Machine Learning Applications in the Diagnosis of Benign and Malignant Hematological Diseases
 , David Shyr2, Anthony D. Sung3,
, David Shyr2, Anthony D. Sung3,  , Shahrukh K. Hashmi4, 5, *
, Shahrukh K. Hashmi4, 5, *- DOI
- 10.2991/chi.k.201130.001How to use a DOI?
- Keywords
- Hematology; machine learning; artificial intelligence
- Abstract
The use of machine learning (ML) and deep learning (DL) methods in hematology includes diagnostic, prognostic, and therapeutic applications. This increase is due to the improved access to ML and DL tools and the expansion of medical data. The utilization of ML remains limited in clinical practice, with some disciplines further along in their adoption, such as radiology and histopathology. In this review, we discuss the current uses of ML in diagnosis in the field of hematology, including image-recognition, laboratory, and genomics-based diagnosis. Additionally, we provide an introduction to the fields of ML and DL, highlighting current trends, limitations, and possible areas of improvement.
- Copyright
- © 2020 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
The fields of machine learning (ML), deep learning (DL), and artificial intelligence (AI) are speculated to change the way that we practice medicine [1–3]. The current medical literature in ML’s and DL’s use is exponentially expanding; however, most applications of these technologies are still limited, with a lack of prospective and real-life applications of the most proposed algorithms [4]. The integration of ML and DL in clinical care is faced with multiple ethical and logistic concerns [5]. However, it is reasonable to say that the field is promising, given the considerable amount of data generated by the current healthcare system and the ability of ML and DL to analyze, process, and identify patterns.
The field of hematology (benign and malignant) is also rapidly expanding, including increasing our molecular/genomic understanding and the number of new therapies joining the pipeline [6]. For instance, genomics has become an integral part of oncology, where it provides diagnostic, prognostic, and therapeutic values [7]. Using deep/machine learning in genomics might help overcome the challenge that a large amount of data imposes, leading to improved diagnosis, identification of genomic patterns, and increased efficiency. The integration of these newer technologies in hematology practice holds an opportunity to improve it.
In this review, we introduce AI tools, particularly ML and DL, in hematology diagnosis. We summarize the significant directions of ML and DL in the current hematology literature and hematopoietic cell transplant.
2. BASICS OF AI, ML, AND DL
2.1. Basic Definitions
Artificial intelligence is a general term that describes the use of technology in accomplishing tasks that would usually need human intelligence, for example, voice or image recognition. Machine learning, on the other hand, is a subset of artificial intelligence. The ML technology enables machines to learn from previous data using statistical approaches and algorithms [8]. The performance of ML algorithms generally improves as more data are used.
Machine learning has two main categories: Supervised and unsupervised. Supervised ML is the most widely used in the medical literature. It uses labeled input and output, which can then be applied to an algorithm to create a function that relates between the input and the output, i.e., y = f(x), where y is output, and x is the input. Supervised ML is usually used to accomplish two main tasks: classification (e.g., support vector machine, naïve Bayes classifier) and regression (e.g., non-linear regression, Bayesian linear regression) [8,9]. Additionally, the incorporation of the ensembles method (combining multiple algorithms to produce a more accurate model) to create decision trees (such methods include random forest, XGBoost Bagging) is another important function of such algorithms.
On the other hand, unsupervised machine learning is used to a lesser extent in medicine. Unlike the supervised process, output data are not needed in unsupervised learning. In addition to supervised and unsupervised learning, semi-supervised learning (or reinforcement) is another category of machine learning that uses a combination of labelled and unlabeled data [8,9]. This approach power stems from its ability to adapt through rewards-like systems to more complex environments. Figure 1 illustrates the different types of machine learning with examples on each.
