Advances in the Propolis Chemical Composition between 2013 and 2018: A Review
- DOI
- 10.2991/efood.k.191029.001How to use a DOI?
- Keywords
- Propolis; chemical composition; new compounds; first time; newly isolated
- Abstract
Propolis is a lipophilic sticky substance collected by bees that has been used by humans for centuries. Owing to its healing, antioxidant, and other medicinal properties, its chemical composition has been widely studied. Most pharmacological properties of propolis have been attributed to its phenols and terpenes, mainly flavonoids, phenolic acids, and their derivatives. More than 500 components of propolis were known from different parts of the world until 2012. In this article, 305 new constituents of propolis described between 2013 and 2018 are being reviewed, with 19 additional compounds that were discovered between 2011 and 2012, and were excluded from a similar previous review article. Altogether more than 850 compounds were isolated from propolis until 2018.
- Copyright
- © 2019 International Association of Dietetic Nutrition and Safety. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Propolis or sometimes called “bee glue” is a lipophilic, adhesive, gummy, and resinous substance collected by different species of bees, including honeybees (e.g. Apis mellifera L.) and stingless bees (e.g. Tetragonisca angustula Illiger). Bees use it to seal holes in their hives, smooth out the internal walls, and protect the entrance against intruders. It also acts as a natural antibiotic to prevent bacterial, viral, or fungal infections within the hive [1–4]. Bees collect the resin from the cracks in the bark and leaf buds of different trees, including different species of poplars, conifers (e.g. pines and cypress), birches, alders, willows, palms, chestnuts, and even trees like eucalyptus, acacia, Clusia spp., and Baccharis dracunculifolia DC. Bees add salivary enzymes to the collected resin, mix it with beeswax and use this partially digested material in their hives [5–9].
The name propolis derives from the Hellenistic ancient Greek meaning “suburb/bee glue” or “defense of the city”, depending on the interpretation [5,10]. The use of propolis dates back to at least 300 BC and has been used by Egyptians, Persians, Greeks, and Romans. It was used mainly as a topical cream for cuts, ulcers, wounds, and other dermatological problems, furthermore it was used for mummification by the Egyptians. However, in medieval times the use of propolis was not very popular. It remained mostly as an alternative herbal medicine, mainly in Eastern Europe, especially in Russia, where it later became known as the “Russian penicillin”. The use of propolis was rediscovered again in the Renaissance with the growing popularity of ancient teachings and medicine. The first scientific researches of propolis began in the 19th century with its distillation and were closely connected with the development of chemistry. The first major chemical research was conducted at the beginning of the 20th century with its fractionation. The first isolated constituents from propolis were vanillin, cinnamic acid, and cinnamyl alcohol. Even bigger breakthrough happened at the beginning of 1970s with the advances in chromatographic analytical methods, which enabled isolation of newer and newer components from different propolis samples [10]. By the beginning of 21st century, Marcucci [6] and Bankova et al. [11] registered more than 300 constituents in propolis and just between 2000 and 2012, at least 241 new compounds have been isolated from it. Subsequently, the number of constituents grew to over 500 by 2012 and is growing every year as new components are being discovered in propolis from different regions and plant origins [8]. Despite of the progress in pharmacology, the list of preparations and uses of propolis in today’s time is still enormous, mostly because of its antiseptic, bacteriostatic, antibacterial, antimycotic, antiviral, antiprotozoal, antioxidative, spasmolytic, choleric, astringent, anti-inflammatory, anesthetic, antitumor, immunostimulating, cytostatic, hepatoprotective, and other properties [7,12].
Propolis could be typified in several different ways. According to its “gatherers”, two main types of propolis are known, the first being “normal” propolis that are collected by honeybees and the second being so-called geopropolis that are collected by stingless bees, which also add soil to their propolis mixtures [5,13]. According to the plant sources, propolis has been classified into seven main types [14], including poplar propolis, which is the most widespread type of propolis (Europe, North America, non-tropical regions of Asia) [11], Baccharis or Brazil green propolis [15], Clusia or Brazil red propolis [16], eucalyptus propolis [17], Macaranga or Taiwanese green propolis [18], birch propolis [19], and Mediterranean propolis [20]. Meanwhile Graikou et al. [9] also classified propolis into seven types, but a bit differently: poplar type (Europe, non-tropical parts of Asia, New Zealand, and North America), birch type (Russia), green type (Brazil), red type (Brazil, Cuba, and Mexico), Clusia type (Cuba and Venezuela), Pacific or Macaranga type (Okinawa prefecture in Japan, Taiwan, and Indonesia), and Mediterranean type (Greece, Sicily, and Malta). Nonetheless, classification of propolis differs among authors as more and more types of propolis of different plant origins are being discovered. For example, Park et al. [21] already described about 12 types of Brazilian propolis; however, a few years later, a new, red Brazilian propolis type was added. From all propolis types, poplar and Brazil green propolis are the most commercially available and widely studied because of their strong pharmacological activities [14].
The composition of propolis is highly dependent on its main plant source and season, as well as of the bee species; however, in general it is composed of 50% plant balsam and resin, 30% bees wax, 10% essential and aromatic oils, 5% pollen, and 5% other organic and inorganic molecules. This is especially true for poplar type propolis [7,10,22,23]. The color of propolis depends on its age and primary plant source and varies from yellow, green to red, and dark brown; there have even been reports about transparent propolis [3]. Its chemical composition is extremely diverse. Until 2012, more than 500 constituents have been recorded in propolis from different plant sources and countries [8] and until 2018 this number grew by at least 305. However, each propolis sample contains approximately 80–100 different constituents [10]. Among those are phenolic acids and esters, many types of flavonoids and other phenolic molecules, terpenes, ketones, aromatic aldehydes and alcohols, proteins, fatty acids, waxy acids, amino acids, steroids, stilbenes, sugars, vitamins (B1, B2, B3, B5, B6, C, and E), minerals (at least 35, some only found in traces), and even enzymes (e.g. β-glycosidase) [5,6,8,24–27]. Some groups of compounds, e.g. glycosides, were discovered quite late [28] and some, e.g. alkaloids and tannins, were found only recently [29–31].
Each year, more and more constituents are reported from propolis for the first time, some of them being completely new to science. In this paper, 305 constituents found in propolis for the first time between 2013 and 2018 are being reviewed, with the addition of 19 compounds found between 2011 and 2012 that were not included in Huang et al. [8], a similar review article. The constituents originating from propolis are from different regions and countries around the world, some countries being represented more than once. New constituents were isolated from propolis originating from North America (Mexico and United States), Oceania (Australia 3× and Pitcairn Island), Middle East (Saudi Arabia 2× and Oman), Asia (Thailand 2×, Malaysia 2×, China, Fiji, and Korea), Europe (Portugal, Bulgaria, France, Italy, United Kingdom, and Serbia), South and Middle America (Brazil 6×, Ecuador, Honduras, Bolivia, Chile, and Argentina), and Africa (Cameroon 5×, Algeria 3×, Nigeria 3×, Ghana, Congo, and Egypt). Components isolated from propolis for the first time between 2013 and 2018 (also those missing in Huang et al. [8]) were scouted and summarized from databases including BioMed Central, PubMed, and others, or were found via Google Scholar search engine.
2. PHENOLS AND FLAVONOIDS
Phenols, or sometimes referred to as polyphenols, are one of the most numerous and widely distributed groups of substances in the plant Kingdom. They are products of the secondary metabolism of plants. They can range from simple molecules, such as phenolic acids, to highly polymerized compounds, such as tannins. Their most characteristic feature is their aromatic ring and the alcohol (−OH) group associated with it. Phenols are further divided into at least 18 classes: simple phenols, benzoquinones, phenolic acids, acetophenones, phenylacetic acids, hydroxycinnamic acids, phenylpropenes, coumarins and isocoumarins, chromones, naftoquinones, xanthones, stilbenes, anthraquinones, flavonoids, lignans, neolignans, lignins, and condensed tannins. More than 8000 phenolic structures are known, most of them belong to the subclass of flavonoids (5000) [32,33].
