Association of Patient-Reported Physical Activity on Allogeneic Hematopoietic Cell Transplant Outcomes
 , Joseph Pidala1, Heather Jim3,
, Joseph Pidala1, Heather Jim3,  , Junmin Whiting4, Qianxing Mo4, Asmita Mishra1
, Junmin Whiting4, Qianxing Mo4, Asmita Mishra1- DOI
- 10.2991/chi.k.210221.001How to use a DOI?
- Keywords
- Allogeneic hematopoietic cell transplant; physical activity; stem cell transplantation
- Abstract
Background: Physical function prior to allogeneic hematopoietic cell transplant (HCT) is associated with survival and may be associated with patient physical activity (PA). Tools to evaluate PA prior to HCT are scarce. We aimed to evaluate the impact of easily obtained patient-report of PA prior to HCT on survival.
Methods: HCT recipients between January 1, 2011 and July 5, 2018 and who completed an International Physical Activity Questionnaire Short Form were included. This patient survey captures self-reported activities over the preceding week to determine PA level.
Results: We report a retrospective study of 587 adult (age ≥18) HCT recipients. The median age for the cohort was 57.9 years (range 19.9–76.1) with 149 patients (25.4%) age ≥65. Younger patients reported higher PA (low, median age 59.7 years; moderate, 56.1; high, 55.7; p < 0.001). High activity level was reported by males (66.7%; p < 0.001). Patients with low PA had HCT-comorbidity index (HCT-CI) ≥ 3 (68.1%, p = 0.002). When controlling for HCT-CI and disease risk index, higher PA was associated with improved overall survival (HR 0.954, 95% CI 0.921–0.988, p = 0.009). After adjusting for HCT-CI, higher PA was associated with reduced non-relapse mortality (NRM) (HR 0.931, 95% CI 0.891–0.972, p = 0.0013). Subgroup analysis in adults age ≥65 years also found that PA was lower in this population and associated with NRM mortality (HR 0.95, 95% CI 0.90–0.99, p = 0.041).
Conclusion: Patient-reported PA is a predictor of post-HCT survival. Future studies to validate incorporation of self-report tools to better predict patient-related adverse risk are warranted.
- Copyright
- © 2021 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) offers a potential cure to patients with a hematologic malignancy; the potential benefit is offset by the risk of morbidity and mortality. While improvements in transplant-related care has allowed for older and more frail patients to be offered this intensive therapy, assessing tolerability of the procedure prior to HCT remains a challenge [1]. The current paradigms for patient evaluation prior to HCT include comorbidity appraisal and healthcare provider-rated estimation of performance status, but not patient-reported assessment of their health status [2]. While these useful tools assist in ascertaining medical risk for an HCT recipient, HCT physicians suggest that additional tools beyond both comorbidity and performance status evaluation are needed to better ascertain a patient’s overall health status and functionality [3].
Notably, HCT recipients have demonstrated limitations in functionality, with 19–25% being reported as frail, underscoring the need to evaluate a patients’ physical capacity prior to HCT [4–8]. A current study, Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 1704: Composite Health Assessment Risk Model for Older Adults (CHARM) (https://clinicaltrials.gov/ct2/show/NCT03992352), is aimed at identifying important tools for assessment of frailty in older adults scheduled for HCT, an at risk population. One component of frailty, physical fitness, has been shown to be of particular importance in HCT recipients [4,9–12]. In this population, decreased physical function prior to HCT has been associated with decreased overall survival (OS) and increased non-relapse mortality (NRM) [4,9–12]. In a multi- institutional study evaluating the impact of exercise training on post-transplant quality of life, lower patient-reported physical function prior to allogeneic HCT was found to correlate with lower OS [9]. Decline in physical function, which is the ability to perform instrumental activities of daily living, may be delayed or prevented by increased physical activity, which include structured exercises, as well as routine activities such as carrying groceries or gardening [13,14]. Currently, patient-reported assessment of activity level in a formal and consistent manner is not frequently collected. We aimed to evaluate the impact of patient-reported physical activity prior to HCT on HCT outcomes. We hypothesized that increased physical activity would be associated with improved survival post-transplant.