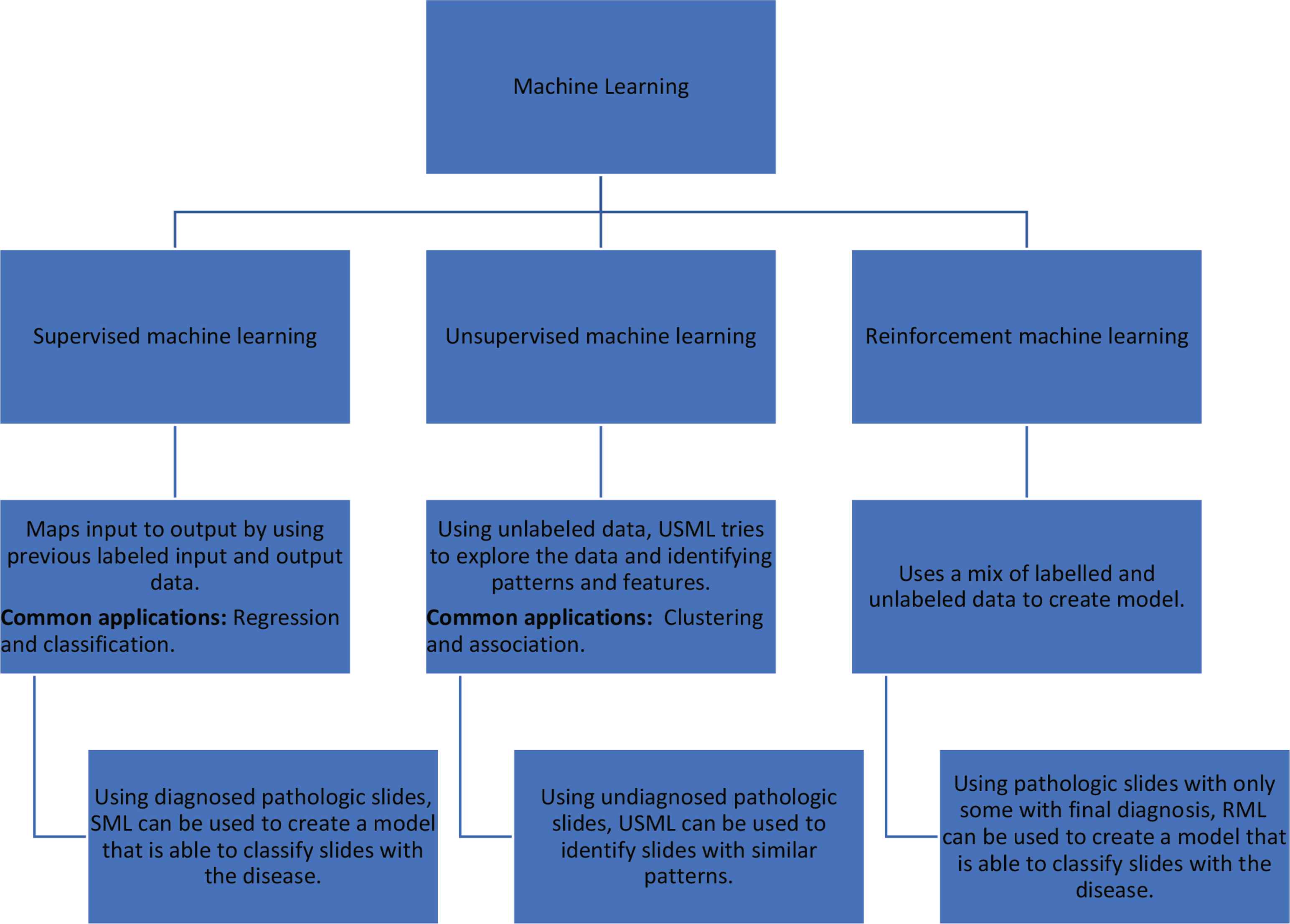
Supervised machine learning and its different subtypes.
