-tetraol, anonaine, and nornuciferine [46]. Moreover, the anxiolytic activity of a leaf hexane extract has been verified in mice through intraperitoneal administration, by measuring exploratory and burying behavior tests. The GABAergic (gamma-aminobutyric acid) antagonist picrotoxin has blocked the effect of the extract, while the GABA-A receptor agonist muscimol has produced a synergistic anxiolytic action with the extract, each used at a sub-threshold dose [47].
By using coupled HPTLC-mass spectrometry, three potential acetylcholinesterase inhibitors have been identified in the fruit peel, namely the alkaloids anonaine, glaucine, and xylopine [48].
4.4.5. Anticancer effect
An ethanol macerate from seeds has shown antiproliferative activity against a gastric adenocarcinoma cell line, inducing overexpression of the p53 upregulated modulator of apoptosis (PUMA) gene. Comparison with the effect on normal human gastric epithelial cells has revealed a selectivity index greater than that of cis-platin [49]. Similarly, an ethanolic seed extract has been tested for pro-apoptotic induction in acute myeloid leukemia cells, viz. the KG-1, Monomac-1, and U937 cell lines, revealing the upregulation of pro-apoptotic proteins operating upstream of intrinsic and extrinsic pathways of apoptosis. It has also been found that these effects are selective, because only a slight decline of cell proliferation has been observed on normal mesenchymal cells [50]. The acetogenins annomolin and annocherimolin, and the cyclic peptides cherimolacyclopeptides, isolated from seeds, have been identified as strong cytotoxic agents against different tumor cell lines [33,41,51].
4.4.6. Skin protection
Proanthocyanidins consisting prevalently of (epi)catechin units linked by B-type interflavan bonds, purified from the fruit pericarp, have shown strong in vitro inhibition of monophenolase and diphenolase activities of tyrosinase, revealing potential as depigmenting agents [52]. A fruit extract has been patented as fibroblast activator for skin treatment [53].
5. FINGER LIME
Citrus australasica F. Muell. (Rutaceae), syn. Microcitrus australasica var. australasica (F. Muell.) Swingle.
5.1. Features
Shrub or small tree, 2–6 m high, with compact crown and axillary straight spines. Leaves are borne on short wingless petioles, small, obovate or elliptic, with a cuneate base and margins crenate towards the notched apex. Many oil glands are present, giving an aromatic smell to the crushed leaves. Flower buds are pink, while opened flowers show white petals. Stamens are numerous (up to 25) and the ovary shows 5–7 locules, each with 8–16 ovules. Fruits are cylindrical, finger-shaped, 4–8 cm long, and variable in color from green-yellow to pink-reddish (Figure 1E and 1F) [57].
The species is endemic of the northeast coast of New South Wales and southern Queensland, Australia [57]. It is largely cultivated in the Byron Bay and in the Bangalow area of northern New South Wales by a limited number of horticulturalists specialized in Australian native species (https://www.fondazioneslowfood.com/en/ark-of-taste-slow-food/finger-lime-2/). Plantations are also present in different world areas with a suitable climate, especially in California at around 15,000 trees, but also in Israel, Spain, and France, and more recently, in southern Italy and eastern Thailand [58].
The fruit contains many spherical juice vesicles that are effervescent and resemble caviar. These vesicles or “pearls” are known as “lime caviar”, or “citrus caviar”, have a flavor slightly sweeter than lemon and are becoming popular as gourmet food, seafood garnish, and cocktail component. Therefore, finger lime is highly sought for by chefs and foodies of top restaurants and for the production of cordials, marmalades and desserts [59]. It is the world’s most lucrative fruit, while in Europe with growing demand its price has reached 125 Euros per kg of good quality product [58]. For this reason, many new cultivars of Australian finger lime are continually being developed and selected, with color varying from blue-green to pink-red, e.g. C. australasica var. sanguinea [60]. Commercial hybrids have been raised between C. australasica and Citrus limonia (rangpur lime), and between C. australasica and Citrus microcarpa (calamondin), obtaining ‘blood lime’ and ‘sunrise lime’, respectively [61]. About 50% of Australian finger lime production is currently exported to European and Asiatic markets [60].