Phenols are also the most abundant constituents in propolis, especially in those of poplar origin. On average, they represent around 28 ± 9% of whole mass of poplar type propolis, of which 8 ± 4% are flavones/flavonols and 6 ± 2% are flavanones/dihydroflavonols. The isolated phenols belong to many different classes of compounds, such as flavonoids, lignans, stilbenes, phenylpropanoids (including different acids), and others, among which flavonoids are the most important molecules in propolis [8,11,34]. Ghisalberti [5] mentioned more than 20 isolated phenols in propolis until the year 1979 and until 1987, at least 59 different phenol constituents have been found in propolis samples [24]). The number rapidly grew and Marcucci [6] reported 100 phenol constituents isolated from propolis until the year 1995. From 1995 to 2000, Bankova et al. [11] reported 40 new phenols and from 2000 to 2012, astounding 184 new phenols have been isolated [8]. Additional six were found in 2011 [35] and thirteen in 2012 [36,37], which were previously not included in Huang et al. [8]. Altogether, at least 330 phenols have been isolated in propolis until the year 2012 and despite those numbers, just between 2013 and 2018, 92 flavonoids (including their glycosides) and altogether 218 new phenols were isolated from propolis, which brings the final number of isolated phenols from propolis to at least 548 until 2018.
From all the constituents, phenols (such as flavonoids, lignans, caffeoylquinic acid derivatives, and hydroxycinnamic acid derivatives) and terpenes are also thought to be the main active molecules of propolis from temperate climates, whereas for the tropical regions and also some Mediterranean regions, the predominant active constituents of propolis are phenols, different from those found in poplar propolis (prenylated ρ-coumaric and cinnamic acids, lignans, stilbenes), and diterpenic acids [1,3,8,9,11,34]. Owing to their abundance and activity, phenols are regarded as the most important constituents of propolis [38,39].
As mentioned, among phenols, flavonoids are the most important propolis constituents, acting as the main biologically active ingredients. They are also used in determining the quality of propolis samples [39]. Walker and Crane [24] reported at least 40 known flavonoids from propolis, whereas Marcucci [6] reported at least 44 in 1995. Bankova et al. [11] mentioned seven newly isolated flavonoids in propolis between 1995 and 2000, but just between 2000 and 2012, an astounding 113 new flavonoids were isolated [8]. Despite high numbers of already isolated flavonoids, 92 (including their glycosides) were discovered in propolis for the first time between 2013 and 2018. According to their chemical structure, isolated flavonoids are classified into 11 subclasses: flavans, isoflavans, flavanones, flavanonols, flavones, isoflavones, isodihydroflavones, flavonols, chalcones, dihydrochalcones, and neoflavonoids (Figure 1). Besides flavonoids, their glycosides are also being discovered in propolis, although until 2012 they were considered very rare. Only two flavonoid glycosides were isolated from propolis until 2009 and until 2012 their number grew only by one [8,40]. Yet in the past 6 years, 57 flavonoid glycosides were isolated from propolis for the first time, making flavonoid glycosides an important group of compounds in propolis samples. In 2004, some speculations were made that propolis samples could also contain anthocyanidins, although they have yet to be reported in propolis [41].
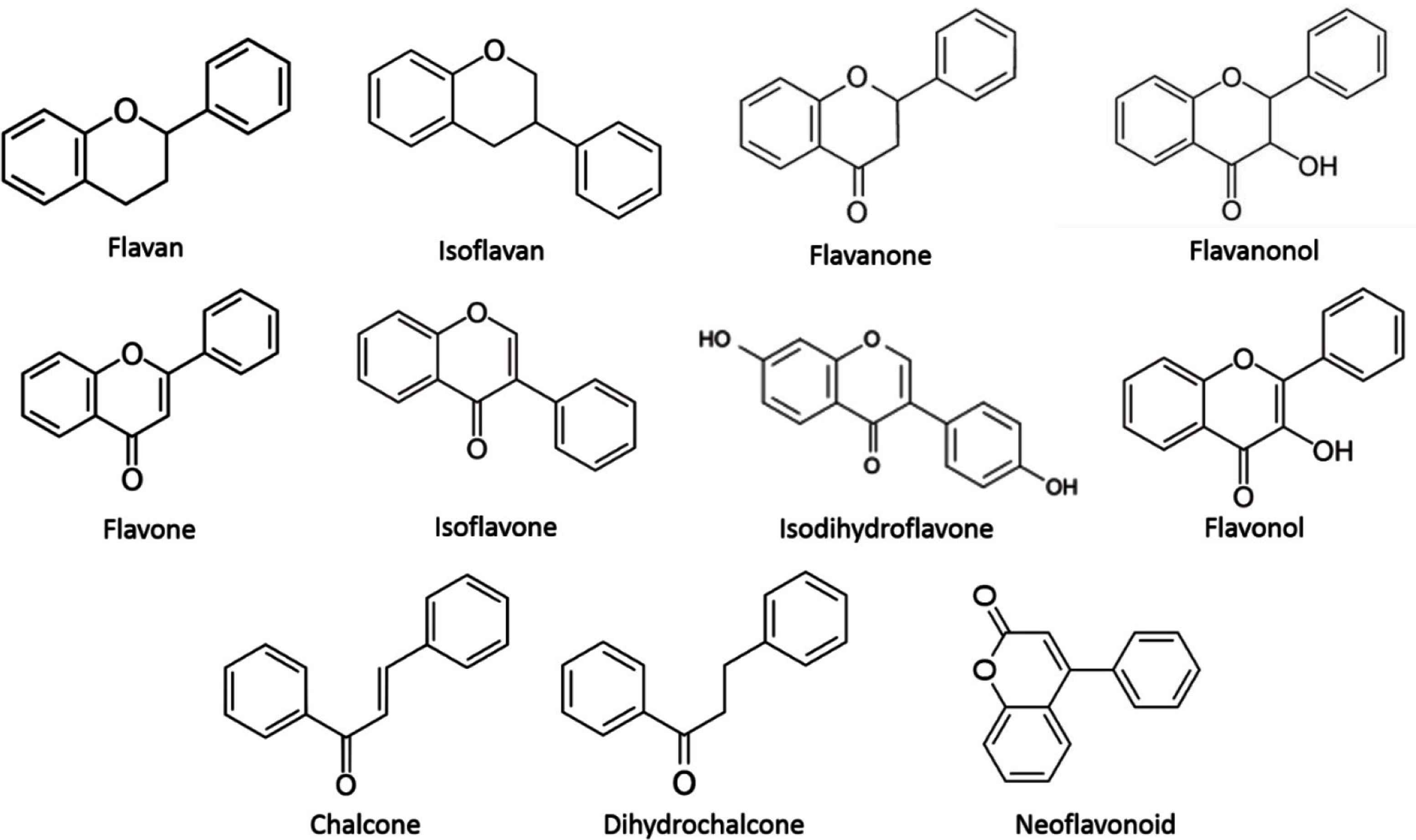
Flavonoid groups/classes isolated from different propolis samples around the world.
Among the 92 newly isolated flavonoids, their glycosides (57) are one of the biggest discoveries in the recent years because of their earlier rarity. They were isolated from European (Serbia, United Kingdom, and Portugal) and South American (Brazil) samples. Some other new flavonoid compounds were also isolated from Middle East (Oman and Saudi Arabia), Europe (France and Serbia), Asia (Thailand, Korea, and Fiji), Middle and South America (Ecuador, Argentina, and Brazil), and Africa (Algeria, Congo, Cameroon, and Nigeria). The newly isolated flavonoids are listed in Table 1.