Deep learning is a subset of ML which approaches problems in a fashion that is more similar to the human approach. In deep learning, algorithms can deduce the data’s features/patterns through multiple layers of processing (i.e., neural networks) [10]. Two very frequently used neural network approaches include convolutional neural network (CNN) and recurrent neural networks (RNN). CNN is a feed-forward neural network method that is mainly used in medical imaging processing, including pathologic diagnosis of hematologic cancers. In RNN, outputs of prior steps are fed as inputs in subsequent steps. This approach is mainly used in time series analysis and sequence classification (Figure 2). Similar to ML, DL algorithms can use labeled and unlabeled data. The DL popularity has increased in the medical literature in recent years.
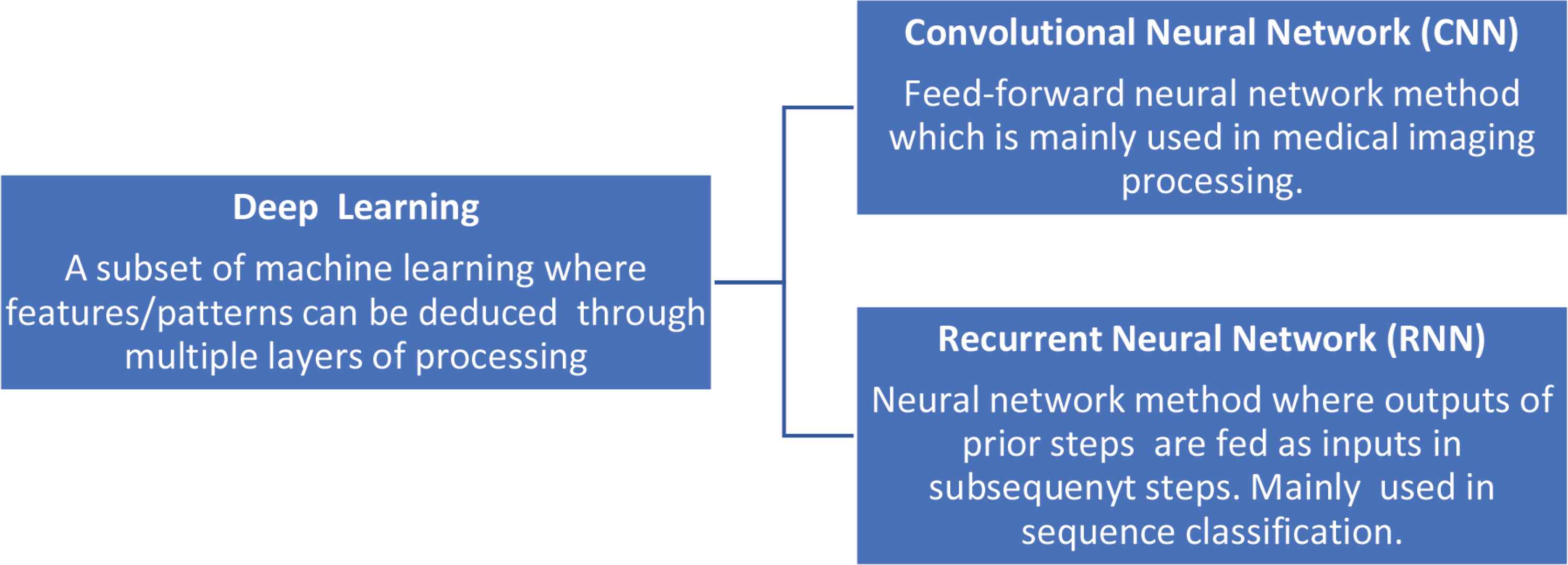
Deep learning with description of two types of neural networks frequently used in medical literature.
2.2. Models’ Evaluation
Applying the different ML and DL methods described above to different medical questions requires measures to ensure that the models are accurate and valid. It is important to note that every model is usually based on a set of data called ‘training set.’ After creating the model, it is generally validated with either another set of data, “validation set”, or using the original data set itself. However, the usual approach is to start with the validation with the original data set, and then use an external “test set” sample.