5.2. Chemical Composition
The composition in nutrients of the fruit edible portion has been reported to include (expressed in g per 100 g FW) 65.5 moisture, 12.4 sugar, 4.9 fat, 2.5 protein, 0.7 ash, 14 dietary fibre. Reported amounts of minerals and vitamin (expressed in mg per 100 g FW) are the following: 50 Ca, 0.4 Cu, 0.8 Fe, 31 Mg, 290 K, 9 Na, 0.3 Zn, and 0.42 niacin equivalents [62].
The fruit contains a low level of phenolics, meaning that its antioxidant capacity is relatively weak. On the other hand, it is high in citric acid and is a good source of vitamin C, especially the pink variety, showing a threefold level of vitamin C with respect to mandarin [63]. A study on the phenolic composition has identified and quantified 31 phenolics, one secoiridoid derivative, and one neolignan glycoside. Among these compounds, seven have been first reported in a citrus fruit: isorhamnetin 3-O-(6′-acetyl)-galactoside, quercetin 3-O-sinapoyl-sophoroside, 3-O-methyl-5-pentylresorcinol-O-[β-D-glucopyranosyl-(1→6)]-β-D-glucopyranoside, (7S,8S)-4,7,9,9′-tetrahydroxy-3,3′ dimethoxy-8-4′-oxyneolignan-9′-OD-glucopyranoside, lyoniresinol 9′-O-glucoside, lyoniresinol 2α-O-β-glucoside, and lonicerjaponin B. In addition, it has been found that the fruit antioxidant properties change significantly in relation to different cultivars, with ‘XiangBin’ exhibiting better antioxidant capacities than ‘LiSiKe’, especially in the peel [64].
In the red finger lime (C. australasica var. sanguinea) phenolics have been quantified as follows: 40.9% flavones/flavonols, 2.5% psoralen, and 22.1% coumarin/cinnamic acid in the flavedo, 11.2% flavones/flavonols, and 74.1% coumarin/cinnamic acid in the juice [65]. In this variety, cyanidin 3-glucoside has been reported as the main anthocyanidin glucoside [66].
The major constituents of the essential oil obtained from the fruit peel of C. australasica var. sanguinea are bicyclogermacrene (25.9%), α-pinene (10.2%), and spathulenol (9.8%) [67], while in C. australasica major constituents are sabinene (19.6%) and limonene (51.1%) [68]. In the C. australasica peel extract, limonene and isomenthone have been found as major volatile compounds, while new molecules identified in a Citrus species include 6-methyloctyl acetate, citronellyl citronellate, cis-isoascaridole, 1,2:5,6-diepoxy-p-menthane, 2,3-epoxy-p-menthan-6-one, cis- and trans-p-menth-1-en-3-ol-6-one, and 1,2-epoxy-p-menthan-5-one [69]. The variability in volatile constituents is probably responsible for the distinct flavors of the fruit peel in different varieties, such as raspberry, floral, melon, apple, and cider [58]. New chemotypes, never reported before for Citrus species, have been characterized for three cultivar of C. australasica, namely the limonene/sabinene chemotype for cv. Alstonville, limonene/citronellal/isomenthone for cv. Judy’s Everbearing, and limonene/citronellal/citronellol for cv. Durham’s Emerald [69]. Besides the peel essential oil, also the leaf essential oil has been studied, showing that the principal components are bicyclogermacrene (19–28%), germacrene-D (2–8%), δ-elemene (0.5–11%) and limonene (12–24%) [70].
5.3. Traditional Uses
Traditional uses of the fruit as food by aboriginal communities is reported by the Australian Native Food & Botanicals web site (https://anfab.org.au/main.asp?_=Finger%20Lime). Unfortunately, this kind of information is limited because many Australian settlements of this rare citrus have been destroyed during land clearing by colonizing European farmers. The species has been also popularly used for woodturning, due to its hard, dense, and fine-grained wood [71].