| No. | Chemical name | Geographic location | References |
|---|---|---|---|
| Flavans | |||
| 1 | Fisetinidola | Oman | Popova et al. [42] |
| 2 | 2,3-trans-3,4-trans Mollisacacidina | Oman | Popova et al. [42] |
| 3 | 2,3-trans-3,4-cis Mollisacacidina | Oman | Popova et al. [42] |
| 4 | 3,4-Dihydro-2-(3,4-dihydroxyphenyl)-2H-chromene-3,7-diola,1 | Saudi Arabia | Almutairi et al. [43] |
| 5 | 8-[E-phenylprop-2-en-1-on]-5-methoxy-(±)-catechina,1 | France | Boisard et al. [44] |
| Flavanones | |||
| 6 | 8-[1-(4′-Hydroxy-3′-methoxyphenyl)prop-2-en-1-yl]-(2S)-pinocembrina,1 | Thailand | Athikomkulchai et al. [45] |
| 7 | 5,4′-Dihydroxy-7,3′-dimethoxyflavanonea | Ecuador | Cuesta-Rubio et al. [46] |
| 8 | Mepuberinb | Brazil | Cisilotto et al. [47] |
| Flavanonols | |||
| 9 | Pinobanksin 3-(E)-caffeatea | Algeria | Piccinelli et al. [48] |
| 10 | 3,5,4′-Trihydroxy-7,3′-dimethoxy flavanonola | Ecuador | Cuesta-Rubio et al. [46] |
| Flavones | |||
| 11 | Psiadiarabina | Saudi Arabia | Almutairi et al. [43] |
| 12 | Tangeritina | Serbia | Ristivojević et al. [49] |
| 13 | 5,7-Dihydroxy-6,4′-dimethoxyflavone (pectolinarigenin)a | Algeria | Segueni et al. [50] |
| 14 | 6,7-Dihydroxy-7,4′-dimethoxyflavone (ladanein)a | Algeria | Segueni et al. [50] |
| 15 | 5,7-Dihydroxy-2-(3,4-dihydroxyphenoxy)-4H-chromen-4-one (2-phenoxychromone)a,1 | Brazil | Mitsui et al. [51] |
| Flavonoles | |||
| 16 | Pinobanksin-O-hexenoatea | Portugal | Falcão et al. [52] |
| 17 | 3,5,3′,4′-Tetrahydroxy-6,7-dimethoxy flavone (eupatolitin)a | Ecuador | Cuesta-Rubio et al. [46] |
| Chalcones | |||
| 18 | (E)-4′-metoxy-4,2′-dihidroxy-3′-(2″,3″-dihydroxy-3″-methylbutyl)-chalcone (Jejuchalcone A)a,1 | Korea | Shimomura et al. [53] |
| 19 | (E,E,E)-4,2′,4′-trihydroxy-3′-(7″-hydroxy-3″,7″-dimethyloct-2″,5″-dienyl)-chalcone (Jejuchalcone B)a,1 | Korea | Shimomura et al. [53] |
| 20 | (E,E)-4,2′,4′-trihydroxy-3′-(5″-hydroxy-3″,7″-dimethyloct-2″,6″-dienyl)-chalcone (Jejuchalcone C)a,1 | Korea | Shimomura et al. [53] |
| 21 | (E)-4′-metoxy-4,3″,4″-trihydroxy-2″,2″-dimethyldihydropyrano-(2′,3′)-chalcone (Jejuchalcone D)a,1 | Korea | Shimomura et al. [53] |
| 22 | (E)-4′-metoxy-4,3″-dihidroxy-2″-(1′″-hydroxyisopropyl)-dihydrofurano-(2′,3′)-chalcone (Jejuchalcone E)a,1 | Korea | Shimomura et al. [53] |
| 23 | (E)-4,4′-dihydroxy-2″-(1′″-hidroxy-1′″,5′″-dimethylhex-4′″–enyl)-dihydrofurano-(2′,3′)-chalcone ((−)-Jejuchalcone F)a,1 | Korea | Shimomura et al. [53] |
| 24 | (E)-4,2′-dihydroxy-2″-methyl-2″-(3′″,4′″-dihydroxy-4′″-methylpentanyl)-2H-pyrano-(3′,4′)-chalcone ((+)-Jejuchalcone G)a,1 | Korea | Shimomura et al. [53] |
| 25 | (−)-(E)-4,2′-dihydroxy-2″-methyl-2″-(3′″,4′″-dihydroxy-4′″-methylpentanyl)-2H-pyrano-(3′,4′)-chalcone ((−)-Jejuchalcone H)a,1 | Korea | Shimomura et al. [53] |
| Flavonoid glycosides | |||
| 26 | Quercetin-3-O-glucuronidea | Portugal | Falcão et al. [52] |
| 27 | Quercetin-3-O-glucosidea | Portugal | Falcão et al. [52] |
| 28 | Kaempferol-3-O-rutinosidea | Portugal | Falcão et al. [52] |
| 29 | Isorhamnetin-O-pentosidea | Portugal | Falcão et al. [52] |
| 30 | Quercetin-3-O-rhamnosidea | Portugal | Falcão et al. [52] |
| 31 | Isorhamnetin-O-glucuronidea | Portugal | Falcão et al. [52] |
| 32 | Kaempferol-methyl ether-O-glucosidea | Portugal | Falcão et al. [52] |
| 33 | Isorhamnetin-O-acetylrutinosidea | Portugal | Falcão et al. [52] |
| 34 | Rhamnetin-O-glucuronidea | Portugal | Falcão et al. [52] |
| 35 | Quercetin-dimethyl ether-O-rutinosidea | Portugal | Falcão et al. [52] |
| 36 | Quercetin-dimethyl ether-O-glucuronidea | Portugal | Falcão et al. [52] |
| 37 | Kaempferol-O-ρ-coumaroylrhamnosidea | Portugal | Falcão et al. [52] |
| 38 | Caffeic acid 4-O-glucosidea | Brazil | Righi et al. [54] |
| 39 | Caffeic acid 4-O-arabinosidea | Brazil | Righi et al. [54] |
| 40 | Caffeic acid 4-O-xylosidea | Brazil | Righi et al. [54] |
| 41 | Dimethoxy-luteolin-glucosidea | Brazil | Righi et al. [54] |
| 42 | Methylkaempferol-O-rutinosidea | Brazil | Righi et al. [54] |
| 43 | Naringenin-C-glucosidea | Brazil | Righi et al. [54] |
| 44 | Apigenin-O-rutinosidea | Brazil | Righi et al. [54] |
| 45 | Delphinidin arabinosidea | Brazil | Righi et al. [54] |
| 46 | Catechin arabinosidea | Brazil | Righi et al. [54] |
| 47 | Apigenin-di-C-glucosyl rhamnosidea | Brazil | Righi et al. [54] |
| 48 | Apigenin-C-rhamnoside (isomer 1)*,a | Brazil | Righi et al. [54] |
| 49 | Apigenin-6,8-di-C-glucoside (vicenin-2)a | Brazil | Righi et al. [54] |
| 50 | Apigenin-C-rhamnosyl arabinosidea | Brazil | Righi et al. [54] |
| 51 | Apigenin-6-C-glucosyl-8-C-arabinose (isoschaftoside)a | Brazil | Righi et al. [54] |
| 52 | Luteolin-O-glucuronidea | Brazil | Righi et al. [54] |
| 53 | Apigenin-8-C-glucosyl-6-C-arabinose (schaftoside)a | Brazil | Righi et al. [54] |
| 54 | Luteolin-6,8-di-C-glucoside (lucenin-2)a | Brazil | Righi et al. [54] |
| 55 | Apigenin-C-rhamnoside (isomer 2)a,* | Brazil | Righi et al. [54] |
| 56 | Luteolin acetylglucosidea | Brazil | Righi et al. [54] |
| 57 | Chrysoeriol-C-glucosidea | Brazil | Righi et al. [54] |
| 58 | Dimethoxy naringenin-diglucosidea | Brazil | Righi et al. [54] |
| 59 | Apigenin-di-O-glucosidea | Brazil | Righi et al. [54] |
| 60 | Quercetin-O-arabinosidea | Brazil | Righi et al. [54] |
| 61 | Isorhamnetin-glucosidea | Brazil | Righi et al. [54] |
| 62 | Apigenin-O-glucuronidea | Brazil | Righi et al. [54] |
| 63 | Naringenin-4′-O-β-glucopyranosideb | Brazil | Da Silva et al. [55] |
| 64 | Myricetin-3-O-β-glucopyranosideb | Brazil | Da Silva et al. [55] |
| 65 | Chrysin glycoside formate adducta,* | United Kingdom | Saleh et al. [56] |
| 66 | Galangin glycosidea,* | United Kingdom | Saleh et al. [56] |
| 67 | 7-Methoxy-5-hydroxy-8-C-flavone rhamnosideb | Brazil | Coelho et al. [57] |
| 68 | Acacetin-di-C-acetyl dirhamnosideb | Brazil | Coelho et al. [57] |
| 69 | Apigenin-6,8-di-C-malonyl glucoside dihexoside (isomer 1)b,* | Brazil | Coelho et al. [57] |
| 70 | Apigenin-6,8-di-C-malonyl glucoside dihexoside (isomer 2)b,* | Brazil | Coelho et al. [57] |
| 71 | Apigenin-di-C-malonyl trihexoside (isomer 1)b,* | Brazil | Coelho et al. [57] |
| 72 | Acacetin-di-C-malonyl trihexosideb,* | Brazil | Coelho et al. [57] |
| 73 | Apigenin-di-C-malonyl trihexoside (isomer 2)b,* | Brazil | Coelho et al. [57] |
| 74 | Acacetin-8-C-arabinoside-7-O-rhamnosideb | Brazil | Coelho et al. [57] |
| 75 | Apigenin-di-C-malonyl trihexoside (isomer 3)b,* | Brazil | Coelho et al. [57] |
| 76 | Catechin rhamnosideb | Brazil | Coelho et al. [57] |
| 77 | Chrysin-8-C-rhamnoside-7-O-rhamnosideb | Brazil | Coelho et al. [57] |
| 78 | Luteolin-8-C-caffeoyl rhamnosideb | Brazil | Coelho et al. [57] |
| 79 | Caffeoylquinic acid-O-arabinosideb | Brazil | Coelho et al. [57] |
| 80 | Apigenin-7-O-glucoside (apigetrin)a | Serbia | Ristivojević et al. [49] |
| 81 | Apigenin 8-C-xyloside-6-C-glucoside (vicenin 3)b | Brazil | Cisilotto et al. [47] |
| 82 | Apigenin 6-C-xyloside-8-C-glucoside (vicenin 1)b | Brazil | Cisilotto et al. [47] |
| Prenylated flavonoids | |||
| 83 | 7-O-methyl-8-prenylnaringenina | Oman | Popova et al. [42] |
| 84 | 3′,8-Diprenylnaringenina | Oman | Popova et al. [42] |
| 85 | 8-Prenyl-5,7-dihydroxy-3′-(3-hydroxy-3-methylbuthyl)-4′-methoxyflavanonea | Oman | Popova et al. [42] |
| 86 | Lonchocarpol Aa | Congo and Cameroon | Papachroni et al. [58] |
| 87 | 6,8-Diprenyl-eriodictyola | Congo | Papachroni et al. [58] |
| 88 | 6,8-Diprenyl-aromadendrina | Cameroon | Papachroni et al. [58] |
| 89 | Lespedezaflavanonea | Cameroon | Papachroni et al. [58] |
| 90 | Glyasperin Aa | Fiji | Trusheva et al. [59] |
| 91 | 8-Prenylnaringenina | Nigeria | Omar et al. [60] |
| 92 | 6-Prenylnaringenina | Nigeria | Omar et al. [60] |
The molecular structure of the compound is not completely defined.