The hold-out approach involves the process by which the data are usually split into training and validation sets. Typically, the validation set is a new set of data that is not a subset of the training set. This approach ensures that the data can be applied to another/new set of data. A discrepancy in accuracy between the training and validation sets indicates ‘overfitting.’ Overfitting is a phenomenon where the algorithm is modeled very well on the training set to a point where it is not generalizable to other data [8,11]. However, another approach to validation is using the training set itself. A commonly used method is ‘cross-validation.’ In cross-validation, the training set will usually be split into multiple subsets on which the model will be run to confirm accuracy. The number of subsets is generally dictated by how many k-folds of cross-validation, where k is the number of subsets [11].
Moreover, models are evaluated by different metrics. Similar to the evaluation of diagnostic tests, classification algorithms (the majority of diagnostic algorithms) can be assessed based on their ability to identify samples with a specific diagnosis (Figure 3). Thus, concepts such as ‘accuracy’ and ‘precision’ can still be used to evaluate algorithms [12,13]. On the other hand, “recall” can be calculated by the ratio of positive results identified by the algorithm to all the positive samples. Moreover, F1 (F-measure) is a harmonic mean of precision and recall used to measure the not correctly classified results [11].
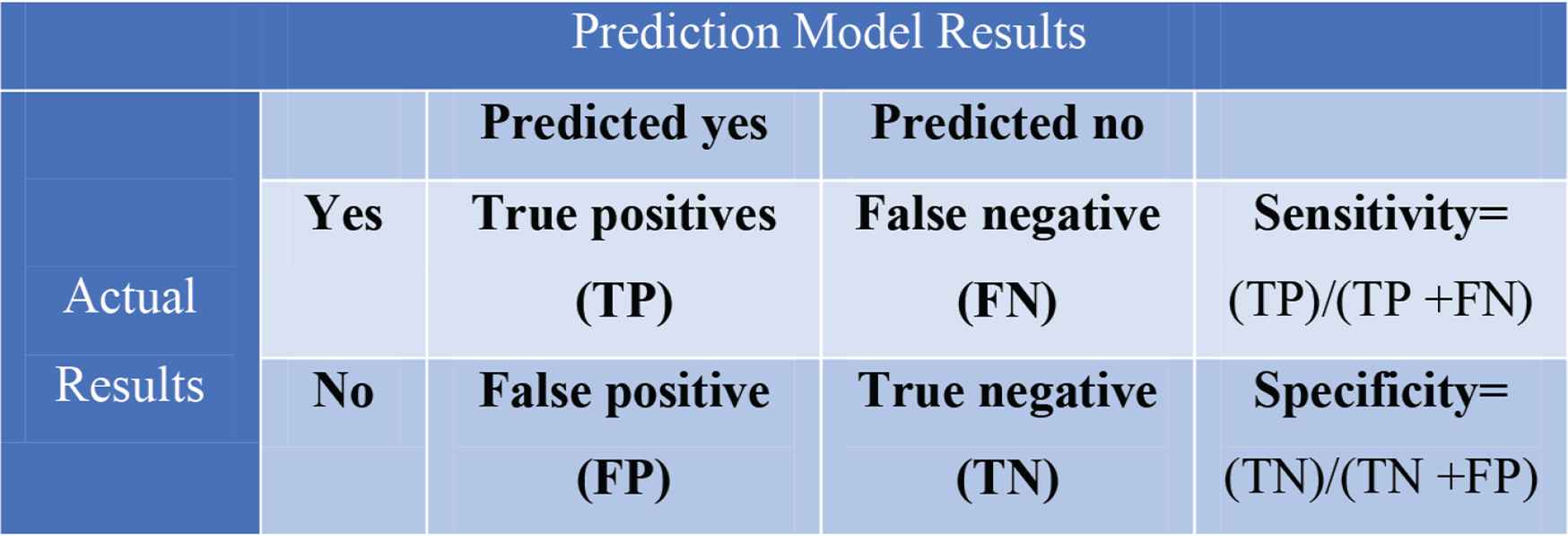
Confusion matrix.