5.4. Therapeutic Properties
5.4.1. Anti-inflammatory effect
Safe doses of fruit extracts have inhibited the lipopolysaccharide-induced release of nitric oxide in immortalized murine microglial BV-2 cells, and in addition have lowered the concentration of inflammatory factors, such as IL-1β, IL-6 and TNF-α, due to the modulation of JAK2/STAT3, NF-κB/IκB, and Toll-like Receptor (TLR) pathways [64]. These data show potentialities of the fruit for the treatment of inflammation and neuronal cell protection.
5.4.2. Antimicrobial and antiparasitic effects
Fruit pearls have been used for medicinal purposes and applied topically as an antiseptic. (https://www.specialtyproduce.com/produce/Finger_Limes_6704.php). In addition, aqueous, ethanol and peptide extracts of a plant mixture consisting of four indigenous Australian plants, including leaves of C. australasica, showed antibacterial and antioxidant activities [72].
5.4.3. Skin protection
An in vitro study carried out on a fruit extract has shown the presence of Alpha Hydroxy Acids (AHA) that improve skin exfoliation through activation of Transient Receptor Potential Vanilloid-3 (TRPV3), a transmembrane channel expressed by keratinocytes that permits the passage of cations such as Ca2+. The proposed mechanism involves AHA entering into keratinocytes causing intracellular acidification, TRPV3-mediated Ca2+ overload, and consequent skin desquamation. Clinical evaluation indicates that this extract shows an improvement of skin exfoliation and renewal without adverse effects (https://asia.in-cosmetics.com/__novadocuments/61672?v=635464898432770000).
6. CARAMBOLA
Averrhoa carambola L. (Oxalidaceae).
6.1. Features
The name of the genus Averrhoa derives from the ancient physician Ibn-Ruschd (1126–1198), known as Averroes. The plant is an evergreen tree, commonly 3–5 m tall and rarely up to 10 m, showing a rounded crown of drooping branches bearing pinnate leaves with a single terminal leaflet. Leaves are sensitive to light and touch, making them to fold up in the dark or when disturbed. Flowers are lilac, downy, bell shaped, and form loose panicles at the end of branches. Fruits are orange-yellow, smelling of oxalic acid, with a 5-angled, oblong shape, about 12.5 cm long and 9 cm wide (Figure 1G and 1H). The typical star shape of the fruit when cut gives the common name “star fruit” to the plant.
The species is native from Indonesia, India, and Sri Lanka. It is cultivated as a commercial crop in Malaysia, Taiwan, and in other tropical areas. The fruit can be eaten raw or used in the preparation of dessert dishes, juice, jelly, and jam. Different cultivars yield fruits differing in flavor, texture and taste, some being acidic, and others sweet. The plant is also grown as an ornamental shade tree [73,74].
6.2. Chemical Composition
A number of secondary metabolites with potential bioactive properties have been found in various portions of the plant. The plant is highly rich in oxalic acid that forms calcium oxalate crystals [75]. Carotenoids are abundant in aerial organs, mainly lutein, violaxanthin and β-carotene [76], while in the fruit an uncommon carotenoid pattern including phytofluene, ζ-carotene, β-cryptoflavin, and mutatoxanthin, has been reported [77]. Carotenoid-derived norisoprenoids are present in the fruit, including two new C13 and C15-norisoprenoids, (5R,6S,7E,9R)-5,6,9-trihydroxy-7-megastigmene 9-O-β-d-glucoside, and (6S,7E,10S)-Δ9,15-10-hydroxyabscisic alcohol, respectively, contributing to its flavor [78,79].