Constituent isolated from the honeybee propolis (from the genus Apis sp.).
Constituent isolated from the stingless bee propolis (from genera Scaptotrigona sp. or Melipona sp.).
Newly discovered compound. Compounds already mentioned in Huang et al. [8] are excluded.
Flavonoids identified in propolis for the first time since 2011
Among other 126 isolated “non-flavonoid” phenols, compounds from stilbenes and phenolic acids groups were the most abundant. Five of the isolated phenols were found in 2011 [35], whereas one phenolic acid ester [36] and twelve phenylpropanoids [37] were isolated in 2012. All of them are included in this review as they were excluded from the previous review article [8]. Otherwise, phenols were isolated from propolis from Europe (Italy, Portugal, Serbia, and United Kingdom), South and Middle America (Chile, Honduras, Brazil, and Argentina), Africa (Egypt, Nigeria, Ghana, Algeria, and Cameroon), Asia (Thailand, Fiji, China, and Malaysia), Middle East (Saudi Arabia), Oceania (Australia), and North America (United States and Mexico). The phenols are listed in Table 2.
| No. | Chemical name | Geographic location | References |
|---|---|---|---|
| Phenolic glycerides | |||
| 93 | Caffeoyl glycerola | Serbia | Ristivojević et al. [49] |
| 94 | Tricoumaroyl glycerola | Serbia | Ristivojević et al. [49] |
| 95 | Coumaroyl feruloyl glycerol (isomer 1)a,* | Serbia | Ristivojević et al. [49] |
| 96 | Coumaroyl feruloyl glycerol (isomer 2)a,* | Serbia | Ristivojević et al. [49] |
| 97 | Dicaffeoyl coumaroyl glycerola | Serbia | Ristivojević et al. [49] |
| 98 | Dicaffeoyl feruloyl glycerola | Serbia | Ristivojević et al. [49] |
| Phenolic acid esters | |||
| 99 | (E)-cinnamyl-(Z)-cinnamatea,1 | Honduras | Lotti et al. [36] |
| 100 | Decyl caffeatea | Egypt | El-Hady et al. [61] |
| 101 | Caffeic acid phenacetyl estera,* | United Kingdom | Saleh et al. [56] |
| 102 | Caffeic acid sesquiterpene ester (isomer 1)a,* | United Kingdom | Saleh et al. [56] |
| 103 | Caffeic acid sesquiterpene estera,* | United Kingdom | Saleh et al. [56] |
| 104 | Methylgalangin hydroxypropionyl estera,* | United Kingdom | Saleh et al. [56] |
| 105 | Caffeic acid monoterpene(geranyl) estera,* | United Kingdom | Saleh et al. [56] |
| 106 | Methyl methylene dioxy kaempferol hexanoyl ester (isomer 1)a,* | United Kingdom | Saleh et al. [56] |
| 107 | Methyl methylene dioxy kaempferol hexanoyl ester (isomer 2)a,* | United Kingdom | Saleh et al. [56] |
| 108 | Caffeic acid sesquiterpene ester (isomer 2)a,* | United Kingdom | Saleh et al. [56] |
| Xanthones | |||
| 109 | α-Mangostinb | Thailand | Sanpa et al. [4] |
| 110 | γ-Mangostinb | Thailand | Sanpa et al. [4] |
| 111 | Mangostaninb | Thailand | Sanpa et al. [4] |
| 112 | 8-Deoxygartaninb | Thailand | Sanpa et al. [4] |
| 113 | Gartaninb | Thailand | Sanpa et al. [4] |
| 114 | Garcinone Bb | Thailand | Sanpa et al. [4] |
| 115 | Furofuran lignan methylpinoresinolb | Thailand | Sanpa et al. [4] |
| Phenylpropanoids | |||
| 116 | 2-Acetyl-1-feruloyl-3-caffeoylglycerola,1 | China | Shi et al. [37] |
| 117 | (+)-2-Acetyl-1-caffeoyl-3-cinnamoylglycerola,1 | China | Shi et al. [37] |
| 118 | (−)-2-Acetyl-1-caffeoyl-3-cinnamoylglycerola,1 | China | Shi et al. [37] |
| 119 | (+)-2-Acetyl-1-(E)-coumaroyl-3-(E)-cinnamoylglycerola,1 | China | Shi et al. [37] |
| 120 | (+)-2-Acetyl-1-(E)-feruloyl-3-(E)-cinnamoylglycerola,1 | China | Shi et al. [37] |
| 121 | (−)-2-Acetyl-1-(E)-feruloyl-3-(E)-cinnamoylglycerola,1 | China | Shi et al. [37] |
| 122 | 2-Acetyl-1,3-dicinnamoylglycerola,1 | China | Shi et al. [37] |
| 123 | (−)-2-Acetyl-1-(E)-cinnamoyl-3-(3″(ζ),16″)-dihydroxy-palmitoylglycerola,1 | China | Shi et al. [37] |
| 124 | 2-Acetyl-1,3-dicaffeoylglycerola | China | Shi et al. [37] |
| 125 | 2-Acetyl-1-caffeoyl-3-coumaroylglycerola | China | Shi et al. [37] |
| 126 | 2-Acetyl-1-feruloyl-3-coumaroylglycerola | China | Shi et al. [37] |
| 127 | 2-Acetyl-1,3,-diferuloylglycerola | China | Shi et al. [37] |
| 128 | 6-O-p-coumaroyl-D-galactopyranoseb,1 | Brazil | De Souza et al. [13] |
| 129 | 6-O-cinnamoyl-1-O-p-coumaroyl-β-D-glucopyranoseb | Brazil | De Souza et al. [13] |
| 130 | Dicoumaroyl glyceroa,* | United Kingdom | Saleh et al. [56] |
| 131 | Acetylcoumaroyl glycerola,* | United Kingdom | Saleh et al. [56] |
| 132 | Boropinic acida | Italy | Taddeo et al. [62] |
| 133 | 4′-Geranyloxyferulic acida | Italy | Taddeo et al. [62] |
| 134 | 7-Isopentenyloxycoumarina | Italy | Taddeo et al. [62] |
| 135 | Auraptenea | Italy | Taddeo et al. [62] |
| Phenylpropanoid glycosides | |||
| 136 | Scopolina | Algeria | Soltani et al. [31] |
| Phenolic glycosides | |||
| 137 | Torachrysone-O-hexoseb,* | Malaysia | Zhao et al. [63] |
| 138 | Torachrysone-O-(acetyl)-hexoseb,* | Malaysia | Zhao et al. [63] |
| 139 | Torachrysone-O-(galloyl)-hexoseb,* | Malaysia | Zhao et al. [63] |
| 140 | Gallic acid-hexoseb,* | Malaysia | Zhao et al. [63] |
| Stilbenes | |||
| 141 | (E)-4-(3-methyl-2-buten-1-yl)-3,4′,5-trihydroxy-3′-methoxystilbenea,1 | Australia | Duke et al. [64] |
| 142 | (E)-2-(3-methyl-2-buten-1-yl)-3,4′,5-trihydroxystilbene (2-prenylresveratrol)a | Australia | Duke et al. [64] |
| 143 | (E)-2,4-bis(3-methyl-2-buten-1-yl)-3,3′,4′,5-tetrahydroxystilbenea,1 | Australia | Duke et al. [64] |
| 144 | (E)-2-(3-methyl-2-buten-1-yl)-3-(3-methyl-2-butenyloxy)-3′,4′,5-trihydroxystilbenea,1 | Australia | Duke et al. [64] |
| 145 | (E)-2,6-bis(3-methyl-2-buten-1-yl)-3,3′,5,5′-tetrahydroxystilbenea,1 | Australia | Duke et al. [64] |
| 146 | (E)-2,6-bis-(3-methyl-2-buten-1-yl)-3,4′,5-trihydroxy-3′-methoxystilbenea,1 | Australia | Duke et al. [64] |
| 147 | (E)-5-(2-(8-hydroxy-2-methyl-2-(4-methylpent-3-en-1-yl)-2H-chromen-6-yl) vinyl)-2-(3-methylbut-2-en-1-yl)benzene-1,3-diola,1 | Ghana | Almutairi et al. [65] |