Using the algorithms’ specificity and sensitivity, the receiver operator curve (ROC) can be drawn using a true-positive and false-positive rate. Additionally, the area under the curve (AUC) can be calculated, an operation which has been widely used for years [14] and is now used to evaluate classification algorithms with binary results. There are no exact cut-offs to interpret AUC values; however, AUC of 0.50 and below usually indicates a low classification ability. Table 1 demonstrates the different definitions.
| Index | Description | Comments |
|---|---|---|
| Accuracy | Reflects the models’ ability to achieve correct predictions | (TP + TN)/(TP + TN + FN + FP) |
| Precision | It is the ratio of the true positive results to the total positive results | TP/(TP + FP) |
| Recall | It is the ratio of the true positive results to the total results that are supposed to be positives | TP/(TP + FN) |
| F1 score | A measure that uses precision and recall to identify the not correctly classified results |
|
| The area under the curve | It is the area under the receiver operating characteristic curve, which plots two parameters; true positive rate (sensitivity) over false-positive rate (1-specificity)—used to assess the performance of binary classification models |
TP, true positive; TN, true negative; FP, false positive; FN, false-negative.
Definitions of frequently used evaluation matrices
3. ML AND DL IN THE DIAGNOSIS OF HEMATOLOGICAL DISEASES
The use of ML and DL tools has been investigated in different areas of hematologic diagnoses, including laboratory, histopathology, flow cytometry, and molecular data. The applications of ML and DL in hematology are many. In the discussion below, we highlight ML’s and DL’s multiple applications in diagnosing hematologic diseases. Table 2 lists selected examples of these.
| Hematologic disease | Application | Diagnostic modality | Methodology | References |
|---|---|---|---|---|
| Acute leukemia | Diagnosing AML using histopathology slides | Microscopic | ML-supervised (SVM) | Kazemi et al. [23] |
| Diagnosing ALL using histopathology slides | Microscopy | ML-supervised (Multiple algorithms) | Rawat et al. [22] | |
| Using gene expression profiling (GEP) to diagnose AML | GEP | Multiple | Warnet-Herresthal et al. [39] | |
| Chronic leukemia | Differentiating normal cytometry from flow cytometry indicating CLL | Flow cytometry | Multiple supervised and unsupervised algorithms | Lakoumenta et al. [37] |
| Myeloma | Detecting bone metastasis in myeloma patients | Radiology | ML-supervised (SVM) | Xu et al. [27] |
| Diagnosis of MM | Mass spectrum | DL | Deulofeu et al. [38] | |
| Lymphoma | Differentiating different types of lymphoma | Microscopy | DL (CNN) | Achi et al. [19] |
| Grading follicular lymphoma into a high or low grade | Microscopy | ML-supervised (k-NN) | Fauzi et al. [20] | |
| Using GEP to classify large B-cell lymphoma (DLBCL) | GEP | ML-supervised (SVM) | Zhao et al. [40] | |
| Myelodysplastic syndrome | Detecting MRD in AML and MDS | Flow cytometry | ML-supervised (SVM) | Ko et al. [36] |
| Anemia | Diagnosing anemia via fundoscopic images | Fundoscopic images | DL (CNN) | Mitani et al. [26] |
| Using cell population data parameters to improve the detection of liver disease and anemia in samples with abnormal scattergrams | Laboratory data | ML-supervised (Multiple algorithms) | Bigorra et al. [34] |
ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; MM, multiple myeloma; MDS, myelodysplastic syndrome; MRD, minimal residual disease.