2. MATERIALS AND METHODS
2.1. Patients
Data were retrospectively collected from the Moffitt Cancer Center (MCC) Health Research Informatics and BMT databases after approval by the Institutional Internal Review Board. Patients who underwent HCT from January 1, 2011 to July 5, 2018 at MCC and who completed the International Physical Activity Questionnaire Short Form (IPAQ) were included. This measure was captured as part of a new patient questionnaire that is given to all new consultations at MCC. Completion of this questionnaire by the any individual patient is per the patient’s discretion and not mandated. Patients were excluded if they had more than one allogeneic HCT or had incomplete (n = 896) or missing (n = 20) IPAQ questionnaire.
2.2. Measures
International Physical Activity Questionnaire Short Form is a 7-item questionnaire which captures patients’ self-reported activities over the preceding week [15]. Patients provide the number of days and the amount of time they performed each of the following intensities of physical activity over a typical week: mild (i.e. walking at least 10 min), moderate (i.e. carrying light load, bike riding at regular pace), or vigorous (i.e. heavy lifting, aerobics, fast bike riding). Utilizing this information, the IPAQ scoring algorithm allows for calculation of a patient’s energy expenditure in metabolic task equivalent (MET)-minutes (MET-min) per week. A MET is the ratio between an activity’s metabolic requirement compared to the metabolic requirement of sitting [16]. Based on the total MET-min, the IPAQ provides the ability to categorize patients into various physical activity levels: low, moderate, or high. An approximate equivalent of high level of physical activity is vigorous activity such as aerobics for at least 0.5 h a day or moderate intensity activity such as biking at a regular pace for at least 1 h a day. An equivalent approximation of a moderate level of physical activity is moderate intensity activity for at least 0.5 h a day.
Demographics were captured from HCT data repository at MCC. Comorbidities were captured by HCT-comorbidity index (HCT-CI) [17]. HCT-CI score was categorized into low (score 0), intermediate (score 1–2), and high (≥3). Disease risk index was utilized to identify risk of poor transplant-related outcomes related to underlying disease [18].
2.3. Statistical Analysis
Activity level was calculated utilizing the IPAQ scoring algorithm and was evaluated as continuous and categorical variables. When physical activity was evaluated as a continuous variable, log2 transformation of the physical activity value was performed. The association between activity level and continuous variables was analyzed utilizing the analysis of variance method. The association between activity level and categorical variables was analyzed utilizing the Chi-square test. Univariate analysis was performed utilizing the Cox proportional hazards model for OS and the sub-distribution hazards model for NRM. The OS endpoint was date of death or date of last contact. NRM was calculated as death without relapse, with death in relapse as a competing risk. Multivariable analysis was performed with a selection of variables which impact OS and NRM post-transplant (gender, HCT-CI, regimen intensity, Disease Risk Index) or have been shown to correlate with physical activity [forced vital capacity in 1 second (FEV1)] [17–19]. Backward elimination was performed to remove variables with overall p > 0.05 in the final model.
As IPAQ is validated in patients 18–65 years of age, to support the importance of physical function in older adult HCT recipients post-hoc subgroup analysis was performed in patients age ≥65 years utilizing the same methods as above [13,20,21]. Spearman’s correlation was utilized to evaluate the correlation between physical activity and time between survey completion to HCT. All analyses were formed with SAS version 9.4 (SAS Institute Inc., Cary, NC).