| 148 | 5-((E)-3,5-dihydroxystyryl)-3-((E)-3,7-dimethylocta-2,6-dien-1-yl)benzene-1,2-diola,1 | Ghana | Almutairi et al. [65] |
| 149 | Schweinfurthin Ca | Nigeria | Zhang et al. [66] |
| 150 | Mappaina | Nigeria | Zhang et al. [66] |
| 151 | Geranyl stilbenoida,* | Nigeria | Zhang et al. [66] |
| 152 | Solomonin Ba,1 | Fiji | Trusheva et al. [59] |
| 153 | Solomonin Ca,1 | Fiji | Trusheva et al. [59] |
| Lignans | |||
| 154 | Meso-(rel 7S,8S,7′R,8′R)-3,4,3′,4′-tetrahydroxy-7,7′-epoxylignana | Argentina | Agüero et al. [35] |
| 155 | (7S,8S,7′S,8′S)-3,3′,4′-trihydroxy-4-methoxy-7,7′-epoxylignana | Argentina | Agüero et al. [35] |
| Phenolic acids | |||
| 156 | Caffeic acid derivative 1a,* | Portugal | Falcão et al. [52] |
| 157 | Caffeic acid derivative 1 (isomer)a,* | Portugal | Falcão et al. [52] |
| 158 | Caffeic acid derivative 2a,* | Portugal | Falcão et al. [52] |
| 159 | Ferulic acid derivativea,* | Portugal | Falcão et al. [52] |
| 160 | Sandaracopimaric acida | Saudi Arabia | Jerz et al. [67] |
| 161 | (E)-3-hydroxy-1,7-diphenylhept-1-ene-5-acetatea | Chile | Nina et al. [68] |
| 162 | (E)-5-hydroxy-1,7-diphenylhept-1-ene-3-acetatea | Chile | Nina et al. [68] |
| 163 | Caffeic acid hextrieneoatea,* | United Kingdom | Saleh et al. [56] |
| 164 | Benzoyl dihydroxyphenylpropionic acida,* | United Kingdom | Saleh et al. [56] |
| 165 | Benzoyl hydroxyphenylacetic acida,* | United Kingdom | Saleh et al. [56] |
| 166 | Hydroxy phenyl acetyl dihydroxyphenylacetic acida,* | United Kingdom | Saleh et al. [56] |
| 167 | Pinobanksin phenyl propionate (isomer 1)a,* | United Kingdom | Saleh et al. [56] |
| 168 | Dimethyl pinocembrin benzoatea,* | United Kingdom | Saleh et al. [56] |
| 169 | Pentenoyl hydroxyphenylpropionic acida,* | United Kingdom | Saleh et al. [56] |
| 170 | Pinobanksin phenyl propionate (isomer 2)a,* | United Kingdom | Saleh et al. [56] |
| 171 | Pinobanksin benzoatea,* | United Kingdom | Saleh et al. [56] |
| 172 | Pinobanksin phenyl propionate (isomer 3)a,* | United Kingdom | Saleh et al. [56] |
| 173 | Methyl pinobanksin acetatea,* | United Kingdom | Saleh et al. [56] |
| 174 | Pinobanksin caffeatea,* | United Kingdom | Saleh et al. [56] |
| 175 | Caffeoyldimethyl pinocembrina,* | United Kingdom | Saleh et al. [56] |
| 176 | Methyl chrysin acetate derivativea,* | United Kingdom | Saleh et al. [56] |
| 177 | Pinobanksin dimethyl cinnamatea,* | United Kingdom | Saleh et al. [56] |
| 178 | (4R,5R,9R,10R)-13-hydroxypodocarp-8(14)-en-19-oic acidb | Brazil | Cisilotto et al. [47] |
| Other phenols | |||
| 179 | Nordihydroguaiaretic acida | Argentina | Agüero et al. [35] |
| 180 | 3′-Methyl-nordihydroguaiaretic acida | Argentina | Agüero et al. [35] |
| 181 | 4′-Methyl-nordihydroguaiaretic acida | Argentina | Agüero et al. [35] |
| 182 | (Ε)-cinnamyl-(E)-cinnamylidenatea,1 | Thailand | Athikomkulchai et al. [45] |
| 183 | Kaempferol-dimethyl ethera | Portugal | Falcão et al. [52] |
| 184 | 5-Hexadecylresorcinola | Cameroon | Kardar et al. [69] |
| 185 | 5-(10′Z-pentadecenyl)-resorcinola | Cameroon | Kardar et al. [69] |
| 186 | 5-(12′Z-heptadecenyl)-resorcinola | Cameroon | Kardar et al. [69] |
| 187 | 5-(14′Z-heptadecenyl)-resorcinola,1 | Cameroon | Kardar et al. [69] |
| 188 | 5-(14′Z-nonadecenyl)-resorcinola | Cameroon | Kardar et al. [69] |
| 189 | 3-Undecyl phenola | Cameroon | Kardar et al. [69] |
| 190 | 3-Tetradecylphenola | Cameroon | Kardar et al. [69] |
| 191 | 3-Pentadecylphenola | Cameroon | Kardar et al. [69] |
| 192 | 3-Hexadecylphenola | Cameroon | Kardar et al. [69] |
| 193 | 3-Heptadecylphenola | Cameroon | Kardar et al. [69] |
| 194 | 3-Nonadecylphenola | Cameroon | Kardar et al. [69] |
| 195 | 3-(10′Z-pentadecenyl)-phenola | Cameroon | Kardar et al. [69] |
| 196 | 3-(12′Z-pentadecenyl)-phenola,1 | Cameroon | Kardar et al. [69] |
| 197 | 3-(8′Z-heptadecenyl)-phenola | Cameroon | Kardar et al. [69] |
| 198 | 3-(12′Z-heptadecenyl)-phenola | Cameroon | Kardar et al. [69] |
| 199 | 3-(14′Z-heptadecenyl)-phenola | Cameroon | Kardar et al. [69] |
| 200 | 3-(13′Z-nonadecenyl)-phenola,1 | Cameroon | Kardar et al. [69] |
| 201 | 3-(14′Z-nonadecenyl)-phenola,1 | Cameroon | Kardar et al. [69] |
| 202 | Deperoxidized derivative of plukenetione Ca,1 | Cameroon | Almutairi et al. [65] |
| 203 | 1,3-Dihydroxy-5-heptadecenylbenzenea | Egypt | El-Hady et al. [61] |
| 204 | 1,3-Dihydroxy-5-heptadecylbenzene (C17:0) derivatea | Egypt | El-Hady et al. [61] |
| 205 | 1,3-Dihydroxy-5-heptadecenylbenzene (C19:1) derivatea | Egypt | El-Hady et al. [61] |
| 206 | (E)-4-(3′-ethoxyprop-1′-enyl)phenol (Ethyl p-coumaroyl ether)a | United States | Savka et al. [70] |
| 207 | Coumaric acid cinnamyl ethera,* | United Kingdom | Saleh et al. [56] |
| 208 | Dimethyl kaempferol phenethyl ethera,* | United Kingdom | Saleh et al. [56] |
| 209 | Dihydroxy propionyl pinocembrin methyl ethera,* | United Kingdom | Saleh et al. [56] |
| 210 | Pinocembrin methyl ether (isomer 1)a,* | United Kingdom | Saleh et al. [56] |
| 211 | Dimethyl galangin phenacetyl ethera,* | United Kingdom | Saleh et al. [56] |
| 212 | Pinocembrin methyl ether (isomer 2)a,* | United Kingdom | Saleh et al. [56] |
| 213 | Hexadieneoyl dimethyl pinobanksina,* | United Kingdom | Saleh et al. [56] |
| 214 | Pinobanksin-5-methylether-3-O-propanoatea | Mexico | Alday et al. [71] |
| 215 | Pinobanksin-5-methylether-3-O-butyratea | Mexico | Alday et al. [71] |
| 216 | Tetragocarbone Ab,1 | Australia | Nishimura et al. [72] |
| 217 | Tetragocarbone Bb,1 | Australia | Nishimura et al. [72] |
| 218 | 3-(2-Hydroxy-4-methoxybenzyl)-6-methoxy-2,3-dihydrobenzofuran (Riverinol)a,1 | Nigeria | Omar et al. [60] |
The molecular structure of the compound is not completely defined.