Selected examples of ML and DL use in the diagnosis of hematologic disease
3.1. Image-based Diagnosis and Recognition
Image recognition and diagnosis are essential in multiple diseases, including malignant and benign hematologic diseases. The increase in ML and DL utilization could be attributed to the increased awareness and the efforts to digitalize histopathology slides, including whole slide imaging (WSI) [15,16]. The primary ML function used in image-based diagnosis is the classification of histopathology slides.
The approach of using ML in image recognition and classification usually starts with pre-processing the images, which includes digitally labeling the slides. Slide labeling is particularly important with supervised machine learning, which generally requires the labeling of both the input and the output, a process that introduces a logistical limitations, given the required resources to label many slides [17]. Following that, images are usually segmented into different parts (i.e., cytoplasm and nucleus), followed by identifying features (feature extraction). The ML algorithm can then be applied to the sample (usually with known output), creating a model that can classify images based on their features. The majority of models in the slide-based diagnosis use a binary approach (diagnosis/no diagnosis), which significantly simplifies the real-life complexity of histopathology diagnosis [18]. Subsequently, all models have to be validated internally or externally to ensure applicability. The use of ML and DL is not a guarantee for developing an accurate model, as many factors play a role in achieving higher accuracy models.
Multiple studies have reported on the application of ML and DL in lymphoma. In one of these, Achi et al. [19] utilized the DL methodology, particularly CNN and WSI from two different databases. The established model was able to diagnose three different types of lymphomas (diffuse large B-cell lymphoma, Burkitt lymphoma, and small lymphocytic lymphoma) with an accuracy of 95%, illustrating the utility of ML in more complex medical questions. As a DL approach, CNN is used for image classification by analyzing each image’s features in multiple deep layers. Usually, the initial layers aim at understanding the simple features of the image, subsequently moving up to more complex and sophisticated feature extraction. K-nearest neighbor (k-NN) is another type of algorithm for tackling both classification and regression problems. Fauzi et al. [20] used k-NN to create a model to grade follicular lymphoma into a high or low grade. The model achieved an acceptable AUC of 0.75.
In leukemia, several studies have reported on ML supervised approaches such as support vector machine (SVM), naïve Bayesian, and random forest. Bigorra et al. [21] and Rawat et al. [22] have used SVM for diagnosing acute lymphoid leukemia (ALL). The AUC achieved by both models was 0.74 and 0.84, respectively. SVM is one of the most commonly used ML supervised approaches. It requires the labeled input and output data to analyze and process the input data, and to perform a classification function, as in the case of histopathology diagnosis. Kazemi et al. [23] used SVM in diagnosis acute myeloid leukemia (AML), achieving a model accuracy of 95%. CNN was primarily used in the histopathology diagnosis of ALL, with models achieving accuracy that is close to 95% [24,25].
The ML utility in image recognition goes beyond the histopathology diagnosis of hematologic diseases. With data from the UK Biobank, Mitani et al. [26] used retinal fundal imaging to diagnose anemia via DL, achieving an AUC of 0.88 when used along with other patients’ data. ML was utilized for detecting bone lesions in multiple myeloma patients via analyzing PET/CT scans using various algorithms, including SVM, k-NN, RF, and CNN [27]. The latter outperformed the other ML algorithms used in that study.
Although the magnitude of practice change by such studies is questionable, they demonstrate the power of DL and ML in analyzing patterns, and the importance of having databases that can provide sufficient data to build algorithms. Furthermore, identifying patterns in imaging using AI could help develop prognostic and diagnostic models [28].
Studies that have been done so far in histopathology diagnosis and image recognition are limited in terms of the sample size, which can be attributed to the lack of multi-institutional database-based studies. However, digital histopathology is a growing field, and WSI’s use may provide multiple opportunities, particularly in ML and DL utilization. The challenge in the AI literature remains to establish whether these models will achieve similar results in real-practice [21,23–25].