In the fruit, most abundant phenolics include gallic, ferulic, protocatechuic, syringic, and p-coumaric acids [80–82]. Other non-flavonoid phenolics include specific alkyl-phenol diglucosides called carambolasides, phenylpropanoids, benzoic acids, naphthoquinones, and simple phenols, [83]. Major flavonoids include quercetin, isoquercetin, procyanidin B2, and proanthocyanidin consisting mainly of epicatechin units [81,82,84,85]. Tetrahydroisoquinoline alkaloids have been also found [86], while oleic acid has been reported as the most abundant fat [85]. Butyl acetate, ethyl decanoate, and hexadecanoic acids have been reported as major volatile components of the fruit [87].
α-Linolenic acid is the most abundant fat in the leaves and oleic acid in the fruit [85]. Flavan-3-ols, 2-diglycosyloxybenzoates, apigenin-derivative C-glycosyl flavones, flavanols, and β-sitosterol have been reported in the leaves [88,89]. Lignans and phenolic glycosides have been isolated from the root [90].
6.3. Traditional Uses
Numerous traditional medicine uses have been reported for the fruit, root, leaves and flowers [91]. The Traditional Chinese Medicine considers the fruit useful for treating high blood pressure and diabetes, the leaves to treat rheumatism, and the flowers to relieve coughs [92]. In Nepal the seeds are used as emmenagogue, galactagogue, and abortifacient, while powder obtained by grinding the seeds is used to treat asthma, colic, and jaundice [93].
6.4. Therapeutic Properties
6.4.1. Antioxidant effect
Variations in bioactive compounds and antioxidant activity of the fruit have been investigated in relation to different ripening stages, showing that total phenolic and flavonoid contents, β-carotene, and γ- and δ-tocopherol are prominent in unripe fruit, while total carotenoid content, and α- and β-tocopherol are more abundant in the ripe fruit [94]. Variations in bioactive compounds and antioxidant activity have been also shown in fruits of different cultivars from Malaysia [95]. Moreover, the residue that is discarded during fruit juice production has been found to contain higher antioxidant activity than the juice, suggesting its possible use in functional food products [96].
Different studies have indicated that the strongest antioxidant compounds are procyanidin-type proanthocyanidins in both fruit and leaves [85,97]. Other fruit constituents that have been reported for their antioxidant activity are newly isolated dihydrochalcone C-glycosides and known flavonoid glycosides, for which a beneficial effect to human health has been suggested [98].
6.4.2. Antimicrobial effect
In a study concerning antimicrobial activities against different strains, including E. coli and Staphylococcus aureus, green fruits have shown better effects than ripe ones [99]. In addition, the antibacterial activity of the fruit juice and ethanolic fruit extract have been tested against gram-positive and gram-negative bacteria, viz. Bacillus cereus, Pseudomonas aeruginosa, Salmonella typhi, Shigella boydii, Salmonella typhi, S. aureus, and E. coli, showing that the extract is more effective than the juice, and that the most sensitive species are S. typhi and S. aureus [100].
In a study on the antimicrobial activity of the leaf, the aqueous extract has exhibited highest activity against S. typhi, while the methanol extract has exerted highest antifungal activity against Candida krusei [101].
6.4.3. Anti-inflammatory effect
Various fractions of an ethanolic leaf extract, viz. hexane, ethyl acetate, and butanol ones, have been tested on a mice model of inflammation consisting of croton oil-induced ear edema. Best results were obtained with the ethyl acetate fraction, inducing a reduction of edema and the inhibition of myeloperoxidase. In contrast, apigenin derivatives isolated from this fraction did not produce significant anti-inflammatory effects [102]. A clinical study conducted on elderly people has shown that regular consumption of the fruit juice for 4 weeks decreases the plasma levels of the inflammation markers TNF-alpha (TNF-α), nitric oxide, and interleukin 23, and concomitantly produces an increase of physical performance, measured as 6-min walking distance [103].