Constituent isolated from the honeybee propolis (from the genus Apis sp.).
Constituent isolated from the stingless bee propolis (from genera Scaptotrigona sp., Melipona sp., Tetragonula sp., Trigona sp., Tetrigona sp., or. Heterotrigona sp.).
Newly discovered compound. Compounds already mentioned in Huang et al. [8] are excluded.
Phenolic compounds identified in propolis for the first time since 2011
2.1. Terpenoids
Terpenes and terpenoids are the biggest and most diverse group of secondary plant metabolites, which include more than 25,000 compounds. They are molecules composed from one or more isoprene (C5) units. Term terpene refers to a hydrocarbon molecule, whereas term terpenoid refers to hydrocarbon molecule that has been modified (e.g., addition of oxygen). Terpenes are further divided into seven classes: hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), tetraterpenes (C40), and polyterpenes (C45 or more) [73]. They are the second biggest and most important group of compounds and also the most abundant volatile components of propolis [74]. As mentioned before, they are one of the main biologically active substances in propolis and they play a major role in determining its quality. Terpenes were found mainly in tropical propolis, being rarer in poplar propolis type, yet some of them were also isolated from the Mediterranean propolis. Sesquiterpenes are the main group of terpenes found in propolis and are further divided into acyclic, monocyclic, dicyclic, and tricyclic sesquiterpenes. Other important terpenes from propolis are monoterpenes, triterpenes, and diterpenes, latter being the most important terpene from the pharmacological point of view [8,20,40,75].
Walker and Crane [24] mentioned 18 isolated terpenoids from propolis and Marcucci [6] added another 11. Between 2000 and 2012 Huang et al. [8] reported 58 terpenoids isolated from propolis for the first time, whereas between 2013 and 2018 another 46 were reported. In total, at least 133 terpenes were isolated from propolis until 2018. Terpenes isolated between 2013 and 2018 were found in propolis samples from Africa (Cameroon, Algeria, Egypt, and Nigeria), Asia (Malaysia and Thailand), South America (Chile, Brazil, and Bolivia), Middle East (Saudi Arabia), and Oceania (Australia and Pitcairn Island). Otherwise, most of the newly isolated terpenoids after 2013 belong to the group of triterpenes. Newly isolated terpenoids are listed in Table 3.
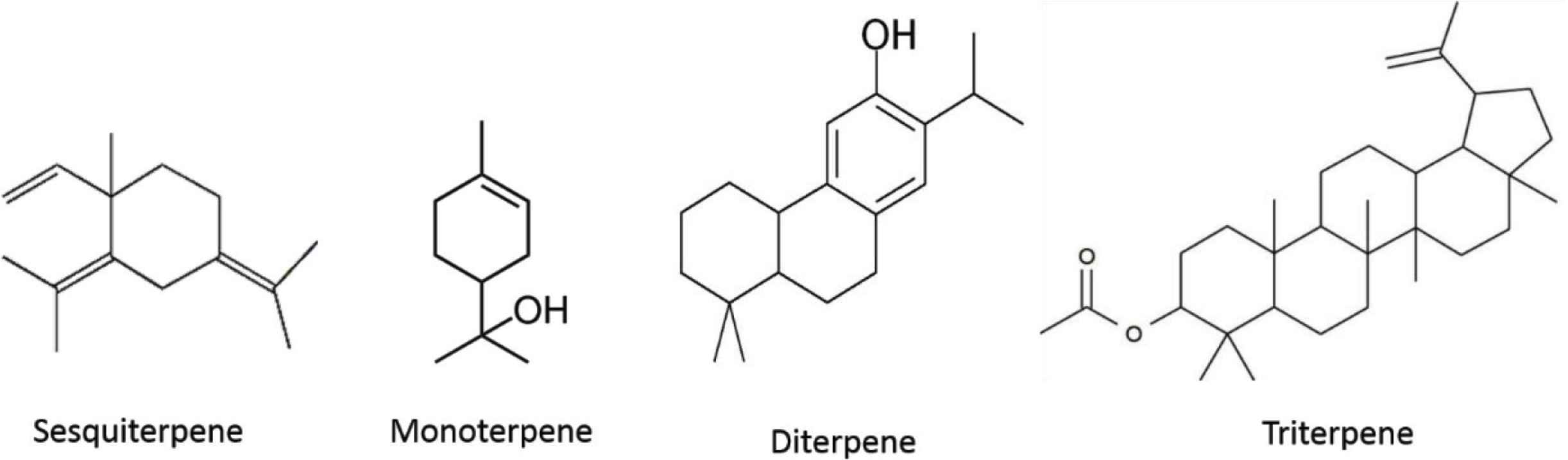
Terpenoid groups/classes isolated from different propolis samples around the world.