3.2. Laboratory-based Diagnosis and Flow Cytometry
Multiple studies have utilized ML and DL in laboratory diagnosis and flow cytometric diagnosis of benign and malignant hematological diseases. The integration of AI models in hematology, particularly laboratory hematology, is not new [29,30]. Several knowledge-based equipment types were developed to aid in simple laboratory diagnoses, for instance, iron deficiency anemia or peripheral smear analysis [31,32].
The current literature provides a good sample of the breadth of the possible applications. For instance, one study has used both regression and classification algorithms to predict ferritin levels and increase its accuracy, as it is usually affected by multiple other biologic processes [33]. The model presented achieved an AUC of 0.97.
Bigorra et al. [34] investigated the use of ML to build a model that can use cell population data parameters to improve the detection of liver disease and anemia in samples with abnormal scattergrams. Multiple algorithms have been used, including random forests, naive Bayes classifiers, k-NN, neural networks, and SVM. The later achieved the highest accuracy. Guncar et al. [35] have used the random forest method to create a model that is able to analyze patterns between the different blood indices (which are usually overlooked) to guide clinicians to the five most possible hematologic diagnoses (both benign and malignant) at an early stage of investigation. The model’s accuracy outperformed the evaluation by internal medicine specialists, and was comparable to that of hematology specialists. The random forest algorithm is another supervised ML algorithm that combines multiple decision trees.
There are also data on the utility of flow cytometry-based ML algorithms yielding diagnostic and prognostic information [36,37]. In one study, the authors used SVM to develop multiple algorithms that can detect minimal residual disease in AML and myelodysplastic syndrome, using data from multiparameter flow cytometry [36]. The model utilized results from 5000 bone marrow samples from 1700 patients, and achieved an AUC of higher than 0.90. Other applications included using mass spectrum as in Deulofeu et al. [38] along with DL (using artificial neural networks) to achieve 100% sensitivity and 95% specificity in diagnosing multiple myeloma.
3.3. Applications of ML in Genomics
With the integration of genomics in cancer diagnosis, prognosis, and treatment, ML and DL have been utilized in improving the diagnosis of multiple cancers, including hematologic malignancies. For instance, Warnet-Herresthal et al. [39] used gene expression profiling to create a system that is able to diagnose AML with no expertise intervention. The authors have used multi-dimensional data with multiple algorithms achieving AUC and accuracy of more than 90%. However, as with many other ML articles, the approach was preliminary, and more prospective studies are needed to establish the utility of these models.
Machine learning and DL approaches have also been utilized in the classification of lymphoma using gene expression profile and DNA microarrays [40,41]. On one study, gene expression profiling was used to classify large B-cell lymphoma into its different molecular subtypes using SVM. The model was able to classify the data into germinal center B-cell like (GCB) and non-GCB with an accuracy of more than 90% in the validation cohort [40].
Given the advancements in genomics and the amount of data generated by the cutting-edge technologies, ML and DL offer the analytical tools to approach and use these data in clinical practice. However, high-quality studies and well-established databases will be needed to build models that will be clinically relevant.
3.4. Other Clinical Uses of ML and DL
The previous discussion has highlighted multiple uses of ML and DL in the diagnosis of hematologic diseases. However, ML and DL tools have been used in various other applications, including prognostic models. These applications are beyond the scope of this article; however, below is a discussion of some examples.
The utilization of AI in hematopoietic cell transplantation (HCT) is increasing [42]. The majority of ML and DL studies in HCT are for prognostication, but multiple studies have tried to tackle other problems, including pre-transplant patient’s selection. Donor/recipient and transplantation characteristics were used to create algorithms to improve pre-transplant match results [43,44]. Nonetheless, AI/ML is complex and dependent on multiple factors; thus, some algorithms had suboptimal accuracy or failed validation [43,45]. Graft vs host disease (GvHD) is a significant cause of morbidity after allogeneic HCT. However, the use of ML and DL models in GvHD diagnosis/prediction is still minimal. Current studies demonstrate these approaches’ potential in improving the accuracy of predicting GvHD and its associated prognosis [46,47]. In a study by Lee et al. [48], a super-learner (combining multiple algorithms) was used to create a model to predict acute GvHD. The algorithm achieved a modest AUC of around 0.60, indicating the importance of data selection in building models.