3. RESULTS
3.1. Baseline Characteristics
A total of 1501 patients received allogeneic HCT at MCC from January 1, 2011 to July 5, 2018 (Figure 1). Of these, 587 underwent one allogenic HCT with complete physical activity data available. The frequency distributions of patient demographics are shown in Table 1. Median age at HCT was 57.9 years (range 19.9–76.1). The majority of patients were age 40–64 years (n = 348, 59.3%), and 25.4% were ≥65 years (n = 149). Subjects were predominantly male (n = 326, 55.5%), had high HCT-CI (n = 320, 54.8%) and intermediate risk by Disease Risk Index (n = 417, 74.3%). The most common HCT was matched unrelated donor (n = 290, 49.4%) and most patients received peripheral blood mobilized stem cells (n = 541, 92.2%). The median follow up was 41.7 months (11.4–267 months).
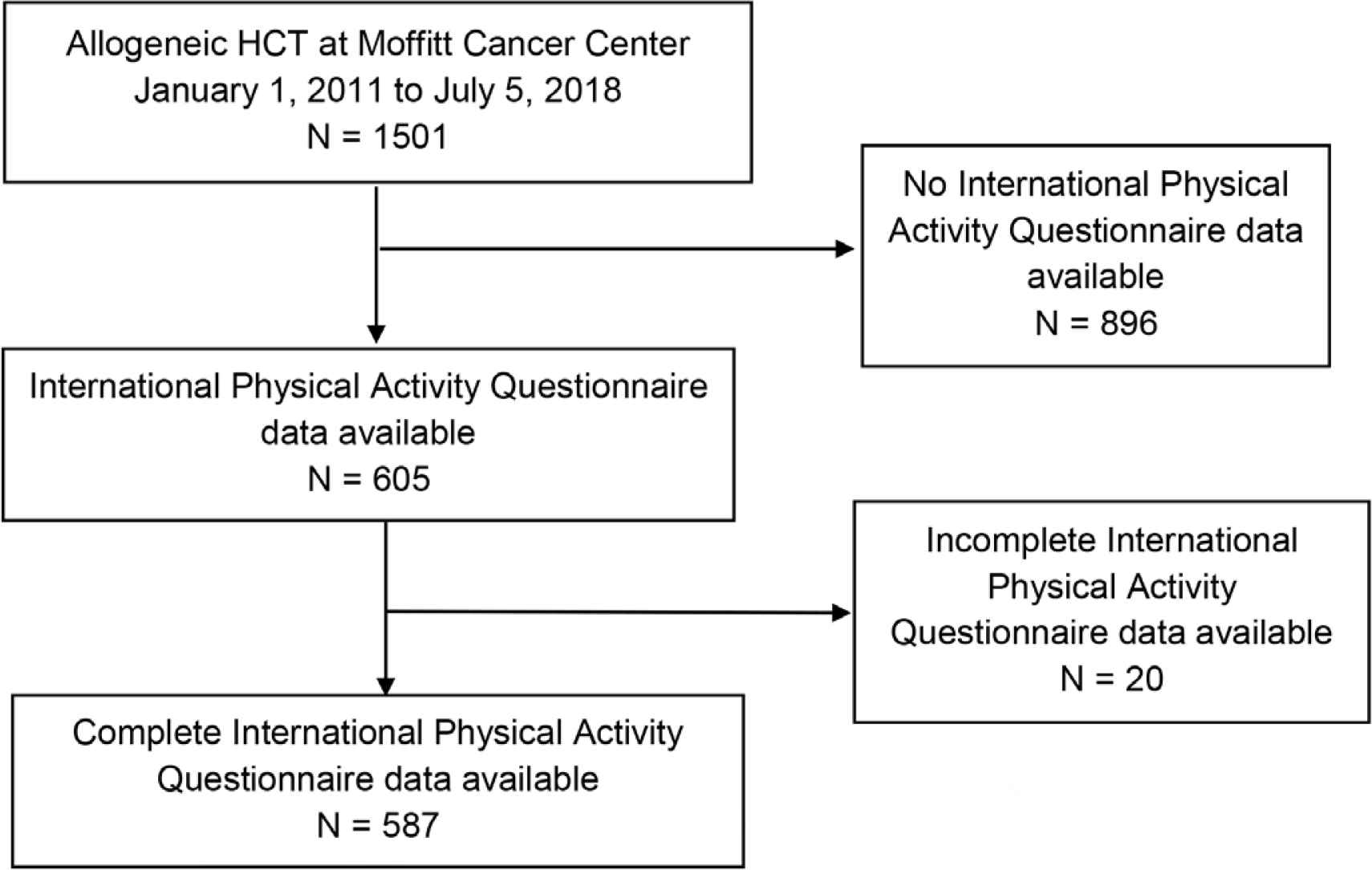
CONSORT diagram.