| No. | Chemical name | Geographic location | References |
|---|---|---|---|
| Monoterpenes | |||
| 219 | 1,8-Terpineola,* | Cameroon | Papachroni et al. [58] |
| Sesquiterpenes | |||
| 220 | β-Panasinsenea | Malaysia | Tuan et al. [76] |
| Diterpenes | |||
| 221 | Cistadiola | Algeria | Piccinelli et al. [48] |
| 222 | 18-Hydroxy-cis-clerodan-3-ene-15-oic acida | Algeria | Piccinelli et al. [48] |
| 223 | Propsiadin ((ent)-2-oxo-kaur-16-en-6,18-diol)a,1 | Saudi Arabia | Almutairi et al. [43] |
| 224 | Psiadina | Saudi Arabia | Almutairi et al. [43] |
| 225 | Poilaneic acida | Chile | Nina et al. [68] |
| 226 | 7,8,18-Trihydroxyserrulat-14-enea,1 | Australia | Aminimoghadamfarouj and Nematollahi [75] |
| 227 | 5,18-Epoxyserrulat-14-en-7,8-dionea,1 | Australia | Aminimoghadamfarouj and Nematollahi [75] |
| 228 | (18RS)-5,18-epoxyserrulat-14-en-8,18-diola | Australia | Aminimoghadamfarouj and Nematollahi [75] |
| 229 | rel-(5S,6S,8R,9R,10S,18R,19S)-18,19-epoxy-2-oxocleroda-3,12(E),14-triene-6,18,19-triol 18,19-diacetate 6-benzoatea,1 | Brazil | Tazawa et al. [77] |
| 230 | Abietinala | Pitcairn Island | Georgieva et al. [78] |
| Triterpenes | |||
| 231 | 3β-Acetoxy-19(29)-taraxasten-20α-ola | Saudi Arabia | Jerz et al. [67] |
| 232 | Pseudotaraxasterol-3β-O-acetatea | Saudi Arabia | Jerz et al. [67] |
| 233 | β-Sitosterola | Nigeria | Odiba et al. [79] |
| 234 | 25-Cyclopropyl-3β-hydroxyurs-12-enea,1 | Cameroon | Sakava et al. [80] |
| 235 | Cycloart-3β-hydroxy-12,25(26)-dienea,1 | Cameroon | Sakava et al. [80] |
| 236 | Lup-20(29)-en-3β-oatea | Cameroon | Sakava et al. [80] |
| 237 | Olean-12-en-3β,28-diol (erythrodiol)a | Cameroon | Sakava et al. [80] |
| 238 | ψ-Teraxasterol-acetatea | Cameroon | Papachroni et al. [58] |
| 239 | Taraxasterol acetatea | Cameroon | Papachroni et al. [58] |
| 240 | 3α-Hydroxy-olean-12-en-30-ola | Cameroon | Papachroni et al. [58] |
| 241 | Bacchara-12,21-dien-3β-ola | Cameroon | Papachroni et al. [58] |
| 242 | Betulinaldehydea | Cameroon | Papachroni et al. [58] |
| 243 | 4,4-Dimethyl-3-oxacholest-5-en-7-onea,1 | Egypt | El-Hady et al. [61] |
| 244 | 9,19-Cyclolanostan-3-ol-24-methylene acetatea,1 | Egypt | El-Hady et al. [61] |
| 245 | Dipterocarpolb | Thailand | Sanpa et al. [4] |
| 246 | 3-O-acetyl ursolic acidb | Thailand | Sanpa et al. [4] |
| 247 | Ocotillone Ib | Thailand | Sanpa et al. [4] |
| 248 | Ocotillone IIb | Thailand | Sanpa et al. [4] |
| 249 | Cabralealactone (isomer 1)b | Thailand | Sanpa et al. [4] |
| 250 | Cabralealactone (isomer 2)b | Thailand | Sanpa et al. [4] |
| 251 | Ursolic aldehydeb | Thailand | Sanpa et al. [4] |
| 252 | Oleanolic aldehydeb | Thailand | Sanpa et al. [4] |
| 253 | Cycloart-24-en-3β,26-diola | Bolivia | Nina et al. [81] |
| 254 | Cycloart-24-en-3-onea | Bolivia | Nina et al. [81] |
| 255 | 24(E)-cycloart-24-en-26-ol-3-onea | Bolivia | Nina et al. [81] |
| 256 | Mangiferonic acid methyl estera | Bolivia | Nina et al. [81] |
| 257 | Lup-20(29)-en-3-onea | Bolivia | Nina et al. [81] |
| 258 | Methyl-3β,27-dihydroxycycloart-24-en-26-oatea,1 | Cameroon | Talla et al. [82] |
| 259 | 20-Hydroxy-24-dammaren-3-oneb | Malaysia | Zhao et al. [63] |
| 260 | 3-Oxo-cycloart-24E-en-21,26-diol-21,26-diacetatea,1 | Pitcairn Island | Georgieva et al. [78] |
| 261 | 3-Oxo-cycloart-24E-en-21,26-diola,1 | Pitcairn Island | Georgieva et al. [78] |
| 262 | 3-Oxo-cycloart-24E-en-21,26-diol-21-acetatea,1 | Pitcairn Island | Georgieva et al. [78] |
| 263 | 3-Oxo-cycloart-24E-en-21,26-diol-26-acetatea,1 | Pitcairn Island | Georgieva et al. [78] |
| 264 | 3-Oxo-cycloart-24-en-26-ala | Pitcairn Island | Georgieva et al. [78] |
Compound needs confirmation.
Constituent isolated from the honeybee propolis (from the genus Apis sp.).
Constituent isolated from the stingless bee propolis (from genera Tetragonula sp., Tetrigona sp., or Heterotrigona sp.).
Newly discovered compound. Compounds already mentioned in Huang et al. [8] are excluded.
Terpenoids identified in propolis for the first time since 2011
2.2. Fatty Acids
Fatty acids are one of the “waxy” nonpolar parts of propolis, and Heinen and Linskens [83] were one of the first researchers who isolated fatty acids (ranging from C7 to C18) from propolis. Until 2018, there were many more found in propolis and some authors reported them as long as C36 [84]. Despite the fact that fatty acids were discovered in propolis relatively soon, most of the authors do not mention them in their articles. They can be present in propolis as glycosides, free fatty acids, different type of esters, or others [56,84]. There are many different types of fatty acids found in propolis: saturated, monounsaturated, polyunsaturated, and even omega-3 and omega-6 fatty acids [85]. As they are not among the most widely reported compounds in propolis, between 2013 and 2018 only two authors reported fatty acids isolated from propolis for the first time. Among those reported, 13 were free fatty acids and 4 were fatty acid glycosides. All of them were from European samples (Bulgaria and United Kingdom). Details can be found in Table 4.
| No. | Chemical name | Geographic location | References |
|---|---|---|---|
| Fatty acids | |||
| 265 | 9-Oxo-10(E)-12(Z)-octadecadienoic acida | Bulgaria | Bilikova et al. [86] |
| 266 | Dihydroxylinoleic acida,* | United Kingdom | Saleh et al. [56] |
| 267 | Dihydroxylinolenic acid (isomer 1)a,* | United Kingdom | Saleh et al. [56] |
| 268 | Dihydroxy eicosenoic acida,* | United Kingdom | Saleh et al. [56] |
| 269 | Hydroxylinolenic acid (isomer 1)a,* | United Kingdom | Saleh et al. [56] |
| 270 | Dihydroxy docosahexenoic acida,* | United Kingdom | Saleh et al. [56] |
| 271 | Hydroxylinolenic acid (isomer 2)a,* | United Kingdom | Saleh et al. [56] |
| 272 | Dihydroxylinolenic acid (isomer 2)a,* | United Kingdom | Saleh et al. [56] |
| 273 | Hydroxylinoleic acida,* | United Kingdom | Saleh et al. [56] |
| 274 | Hydroxyheptadecanoic acid acetatea,* | United Kingdom | Saleh et al. [56] |
| 275 | Hydroxydocosapentaenoic acida,* | United Kingdom | Saleh et al. [56] |
| 276 | Dihydroxylinolenic acid (isomer 3)a,* | United Kingdom | Saleh et al. [56] |
| 277 | Hydroxydocosahexanoic acida,* | United Kingdom | Saleh et al. [56] |
| Fatty acid glycosides | |||
| 278 | Hydroxynonadecanoic acid glucosidea,* | United Kingdom | Saleh et al. [56] |
| 279 | Octadecatriol glucosidea,* | United Kingdom | Saleh et al. [56] |
| 280 | Dihydroxy ecosanoic acid glucosidea,* | United Kingdom | Saleh et al. [56] |
| 281 | Hydroxy ecosanoic acid glucosidea,* | United Kingdom | Saleh et al. [56] |
The molecular structure of the compound is not completely defined.
Constituent isolated from the honeybee propolis (from the genus Apis sp.). Compounds already mentioned in Huang et al. [8] are excluded.
Fatty acids and their glycosides identified in propolis for the first time since 2011
2.3. Alcohols
Propolis, among other things, also contains different types of alcoholic compounds, such as simple alcohols, fatty alcohols, sugar alcohols, sterols, and others [8,24,84]. Between 2013 and 2018, two new alcohols were isolated from propolis samples from Africa (Cameroon) and Middle East (Oman). Table 5 includes only alcohols that were not included in the previous tables.
| No. | Chemical name | Geographic location | References |
|---|---|---|---|
| Alcohols | |||
| 282 | Pinitola | Oman | Popova et al. [42] |
| 283 | 1′-O-eicosanyl glycerola,1 | Cameroon | Talla et al. [82] |
Constituent isolated from the honeybee propolis (from the genus Apis sp.).
Newly discovered compound. Compounds already mentioned in Huang et al. [8] are excluded.
