The Impact of Achieving Complete Remission Prior to Allogeneic Stem Cell Transplantation on Progression-Free Survival in Hodgkin Lymphoma
 , Zinaida Perić1, 2, Sandra Bašić Kinda1, Lana Desnica1, Dino Dujmović1, Ivo Radman Livaja1, Ranka Serventi Seiwerth1, Igor Aurer1, 2,
, Zinaida Perić1, 2, Sandra Bašić Kinda1, Lana Desnica1, Dino Dujmović1, Ivo Radman Livaja1, Ranka Serventi Seiwerth1, Igor Aurer1, 2,  , Radovan Vrhovac1, 2,
, Radovan Vrhovac1, 2, 
- DOI
- 10.2991/chi.k.210704.002How to use a DOI?
- Keywords
- Hodgkin lymphoma; alloHSCT; PET-CT
- Copyright
- © 2021 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
Allogeneic hematopoietic stem cell transplantation (alloHSCT) is a potential curative option for patients suffering from relapsed/refractory (r/r) Hodgkin lymphoma (HL) after autologous stem cell transplantation (ASCT), offering a survival advantage over standard chemotherapy approaches [1]. However, two recently approved new drug treatments for r/r HL after ASCT [antiCD30 antibody-drug conjugate, brentuximab-vedotin (BV) and immune-checkpoint inhibitors (ICI)], demonstrated long-term disease control, with 38% and 16% of patients achieving complete response (CR), respectively [2]. These results have lately triggered much debate whether patients need to undergo alloHSCT at all after achieving response with BV or ICI [3,4].
The role of disease status prior to alloHSCT in HL is still under investigation. It has been previously shown that not just chemosensitivity but rather achieving complete PET-CT negativity pre-transplant is important for long-term outcomes after ASCT [5]. However, studies conducted so far have not systematically examined this question in the alloHSCT setting. It has been shown that patients with chemosensitive disease face less relapse, but there was no difference between patients in CR and partial response (PR) [6]. A study by Reyal et al. [7] assessed the prognostic value of pre-transplant CR evaluated by PET-CT according to the Deauville criteria, and showed it to be of no significance. Recently, Castagna et al. [8] at all have shown significant benefit in overall survival (OS) and progression free survival (PFS) in patients in CR compared to PR, with disease status proving to be an independent predictor of PFS in a multivariate analysis.
To evaluate the importance of achieving PET-CT negativity prior to alloHSCT, we conducted a single-centre retrospective study of 22 consecutive patients who underwent reduced intensity conditioning alloHSCT over a 5-year period (January 2014–April 2019).
Nine female and 13 male patients at a median age of 34 years (range, 19–62) underwent alloHSCT from either HLA identical or HLA haploidentical related donors. The median number of lines of therapy prior to alloHSCT was 4 (range 3–8). Twenty patients (91%) had undergone prior ASCT, 11 of them (55%) relapsing in less than 12 months from transplant. Four patients (18%) were subsequently treated with ICI, receiving a median of four cycles, all achieving CR and then proceeding to alloHSCT. The median time from ICI to transplantation was 79 days (range 64–103). Fifteen patients (68%) were transplanted using haploidentical donors and bone marrow (BM) as a source of cells, and seven (32%) were transplanted from a related HLA identical donor using peripheral blood stem cells (PBSC). Patients receiving transplant from haploidentical donors were conditioned using fludarabine, cyclophosphamide, and total body irradiation (FluCyTBI200 protocol) [9], while those transplanted from matched related donors were conditioned using fludarabine-based reduced intensity conditioning. The disease status at the time of the transplant was evaluated by PET-CT using the Deauville score (a score of 4 or 5 was considered to be a positive finding) [10], with 17 (77%) patients being in CR and five (23%) in PR. Patient and transplantation characteristics are reported in Table 1. There were no significant differences in the number of lines of chemotherapy received between patients in CR and PR. The only difference found was in gender, with more female patients in the PR group.