6.4.4. Cardiovascular protection
In a rat model, detrimental effects on heart caused by isoprenaline-induced ventricular remodeling with endothelial dysfunction have been alleviated by an aqueous extract of the fruit [104]. An aqueous extract of leaves has induced various depressive effects on guinea pig heart, including atrioventricular block and cardiac rate slowing [105]. The same extract, used on guinea pig left atrium and pituitary GH3 cells, has induced hypotensive effects by inhibiting the inotropic action of the L-type Ca2+ channel agonist BAY K 8644, and by blocking the L-type, inward calcium current in GH3 cells [106]. In agreement with these data, the leaf aqueous extract has induced hypotension on normotensive rats, and has inhibited aortic responses to phenylephrine and extracellular Ca2+, suggesting that the hypotensive effect is due at least in part to the inhibition of Ca2+ influx [107].
6.4.5. Antidiabetic effect
Various portions of the plant have long been used to treat diabetes in traditional medicine, while thereafter, this therapeutic property has been confirmed in experimental studies. In healthy rats, oral treatment with a leaf hydroalcoholic extract has induced a lowering of fasting glycemia [108]. Moreover, in a streptozotocin-induced diabetic mouse model, it has been shown that gavage with the fruit juice ameliorates hyperglycemia, hyperlipidemia, and nephropathy [109]. On the same model, ethanol and water extracts of roots have decreased the glucose and lipid serum levels, and in contrast have increased serum insulin. In addition, the treatments have down-regulated caspases and Bax protein involved in apoptosis and increased the anti-apoptotic Bcl-2 protein in pancreas tissue [110]. Besides the use of extracts obtained from plant tissues, an insoluble, fiber-rich fraction, isolated from fruit pomace and tested in vitro, has induced retardation of glucose diffusion, inhibition of α-amylase, and delayed glucose release from starch [111].
Some studies have been focused on specific active compounds isolated from the plant. The cyclohexanedione 2-Dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione (DMDD) isolated from the root has been proved to lessen the hyperglycemia-stimulated, epithelial–mesenchymal transition, an initiating step of diabetic kidney disease, in a proximal tubule epithelial cell line [112]. In a streptozotocin-induced, diabetic nephropathy mouse model, DMDD has mitigated kidney damage and inflammation by inhibiting NF-κB activation induced by the TLR4/Myeloid Differentiation factor (Myd88) signaling pathway [113]. DMDD has also contrasted the obesity and insulin resistance induced in mice by a high-fat diet, by decreasing body and adipose tissue weights, glucose and lipid serum levels, as well as TLR4 and Myd88 expression in adipose tissue, and increasing insulin sensitivity [114]. The flavone apigenin-6-C-β-fucopyranoside from the leaves has been found to exert hypoglycemic activity and to increase muscle and liver glycogen in rats [115].
In the poloxamer 407-induced hyperlipidemic rat model a methanolic leaf extract has reduced serum cholesterol, triglycerides, LDL, VLDL, and the atherogenic index [116]. It has been also reported that a peel extract suppresses differentiation of 3T3-L1 preadipocytes into adipocytes, possibly due to epicatechin downregulation of the CCCAAT/enhancer-binding protein alpha (C/EBP-α) and peroxisome proliferator-activated receptor-gamma (PPAR-γ) genes, and concomitant upregulation of the PPARα receptor gene [117].
6.4.6. Hepatoprotective effect
Acute liver injury induced in mice by carbon tetrachloride (CCl4) has been attenuated by pre-treatment with a root extract through a reduction of free radical production and lipid peroxidation, as shown by consistent variations of inflammation and oxidative stress markers [118]. Comparable results have been found using the same mouse model treated with an aqueous fruit extract [119]. Nonalcoholic hepatic steatosis has been improved in a leptin receptor-deficient mouse strain by treatment with a free phenolic extract that has downregulated the expression of mircoRNA-34a and mircoRNA-33, induced phosphorylation of AMP-activated protein kinase-alpha (AMPK-α), reduced sterol regulatory element-binding protein-1c (SREBP-1c) expression, and downregulated fatty acid synthase and stearoyl-CoA desaturase [120]. The fruit juice has shown inhibitory property on the CYP3A activity in human liver microsomes [121].