Alcohols and related compounds identified in propolis for the first time since 2011
2.4. Alkaloids and their Derivatives
One of the most surprising discoveries regarding propolis in the recent years is definitely the discovery of alkaloids and their derivatives in propolis samples. Neither alkaloids nor nitrogenous compounds (except some vitamins from only a few samples) as such were reported from propolis before 2011–2012 [8,87]. To the best of our knowledge, alkaloids and their derivatives were first isolated from propolis in 2015 [57], when they were isolated from Brazilian propolis. They were later reported again, when they were isolated from Algerian propolis in 2017 [31] and from Brazilian propolis in 2018 [47]. Altogether 16 alkaloids and 5 alkaloid derivatives were isolated from propolis samples from two different countries. Specifics are listed in Table 6.
| No. | Chemical name | Geographic location | References |
|---|---|---|---|
| Alkaloids | |||
| 284 | 7(3-Methoxy-2-methylbutyryl)-9-echimidinylretronecine derivative (1)b,* | Brazil | Coelho et al. [57] |
| 285 | 7(3-Methoxy-2-methylbutyryl)-9-echimidinylretronecine derivative (2)b,* | Brazil | Coelho et al. [57] |
| 286 | Pagicerinea | Algeria | Soltani et al. [31] |
| 287 | Demecolcinea | Algeria | Soltani et al. [31] |
| 288 | Papaverinea | Algeria | Soltani et al. [31] |
| 289 | Aspidospermidinea | Algeria | Soltani et al. [31] |
| 290 | Morphinan-6-one-2-ola | Algeria | Soltani et al. [31] |
| 291 | Thebainea | Algeria | Soltani et al. [31] |
| 292 | N,O-dimethyl stephinea | Algeria | Soltani et al. [31] |
| 293 | Morpholinea | Algeria | Soltani et al. [31] |
| 294 | Lelobanonolineb | Brazil | Cisilotto et al. [47] |
| 295 | 2-[6-(2-Hydroxy-propyl)-1-methyl-[2]-piperidyl]-1-phenylethanoneb | Brazil | Cisilotto et al. [47] |
| 296 | Norlobelanidineb | Brazil | Cisilotto et al. [47] |
| 297 | Norlobelineb | Brazil | Cisilotto et al. [47] |
| 298 | Lobelineb | Brazil | Cisilotto et al. [47] |
| 299 | Lobelanidineb | Brazil | Cisilotto et al. [47] |
| Alkaloid derivatives | |||
| 300 | 5(4H)-thebenidinonea | Algeria | Soltani et al. [31] |
| 301 | 4-(Phenylthioxomethyl)morpholinea | Algeria | Soltani et al. [31] |
| 302 | 4-Methyl-2,6-bis(4-morpholylmethyl)phenola | Algeria | Soltani et al. [31] |
| 303 | 3-[(Trimethylsilyl)oxy]4,5α-epoxy-14- hydroxy-17-(2-propenyl)morphinan-6-onea | Algeria | Soltani et al. [31] |
| 304 | Nicotinaldehydesemicarbazonea | Algeria | Soltani et al. [31] |
The molecular structure of the compound is not completely defined.
Constituent isolated from the honeybee propolis (from the genus Apis sp.).
Constituent isolated from the stingless bee propolis (from genera Scaptotrigona sp. or Melipona sp.). Compounds already mentioned in Huang et al. [8] are excluded.
Alkaloids and their derivatives identified in propolis for the first time since 2011
2.5. Other Compounds
Researchers also reported some new compounds in propolis that do not belong to any of the previously mentioned groups but were still isolated from propolis for the first time. Nineteen new compounds were isolated between 2013 and 2018, with the addition of one compound isolated in 2011 [35], which was not included in the review article by Huang et al. [8]. The newly isolated compounds were found in propolis from South America (Argentina), Africa (Algeria), and Europe (United Kingdom). Specifics are listed in Table 7.
| No. | Chemical name | Geographic location | References |
|---|---|---|---|
| 305 | 4-[4-(4-Hydroxy-phenyl)-2,3-dimethyl-butyl]-benzene-1,2-diola | Argentina | Agüero et al. [35] |
| 306 | Ethoxy sulfonatea,* | United Kingdom | Saleh et al. [56] |
| 307 | 3,4,5-Triphenylpyrazolea | Algeria | Soltani et al. [31] |
| 308 | 3-(4-Methoxyphenyl)benzo[f]quinazolinea | Algeria | Soltani et al. [31] |
| 309 | 2-(4-Methoxyphenyl)-4-[(2-propyn-1-yl)thio]quinazolinea | Algeria | Soltani et al. [31] |
| 310 | 4-Aminobenzo[g]quinazolinea | Algeria | Soltani et al. [31] |
| 311 | 5-(4-Diethylaminobenzylidene)rhodaninea | Algeria | Soltani et al. [31] |
| 312 | Carbamazepinea | Algeria | Soltani et al. [31] |
| 313 | 1-(3H-imidazol-4-yl)-ethanonea | Algeria | Soltani et al. [31] |
| 314 | Nifenazonea | Algeria | Soltani et al. [31] |
| 315 | Podofiloxa | Algeria | Soltani et al. [31] |
| 316 | Brallobarbitala | Algeria | Soltani et al. [31] |
| 317 | Cyclobarbitala | Algeria | Soltani et al. [31] |
| 318 | 6,7,8-Trimethoxy-isoquinolinea | Algeria | Soltani et al. [31] |
| 319 | 1-Butyl-isoquinolinea | Algeria | Soltani et al. [31] |
| 320 | 1-(Phenylthioxomethyl)-2,5-pyrrolidinedionea | Algeria | Soltani et al. [31] |
| 321 | 2-(4-Methoxyphenyl)-2-methyl-1,3-dioxolanea | Algeria | Soltani et al. [31] |
| 322 | 1′H-cholesta-3,5-dieno-[3,4-b]indola | Algeria | Soltani et al. [31] |
| 323 | 3-(3,4-Dimethoxyphenyl)-6-nitro-coumarina | Algeria | Soltani et al. [31] |
| 324 | 10-Butyl-3,7-dinitro-10H-phenothiazinea | Algeria | Soltani et al. [31] |
The molecular structure of the compound is not completely defined.
Constituent isolated from the honeybee propolis (from the genus Apis sp.). Newly discovered compound. Compounds already mentioned in Huang et al. [8] are excluded.
Compounds, not belonging to any previously mentioned groups, identified in propolis for the first time since 2011
Besides compounds mentioned above, there might be some that were not included in this review, either because their structures were not determined [47,60,66], because authors did not pay enough attention to their novelty and they were not specifically labelled as new [13,31,47,53,63,64,67,88], or simply because they were overlooked. In conclusion, actual number of compounds isolated in the recent years could be even higher.
3. CONCLUSION
Until 2000 at least 300 compounds were reported from propolis [6,11] and Huang et al. [8] reported another 241 between 2000 and 2012. Despite these numbers, just between 2013 and 2018 at least 305 compounds were isolated from propolis for the first time, including the first isolation of alkaloids. This number excludes 19 compounds isolated between 2011 and 2012, which were excluded from the previously mentioned review by Huang et al. [8] and were thus included in this article, bringing the total number to 324. Altogether, until 2018 more than 850 compounds are reported from propolis.
Compounds included in this article were isolated from 6 different continents and from 29 different countries (including the 19 added compounds isolated in 2011–2012 mentioned above). New compounds were isolated on more than one occasion from propolis of some countries, most often from Brazil (6×). Most of the compounds belong to the groups of flavonoids (92), phenols (126), and terpenes (46), whereas fatty acids (17), alcohols (2), alkaloids (21), and other compounds (20) represent a minor fraction. Despite the fact that propolis has been intensely studied for at least 30–40 years, new discoveries are being made on a yearly basis and it is not yet known how many more will be discovered in the upcoming years.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
The informations were gathered and the bulk of the article was written by Luka Šturm, while the critical revision and final approval were done by dr. Nataša Poklar Ulrih, whom also submitted the article.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Luka Šturm AU - Nataša Poklar Ulrih PY - 2019 DA - 2019/11/14 TI - Advances in the Propolis Chemical Composition between 2013 and 2018: A Review JO - eFood SP - 24 EP - 37 VL - 1 IS - 1 SN - 2666-3066 UR - https://doi.org/10.2991/efood.k.191029.001 DO - 10.2991/efood.k.191029.001 ID - Šturm2019 ER -