| All patients (n = 22) | CR (n = 17) | PR (n = 5) | p | |
|---|---|---|---|---|
| Age, years (median, range) | 34 (19–62) | 32 (19–62) | 36 (22–38) | 0.809 |
| Sex M/F | 13/9 | 12/5 | 1/4 | 0.045 |
| Number of CT lines (median, range) | 4 (3–8) | 4 (3–7) | 4 (3–8) | 0.663 |
| Previous ASCT (n, %) | 20 (91) | 15 (88) | 5 (100) | NS |
| Stem cell source (n, %) | 0.12 | |||
| PBSC | 7 (32) | 4 | 3 | |
| BM | 15 (68) | 13 | 2 | |
| ATG prophylaxis GVHD (n, %) | 0.12 | |||
| No | 15 (68) | 13 | 2 | |
| Yes | 7 (32) | 4 | 3 | |
| Conditioning regimens (n, %) | 0.12 | |||
| non-myeloablative (NMAC) | 15 (68) | 13 | 2 | |
| Reduced-intensity conditioning (RIC) | 7 (32) | 4 | 3 | |
Patient and transplantation characteristics
With a median follow up of 26.7 months (range 13–60.5), the OS for the entire group was 86% at 12 months (95% CI 75–100), and the PFS at 18 months was 66% (95% CI 48–90) (Figure 1a). In univariate analysis, patients in CR at the time of transplant showed significantly better PFS when compared to patients in PR (80%, 95% CI 62–100 versus 20%, 95% CI 3–100, p = 0.006) (Figure 1b), while there was no significant difference in OS (88%, 95% CI 74–100 versus 80%, 95% CI 52–100, p = 0.70). The cumulative incidence (CI) of acute graft-versus-host disease (aGVHD) grade 2–4 was 27% (95% CI 11–47), with none of the patients having had aGVHD grade ≥3. The CI of chronic (cGVHD) was 18% (95% CI 4–41) at 2 years (Figure 1c), that of relapse was 33% (95% CI 14–53), and of non-relapse mortality (NRM) was 5% (95% CI 0–19; Figure 1d).
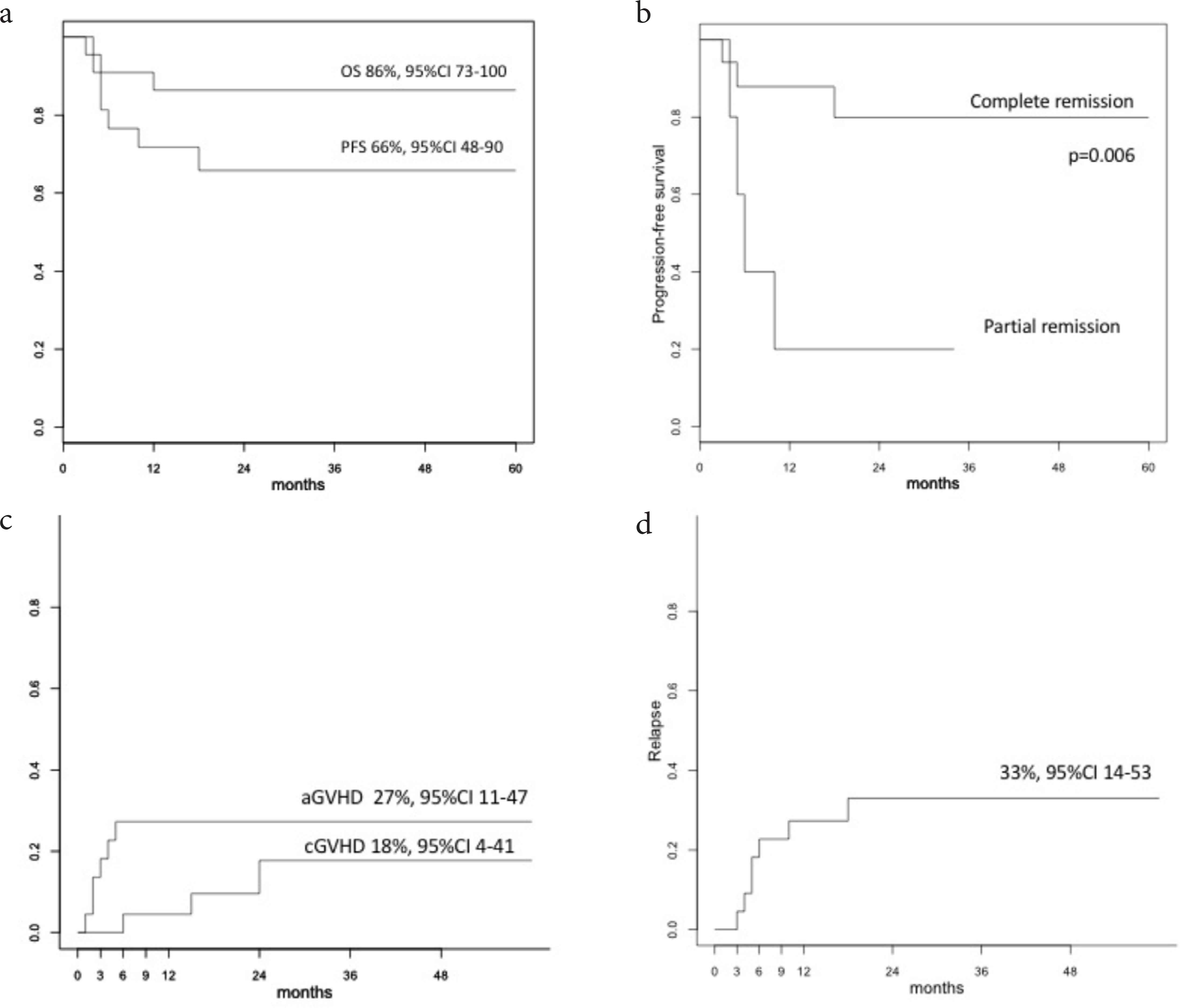
(a) Overall survival (OS) and progression-free survival (PFS). (b) Progression-free survival (PFS) according to disease status. (c) Acute and chronic GVHD cumulative incidence. (d) Non-relapse mortality cumulative incidence.