6.4.7. Neuroprotective effect
The compound DMDD has been tested on the Alzheimer’s disease model APP/PS1 mice subjected to Morris water and Y-type electric mazes. The compound has reversed spatial learning and memory deficit, fear memory deficit, and hippocampal neuron apoptosis. By using PC12 cells cultivated in vitro, it has been observed that DMDD is able to protect against noxious Aβ1-42 effects, including apoptosis, mitochondria membrane potential loss, upregulation of pro-apoptotic Bax, downregulation of anti-apoptotic Bcl-2, and rise in caspase-3 and -9 activities. Moreover, in both APP/PS1 mice and PC-12 cells the Bcl-2/Bax ratio was increased by DMDD [122].
6.4.8. Anticancer effect
Different plant products have shown either preventive or therapeutic effects on various cancer models. A fruit extract used in vivo as a preventive treatment has significantly reduced liver cancer incidence in mice exposed to diethylnitrosamine followed by CCl4 [123]. An ethanolic extract of the plant used in vitro has affected the cell viability of MCF-7 human breast cancer cells more than that of nonmalignant Chang Liver cells, although the cytotoxic effect has proven not particularly strong, with a reported IC50 of 145 μg/ml [124]. In vitro models have also been used to show that the compound DMDD is able to induce apoptosis in different cancer cell lines. In breast cancer cells, the compound has caused G1 phase arrest and promoted oxidative stress, and in addition it has affected the cell growth of cancer cells more than that of normal counterparts [125].
6.4.9. Skin protection
A study based on an in vitro model consisting of human keratinocytes exposed to ultraviolet B rays (UVB) light has reported protective effects of the plant ethanol and aqueous fractions, as revealed by reduced apoptosis rate and lowered caspase-3 activation and DNA damage [126]. As for in vivo studies, topical anti-inflammatory effects of the leaf ethanolic extract, and its hexane, ethylacetic, and butanol fractions, have been tested on a mouse skin inflammation model, consisting of croton oil-induced ear edema. All fractions have lowered edema formation and myeloperoxidase activity, while the ethylacetic fraction has been the most effective agent [102].
6.4.10. Nephrotoxic and neurotoxic effects
The fruit contains high oxalate that upon ingestion of large amounts, especially with an empty stomach, in rare cases produces oxalate nephropathy resulting in acute kidney injury [127,128]. Moreover, neurotoxicity and seizures have also been observed in subjects with chronic renal disease, due to the presence of caramboxin, a compound structurally similar to phenylalanine, which is an agonist of N-methyl-
7. DISCUSSION AND CONCLUSION
The plant species selected in this study have a consolidated tradition as food for the use of their fruits, but also in popular medicines for the use of fruits and other plant portions. Following these notions, the rising importance of these plants as agricultural crops and market products worldwide has stimulated scientific researches on their beneficial properties for the human organism. However, the level of knowledge about the therapeutic properties and the ability to prevent disease of the different species is unbalanced, A. carambola being the most investigated species, while C. australasica being at the opposite extreme (Table 1).