| Characteristic | Overall (n = 587) | Physical activity level | ||
|---|---|---|---|---|
| Low (n = 337) | Moderate (n = 52) | High (n = 198) | ||
| Age (Median, range) | 57.85 (19.91–76.07) | 59.67 (20.3, 75.4) | 56.1 (22.3, 72.4) | 55.74 (19.9, 76.1) |
| Gender | ||||
| Female | 261 (44.5%) | 173 (51.3%) | 22 (42.3%) | 66 (33.3%) |
| Male | 326 (55.5%) | 164 (48.7%) | 30 (57.7%) | 132 (66.7%) |
| KPS | ||||
| 90–100 | 461 (78.5%) | 261 (77.4%) | 42 (80.8%) | 158 (79.8%) |
| ≤80 | 126 (21.5%) | 76 (22.6%) | 10 (19.2%) | 40 (20.2%) |
| HCT-CI* | ||||
| Low | 98 (16.8%) | 44 (13.1%) | 10 (19.6%) | 44 (22.2%) |
| Intermediate | 166 (28.4%) | 84 (25.1%) | 17 (33.3%) | 65 (32.8%) |
| High | 320 (54.8%) | 207 (61.8%) | 24 (47.1%) | 89 (44.9%) |
| Number of prior chemotherapy | ||||
| <2 | 150 (26.8%) | 86 (27%) | 17 (32.7%) | 47 (24.9%) |
| ≥2 | 410 (73.2%) | 233 (73%) | 35 (67.3%) | 142 (75.1%) |
| Missing | 27 | |||
| Regimen intensity | ||||
| NMA | 269 (45.8%) | 165 (49%) | 32 (61.5%) | 84 (42.4%) |
| MAC | 318 (54.2%) | 172 (51%) | 20 (38.5%) | 114 (57.6%) |
| Disease Risk Index** | ||||
| Low | 45 (8.0%) | 25 (7.8%) | 4 (8%) | 16 (8.5%) |
| Intermediate | 417 (74.3%) | 241 (74.8%) | 41 (82%) | 135 (71.4%) |
| High/very high | 99 (17.6%) | 56 (17.4%) | 5 (10%) | 38 (20.1%) |
| Primary disease€ | ||||
| Benign hematologic disorder | 17 (2.9%) | 10 (3%) | 0 (0%) | 7 (3.6%) |
| Lymphoid disorder | 139 (23.9%) | 71 (21.3%) | 9 (17.6%) | 59 (29.9%) |
| Myeloid disorder | 402 (69.2%) | 247 (74.2%) | 35 (68.6%) | 120 (60.9%) |
| Plasma cell disorder | 23 (4.0%) | 5 (1.5%) | 7 (13.7%) | 11 (5.6%) |
| FEV1 | ||||
| >80% | 467 (79.6%) | 268 (79.5%) | 40 (76.9%) | 159 (80.3%) |
| ≤80% | 120 (20.4%) | 69 (20.5%) | 12 (23.1%) | 39 (19.7%) |
| Acute GVHD† | ||||
| 0–I | 297 (53.8%) | 170 (54.1%) | 25 (49%) | 102 (54.5%) |
| II–IV | 255 (46.2%) | 144 (45.9%) | 26 (51%) | 85 (45.5%) |
3 missing;
26 unable to be scored;
6 missing;
35 missing.