This study is a single-centre retrospective analysis of outcomes of alloHSCT in r/r HL, illustrating real-life experience in the treatment of this heavily pre-treated patient population. Our results showed that achieving CR pre-transplant result in significantly better PFS in comparison to achieving PR only. Up-to-now, studies in the alloHSCT setting have usually grouped patients into a chemosensitive (CR + PR) and chemoresistant group, whereas studies which compared outcomes in patients achieving CR and PR have yielded somewhat conflicting results [7,8] Also, there is a possible pitfall in making comparisons with published data, as in Reyal et al.’s study a Deauville 3 was deemed as positive, while in our analysis only Deauville 4 and 5 were considered to be positive findings, a practice usually used in the clinical setting [10].
Not surprisingly, we found no difference in OS between these two groups, since we were able to successfully treat the majority of relapsing patients with BV (re-) treatment and donor lymphocyte infusions. The NRM was quite low, reflecting the low incidence of both aGVHD and cGVHD and, most likely, influenced by the majority of patients having been transplanted using haploidentical donors, post-transplant cyclophosphamide GVHD prophylaxis and BM as the source of cells.
The obvious limitation to this study is the small number of patients included, which hindered us from performing a multivariate analysis. Our cohort also had a limited number of patients in PR (23% of the entire cohort), resulting from our institutional decision not to transplant before a meaningful response is obtained. This also led us to utilize IC in four patients, even though it is not reimbursed in our country. That is, actually, a selection bias, as we aimed for the best response and not the quickest transplant. However, there was no difference in the number of lines of therapy received prior to transplantation when comparing patients in CR and PR (Table 1). With all that said, these results should most certainly be confirmed in a multicentre study in a larger number of patients.
In the era of new potent drugs in r/rHL there has been much debate on whether patients need to undergo alloHSCT at all, after achieving a response with BV or ICI. Both drugs offer the possibility of excellent disease control, and there is probably no need to consolidate the response with alloHSCT in all patients [2]. However, there is currently no available method to enable us to identify patients who will remain in long term CR. Also, ICI have shown great value in disease control, but in a study reported after follow-up of only 18 months, the median duration of response was 16.6 months [11], so long-term disease control might not be achievable using this strategy. A more recent publication examining the effect of nivolumab and subsequent transplant in r/rHL showed superior PFS in a subgroup of patients achieving CR/PR with nivolumab and proceeding to alloHSCT, in comparison to patients not transplanted afterward [12]. However, a randomized study is lacking. As said, the question on whether to transplant or not has been much debated; however, maybe the true question should be “When” and not “Whether” to transplant. Even with the limitation of relatively small patient numbers in our study, the detected difference in the outcomes of patients achieving CR is, in our opinion, if not conclusively important, then, at least, intriguing. Choosing haploidentical donors and BM as a source of cells, aiming at CR prior to alloHSCT and using all available resources to achieve it (including ICI), may result in an excellent long-term survival and, more importantly, long-term disease control. This, of course, needs to be confirmed in a larger cohort of patients and, hopefully, our data, however limited, may instigate this strategy as a valuable direction for future studies.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
ND wrote the article, ZP performed the analysis. All authors reviewed and edited the manuscript.
REFERENCES
Cite this article
TY - JOUR AU - Nadira Duraković AU - Zinaida Perić AU - Sandra Bašić Kinda AU - Lana Desnica AU - Dino Dujmović AU - Ivo Radman Livaja AU - Ranka Serventi Seiwerth AU - Igor Aurer AU - Radovan Vrhovac PY - 2021 DA - 2021/07/15 TI - The Impact of Achieving Complete Remission Prior to Allogeneic Stem Cell Transplantation on Progression-Free Survival in Hodgkin Lymphoma JO - Clinical Hematology International SP - 116 EP - 118 VL - 3 IS - 3 SN - 2590-0048 UR - https://doi.org/10.2991/chi.k.210704.002 DO - 10.2991/chi.k.210704.002 ID - Duraković2021 ER -