| Plant species | Product | Administration | Model | Biological effect | References |
|---|---|---|---|---|---|
| Hylocereus undatus | Fresh fruit | Dietary | Humans | Antidiabetic | [15] |
| Dried fruit | Dietary | Humans | Blood glucose lowering | [14] | |
| Fruit | Dietary | Diabetic rats | Antidiabetic | [18] | |
| Fruit juice | Dietary | High-fat fed mice | Antidiabetic | [19] | |
| H2O fruit extract | Dietary | Rabbit | Microcirculation | [21] | |
| EtOH peel extract | In vitro exposure | Human hepatic carcinoma cells | Antiproliferative | [15] | |
| MetOH fruit extract | In vitro exposure | AGS gastric, HeLa cervical, MCF-7 breast cancer cells | Antiproliferative | [22] | |
| H2O extracts | Wound dressing | Streptozotocin diabetic rats | Wound healing | [9] | |
| Taraxast-20-ene-3α-ol | Intraperitoneal | Rabbit | Microcirculation | [20] | |
| Annona cherimola | MetOH, EtOH, and DMF fruit extracts | In vitro exposure | Human lymphocytes | Antioxidant | [38] |
| Leaf essential oil | In vitro assay | Bacteria, fungi | Antimicrobial | [40] | |
| MetOH leaf extract | In vitro assay | HSV-2-infected MDBK and HEp-2 cells | Antiviral | [42] | |
| EtOH leaf extract/kaempferol | In vitro assay | E. histolytica, G. lamblia | Antiprotozoal | [43] | |
| EtOH leaf extract | Dietary | Streptozotocin diabetic rats | Blood glucose lowering | [29] | |
| EtOH leaf extract | Dietary | Normal rats | Hypoglycemic | [29] | |
| Fruit phenolamides | In vitro assay | α-Glucosidase | Enzyme inhibition | [44] | |
| Leaf decoction/rutin | In vitro assay | Caco-2 cell monolayers | Hypocholesterolemic | [45] | |
| Leaf aporphine alkaloids | Forced swimming test | Mice | Antidepressant | [46] | |
| Hexane leaf extract | Behavior tests | Mice | Anxiolytic | [47] | |
| Anonaine, glaucine, and xylopine alkaloids | In vitro assay | Acetylcholinesterase | Enzyme inhibition | [48] | |
| Ethanol seed macerate | In vitro exposure | Gastric adenocarcinoma cells | Antiproliferative | [49] | |
| EtOH seed extract | In vitro exposure | Leukemia cells | Antiproliferative | [50] | |
| Annomolin and annocherimolin acetogenins | In vitro exposure | PC-3, MCF-7, and HT-29 cancer cells | Antiproliferative | [33] | |
| Cherimolacyclopeptide E | In vitro assay | Bacteria, fungi, earthworms | Antimicrobial, anthelmintic | [41] | |
| Cherimolacyclopeptide E and F | In vitro exposure | KB nasopharyngeal carcinoma cells | Antiproliferative | [51] | |
| Cherimolacyclopeptide E | In vitro exposure | Dalton’s lymphoma ascites and Ehrlich’s ascites carcinoma | Antiproliferative | [41] | |
| Cherimolacyclopeptide E | In vitro exposure | P. aeruginosa, E. coli, C. albicans | Antimicrobial | [41] | |
| Procyanidin-type proanthocyanidins | In vitro assay | Tyrosinase | Enzyme inhibition | [52] | |
| Citrus australasica | Methanolic fruit extract | In vitro exposure | Murine microglial BV-2 cells | Antinflammatory | [64] |
| Averrhoa carambola | Green fruits | In vitro assay | E. coli, S. aureus | Antibacterial | [99] |
| EtOH fruit extract | In vitro assay | Gram-positive and gram-negative bacteria | Antibacterial | [100] | |
| Aqueous leaf extract | In vitro assay | S. typhi | Antibacterial | [101] | |
| MetOH leaf extract | In vitro assay | C. krusei | Antifungal | [101] | |
| ETOAc fraction of EtOH leaf extract | Topic application | Mice ear edema | Anti-inflammatory | [102] | |
| Fruit juice | Dietary | Humans | Anti-inflammatory | [103] | |
| Aqueous fruit extract | Intragastric administration | Rats | Cardioprotective | [104] | |
| Aqueous leaf extract | In vitro exposure | Isolated guinea pig heart | Cardiodepressive | [105] | |
| Aqueous leaf extract | In vitro exposure | Guinea pig left atrium | Hypotensive | [106] | |
| Aqueous leaf extract | Catheter vein administration | Rats | Hypotensive | [107] | |
| EtOH leaf extract | Oral treatment | Rats | Hypoglycemic | [108] | |
| Fruit juice | Gavage | Streptozotocin diabetic mouse | Antidiabetic | [109] | |
| EtOH and aqueous root extracts | Gavage | Streptozotocin diabetic mouse | Antidiabetic | [110] | |