KPS, karnofsky performance status; NMA, nonmyeloablative; MAC, myeloablative; GVHD, graft-versus-host disease.
Patient characteristics
3.2. Self-Reported Physical Activity by IPAQ
A significant difference in age was seen by level of activity, with younger patients reporting a higher activity level (low activity level, median age 59.7 years, range 20.3–75.4; moderate, median 56.1, range 22.3–72.4; high, median 55.7, range 19.9–76.1; p < 0.001) (Table 1). More males reported a high activity level (66.7%, female 33.3%; p < 0.001). Patients with reported low activity level had higher HCT-CI (p = 0.002). No difference in healthcare provider measured Karnofsky Performance Status (KPS) was seen by patient-reported activity level (p = 0.749). The number of chemotherapy regimens received prior to HCT was similar between physical activity levels (p = 0.526) with 75.1% (n = 142) of the patients reporting high activity level having received two or more prior lines of chemotherapy. No difference in activity level was seen by Disease Risk Index (p = 0.556). Although 33.7% (n = 198) of the study population reported high level of physical activity, 19.7% (n = 39) were found to have low FEV1.
3.3. Transplant-Related Outcomes
The median OS for the entire cohort was 33.6 months. In univariate analysis, an increased activity level demonstrated improved hazard ratio for OS (HR 0.94, 95% CI 0.91–0.97, p < 0.001) (Table 2). This finding indicates that with increasing physical activity, as measured by total METs, the likelihood of death decreases by 6%. Older age was associated with an increased risk of mortality (HR 1.02, 95% CI 1.01–1.04, p < 0.001). A KPS of 90–100% was associated with higher OS (versus ≤80%, HR 1.73, 95% CI 1.28–2.34, p < 0.001). Patients with high HCT-CI did not have significant difference in OS compared to those with low HCT-CI (HR 1.40, 95% CI 0.97–2.04, p = 0.073). High or very high Disease Risk Index correlated with worse OS (HR 2.99, 95% CI 1.66–5.38, p < 0.001). In multivariate analysis, when controlling for HCT-CI and Disease Risk Index, greater physical activity remained significantly correlated with improved OS (HR 0.954, 95% CI 0.921–0.988, p = 0.009) (Table 3).
| Patient characteristic | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Physical activity | 0.94 (0.91–0.97) | <0.001 |
| Gender | ||
| Male | – | 0.437 |
| Female | 0.90 (0.69–1.18) | |
| KPS | ||
| ≤80% | 1.73 (1.28–2.34) | <0.001 |
| >80% | – | |
| HCT-CI | ||
| Low | – | <0.001 |
| Intermediate | 0.69 (0.44–1.07) | |
| High | 1.40 (0.97–2.04) | |
| Disease Risk Index | ||
| Low | – | <0.001 |
| Intermediate | 1.70 (0.99–2.93) | |
| High | 2.99 (1.66–5.38) | |
| FEV1 | ||
| ≤80% | 1.27 (0.93–1.73) | 0.134 |
| >80% | – | |
| Regimen intensity | ||
| MAC | 1.28 (0.98–1.67) | 0.074 |
| NMA | – | |
MAC, myeloablative conditioning; NMA, nonmyeloablative.