Several HCT-related databases have been established. They are being used in ML and DL studies, mostly by the European Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research [45,49]. Although they are still in their initial phases, these databases’ use and investment might improve ML and DL utilization [50].
Gal et al. [51] used ML to predict complete remission in AML patients utilizing around 75 genes. The k-NN algorithm was used, and the model was able to achieve an AUC of 0.81. Other examples of prognostic models were published in the literature, including the use of single-nucleotide polymorphism data for the prognosis of MM [52], gene expression to predict prognosis in Hodgkin lymphoma [53], and an ensemble algorithm to develop a model to predict the risk of infection complications in chronic lymphocytic leukemia patients [54].
4. FUTURE DIRECTIONS
The field of AI and ML is a promising field that would help to evolve our practice. This review highlights the different trends and applications in the hematology literature. AI uses are so far limited in terms of quality and quantity [4,18]. “Big data” is difficult to obtain with no sufficient databases with internally homogenous data, particularly in histopathology diagnosis where digital histopathology is still in its childhood. New databases should be developed with governance and data collection processes that allow for optimal use of ML algorithms. In a promising step, the American Society of Hematology has announced the development of a data hub to harness big data [55].
The incorporation of ML tools in medical practice faces many challenges. Technical hurdles include data collection and the need for ‘big data’ to create representative algorithms. However, the challenges facing ML integration are multi-faceted. When data are available, creating a model might be technically feasible, but many other issues must be taken into consideration. For instance, the ethics of data sharing and the use of patient information is a significant issue to be addressed. A similar concern is the possibility of, models’ bias, which may deepen health-care disparities by representing only certain groups and increasing racial biases, has aspects which have been previously reported [56]. These issues will require regulations and guidelines to avoid the misuse of data or an increase in bias. Lastly, to be able to meaningfully implement ML models, there is a growing need for them and their tools to be technically well-performing, transparent and explainable to both providers and patients [57].
The currently available data show a limited number of prospective studies in the field of AI, with no evidence to improve clinical outcomes. The ML models reported on different studies lack reproducibility, limiting their applicability. An accurate ML algorithm may not be reproducible on another population/dataset [58]. The costs of reproducing/replicating ML algorithms are huge, introducing a challenging aspect of implementing them in real-practice [59]. Moreover, current utilization of ML does not always yield significantly better clinical outcomes and results, as demonstrated by a recent review of algorithms in the field of heart failure, indicating that more work is needed to unlock the power of ML [60].
The field ML, particularly in hematology, holds the potential for future impact. This should be facilitated by developing databases/hubs, better governance of data collection, and improving current research practices.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
INM and SKH wrote the first draft of the manuscript. All authors vouched for the accuracy and contents of the manuscript.
DISCLOSURES
SKH has received honorarium from Mallinckrodt, Janssen, Novartis and Pfizer & travel grants from Merck, Takeda, Sanofi and Gilead. ADS has received research support from Merck & Co. and Novartis.
REFERENCES
Cite this article
TY - JOUR AU - Ibrahim N. Muhsen AU - David Shyr AU - Anthony D. Sung AU - Shahrukh K. Hashmi PY - 2020 DA - 2020/12/21 TI - Machine Learning Applications in the Diagnosis of Benign and Malignant Hematological Diseases JO - Clinical Hematology International SP - 13 EP - 20 VL - 3 IS - 1 SN - 2590-0048 UR - https://doi.org/10.2991/chi.k.201130.001 DO - 10.2991/chi.k.201130.001 ID - Muhsen2020 ER -