| MetOH leaf extract | Oral treatment | Hyperlipidemic rat model | Anti-hyperlipidemic | [91] | |
| Peel extract | In vitro exposure | 3T3-L1 preadipocytes | Anti-adipogenesis | [117] | |
| Root extract | Intragastric gavage | CCl4 hepatic injury mice | Hepatoprotective | [118] | |
| Aqueous fruit extract | Intragastric gavage | CCl4 hepatic injury mice | Hepatoprotective | [119] | |
| Fruit extract | Oral treatment | Liver cancer mice | Anticarcinogenic | [123] | |
| EtOH extract | In vitro exposure | MCF-7 cells | Antiproliferative | [124] | |
| EtOH and aqueous plant fractions | In vitro exposure | UVB exposed keratinocytes | Skin protection | [126] | |
| DMDD | Oral gavage | Alzheimer’s APP/PS1 mice | Neuroprotective | [122] | |
| DMDD | In vitro exposure | Breast cancer cells | Antiproliferative | [125] | |
| DMDD | In vitro exposure | Proximal tubule epithelial cells | Nephroprotective | [112] | |
| DMDD | Oral treatment | Streptozotocin diabetic mice | Nephroprotective | [113] | |
| DMDD | Oral treatment | High-fat diet mice | Antidiabetic | [114] | |
| Apigenin-6-C-β-fucopyranoside | In vitro exposure | Rat soleus muscle | Glucose uptake stimulation | [115] |
Biological effects of the different species, their products, and compounds
The complex of the investigated topics reflects primarily the importance of these plants as food. Among the reported studies, those concerning problems most strictly related to the nutrition sphere, such as body redox balance, metabolic syndrome, and hepatoprotective effects, sum up to 51.25%. Studies devoted to anticancer and antimicrobial effects, are next in quantity, representing 12.5%, and 11.25% of total, respectively, anti-inflammatory and neuroprotective effects sum up to 8.75%, and cardiovascular and skin protection are 7.5% each.
Various pharmacological investigations have been focused on specific compounds isolated from plant tissues, assuming a potential role in drug discovery. One of these studies have been conducted on H. undatus, concerning vascular protective triterpenes from leaves [20]. Studies on A. cherimola have identified α-glucosidase inhibitor phenolamides from leaves [44], acetylcholinesterase inhibitor alkaloids from the fruit peel [48], anticancer acetogenins and cyclic peptides from seeds [33,41], and tyrosinase inhibitor proanthocyanidins from the fruit pericarp [52]. Studies on A. carambola have suggested versatile therapeutic uses of the compound DMDD from the root, including the treatment of diabetic kidney disease [113], metabolic syndrome [114], Alzheimer’s disease [122], and breast cancer cells [125], and in addition an antihyperglycemic effect of apigenin-6-C-β-fucopyranoside from the leaves [115]. Concern for possible adverse effects point to nephrotoxic oxalic acid and neurotoxic caramboxin of A. carambola fruit [128,130], but these problems are uncommon and linked to high intake and kidney comorbidity, while no warning is issued for regular consumption by healthy people.
In conclusion, these plant species are spreading across the markets, mostly due to their taste and food properties. However, studies are also indicating that food products, byproducts, and single compounds derived from these plants are able to induce physiological responses in the organism that could be exploited in the prevention of disease or even in the specific treatments of health problems.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
LC and BB contributed in study design. LC, AS, DT and BB contributed in literature search and manuscript writing. JX contributed in manuscript editing.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Laura Cornara AU - Jianbo Xiao AU - Antonella Smeriglio AU - Domenico Trombetta AU - Bruno Burlando PY - 2020 DA - 2020/04/14 TI - Emerging Exotic Fruits: New Functional Foods in the European Market JO - eFood SP - 126 EP - 139 VL - 1 IS - 2 SN - 2666-3066 UR - https://doi.org/10.2991/efood.k.200406.001 DO - 10.2991/efood.k.200406.001 ID - Cornara2020 ER -