Univariate analysis of overall survival
| Patient characteristic | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Physical activity | 0.954 (0.921–0.988) | 0.009 |
| HCT-CI | ||
| Low | – | 0.004 |
| Intermediate | 0.742 (0.472–1.165) | |
| High | 1.318 (0.904–1.921) | |
| Disease Risk Index | ||
| Low | – | <0.001 |
| Intermediate | 1.648 (0.94–2.887) | |
| High/very high | 2.758 (1.5–5.072) | |
Overall survival multivariable analysis
The activity level was associated with NRM (HR 0.92, 95% CI 0.88–0.96, p < 0.001) in univariate analysis (Figure S1 and Table S1). Older age was associated with higher NRM (HR 1.04, 95% CI 1.02–1.06, p < 0.001). Low KPS and high HCT-CI were associated with higher NRM (KPS ≤80 vs >80, HR 2.12, 95% CI 1.47–3.08, p < 0.001; HCT-CI ≥ 3, HR 1.00–2.83, p = 0.048). In multivariable analysis, after adjusting for HCT-CI, greater activity level was a significant predictor of reduced NRM (HR 0.931, 95% CI 0.891–0.972, p = 0.0013) (Table 4).
| Patient characteristic | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Physical activity | 0.931 (0.891–0.972) | 0.0013 |
| HCT-CI | ||
| Low | – | 0.0145 |
| Intermediate | 0.838 (0.455–1.543) | |
| High | 1.523 (0.902–2.572) | |
Non-relapse mortality multivariable analysis
3.4. Older Adults
A subgroup analysis was performed in patients aged ≥65 years. The median age at HCT in this subgroup was 68.5 years (range 65.1–76.1 years). The majority of patients reported low level of physical activity (n = 95, 63.8%; moderate, n = 8, 5.4%; high, n = 46, 30.9%), had high KPS as determined by the treating physician (n = 114, 76.5%), increased HCT-CI (n = 88, 60.3%; intermediate, n = 35, 24%; low, n = 23, 15.8%), and FEV1 ≥ 80% (n = 111, 74.5%). Twenty percent (n = 30) of patients received myeloablative conditioning regimen.
The activity level was not significantly associated with OS (HR 0.97, 95% CI 0.92–1.01, p = 0.173) (Table S2). Regimen intensity and HCT-CI had borderline significance. Greater activity level was significantly associated with reduced NRM (HR 0.95, 95% CI 0.90–0.99, p = 0.041; Table S2). KPS, HCT-CI, and regimen intensity did not impact NRM.
4. DISCUSSION
This is the largest study of patient-reported physical activity prior to allogeneic HCT. Its results suggest that patients with a higher level of physical activity prior to HCT have improved OS and NRM post-transplant. Despite high HCT-CI seen in patients with low physical activity, the above findings remained significant after adjustment for comorbidities. Patients reported high physical activity level despite low KPS or FEV1, suggesting that patient-reported physical activity provides information beyond what is captured by routine pre-transplant evaluation. Patient-reported physical activity likely reflects elements which are not captured on provider- reported KPS.
Our findings are consistent with prior studies demonstrating the importance of physical fitness prior to allogeneic HCT. The gold standard to evaluate physical fitness is maximum oxygen utilized in exercise (VO2max), but is limited by the need for specialized equipment. Utilizing patient-report, the IPAQ measures patient’s physical activity and has been correlated with VO2max [22]. The 6-min walk test (6MWT), an objective measure of physical function which correlates with VO2max, has also been shown to correlate with post-transplant survival [10,23]. Incremental increases in the 6MWT correlate with improved OS and NRM. Serial evaluation of physical activity not only pre- to post-transplant, but during chemotherapy prior to HCT may provide additional information into a patient’s physical resilience.
The importance of physical fitness in HCT is also evident by interventional studies investigating exercise regimens to improve outcomes post-transplant, although the evidence for such interventions is mixed. An exercise intervention study which included self-directed and supervised components showed decreased overall mortality and NRM with exercise, as well as decreased fatigue and improved quality of life (QOL), except for anxiety, in allogeneic HCT recipients [24]. With a focus on strength training, Hacker et al. [25] found exercise intervention during HCT to maintain physical function and improve fatigue in patients who receive allogeneic or autologous HCT. In a multicenter interventional cooperative group trial, self-directed exercise and stress management was studied in adults undergoing allogeneic or autologous HCT [26]. Although that study did not show an improvement on QOL at day +100, a sub-study showed a correlation of higher baseline patient-reported physical function with higher OS and lower NRM in allogeneic HCT recipients, which is consistent with our findings [9]. These studies are limited by the differences in HCT type, autologous versus allogeneic, which carry different risks.
In older adults (age ≥65 years), our analysis suggests that physical activity, measured by the IPAQ, impacts NRM. With the increasing number of older adults receiving allogeneic HCT, there is a rising need to understand patient factors other than chronologic age and provider-rated performance status, which are predictive of survival post-transplant [1]. In older adults undergoing chemotherapy, studies have shown the importance of physical function on survival and risk of chemotherapy toxicity [27–30]. In older adults receiving intensive induction chemotherapy for acute myeloid leukemia, physical function assessed by an objective measure correlated with OS [29]. With incremental increases in physical function, improvement in OS was seen. In allogeneic HCT, multiple single-institution studies have shown that OS and NRM are impacted by pre- transplant physical function, as evaluated by objective and patient- reported measures [4,11,12]. Those studies were limited by the use of different measures to evaluate physical function. In contrast, a multicenter retrospective study did not show a correlation of an objective measure of function, Timed-Up-and-Go, and patient- reported instrumental activities of daily living with OS or NRM [31]. In our study, the correlation between physical activity and NRM in older adults suggests the added utility of IPAQ in evaluating this patient population prior to HCT.
This study is limited by the patients excluded due to lack of or incomplete physical activity data. Physical activity questionnaires were completed as part of a larger set of patient questionnaire at the time of initial consult at our institution. The heterogeneity of time from survey completion to transplant is another limitation. Despite this, we found no correlation between time from survey completion to transplant with physical activity level. Variation seen in time to transplant is consistent with donor identification and proceeding with HCT. Studies are needed to standardize measurement of patient-reported physical function and evaluate how best to incorporate this into HCT evaluation. BMT CTN 1704 CHARM is currently underway and poised to fill these gaps in our knowledge.
5. CONCLUSION
In summary, herein we describe the largest cohort of patient- reported physical activity in allogeneic HCT recipients. Our study demonstrates that patient-reported physical activity is an independent predictor of OS and NRM. Given our findings, we believe patient-reported physical activity may have added benefit for physician assessment, and be reflective of patient functionality reserve prior to transplantation. Future studies are needed to better understand the role of physical activity in HCT, and validation of inclusion of patient-reported physical activity assessment prior to allogeneic HCT is warranted. Such assessments may facilitate treatment plans and interventions in allogeneic HCT recipients to mitigate transplant related excess risk.
CONFLICTS OF INTEREST
RJ, JP, JW, MQ, AM have no conflict of interest to disclose. HJ reports consultant fees from RedHill BioPharma, Janssen Scientific Affairs, and Merck and grants from Kite Pharmaceuticals outside the submitted work.
AUTHORS’ CONTRIBUTION
RJ and AM performed literature search, study design, data collection, data analysis, data interpretation, manuscript writing. JP and HJ participated in study design, data analysis, data interpretation, manuscript writing. JW and QM performed data analysis and participated in manuscript writing.
FUNDING
This work was supported by the
ACKNOWLEDGMENT
Data were collected in part utilizing the Blood and Marrow Transplant Research Analysis Information Network (BRAIN).
SUPPLEMENTARY MATERIALS
Supplementary data related to this article can be found at
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Reena V. Jayani AU - Joseph Pidala AU - Heather Jim AU - Junmin Whiting AU - Qianxing Mo AU - Asmita Mishra PY - 2021 DA - 2021/03/04 TI - Association of Patient-Reported Physical Activity on Allogeneic Hematopoietic Cell Transplant Outcomes JO - Clinical Hematology International SP - 34 EP - 39 VL - 3 IS - 1 SN - 2590-0048 UR - https://doi.org/10.2991/chi.k.210221.001 DO - 10.2991/chi.k.210221.001 ID - Jayani2021 ER -
