Use of Biosimilar Granulocyte Colony-Stimulating Factor for Mobilization in Autologous and Allogeneic Hematopoietic Stem Cell Transplantation
- DOI
- 10.2991/chi.d.191008.001How to use a DOI?
- Keywords
- Hematopoietic stem cell transplantation; Biosimilar; Granulocyte colony-stimulating factor; Stem cell mobilization; Filgrastim; Tbo-filgrastim
- Abstract
The biologic medication filgrastim is approved by the Food and Drug Administration (FDA) to mobilize hematopoietic progenitor cells (HPCs) for collection by leukapheresis for autologous hematopoietic stem cell transplant (HSCT). The FDA-approved biologic tbo-filgrastim is currently used off-label for this indication in both autologous and allogeneic HSCT at the Tennessee Valley Healthcare System. The purpose of this review is to compare the efficacy of filgrastim and tbo-filgrastim for this indication. The primary outcomes were the proportion of autologous patients and allogeneic donors with a CD34+ count ≥15 × 103 cells/uL on day 4 of filgrastim or tbo-filgrastim mobilization. The secondary outcome was the use of plerixafor in the autologous population. A total of 469 subjects were identified for inclusion; 367 underwent mobilization for autologous HSCT and 102 for allogeneic HSCT donation. The primary outcome was achieved in 47.5% of patients who received filgrastim compared to 50.2% who received tbo-filgrastim in the autologous population (p = 0.67). Among donors for allogeneic HSCT, there was no difference between those eligible for collection on day 4 of filgrastim or tbo-filgrastim administration (97.6% vs. 100%, p = 0.41). No significant difference was identified in the number of patients requiring plerixafor use in the autologous HSCT population. The use of the biosimilar tbo-filgrastim for mobilization in either autologous HSCT patients or allogeneic HSCT donors has comparable outcomes to that of the biotherapeutic reference product filgrastim at a reduced cost to the healthcare system.
- Copyright
- © 2019 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) is used to treat several malignant and nonmalignant conditions including multiple myeloma, Hodgkin's/non-Hodgkin's lymphomas, acute myeloid/lymphoid leukemias, and myelodysplastic syndromes [1]. According to the Center for International Blood & Marrow Transplant Research, over 22,000 autologous and allogeneic transplants were performed in the United States in 2017 [2]. Currently, methods for harvesting hematopoietic progenitor cells (HPCs) include surgical removal from bone marrow in the donor's hip, mobilization and leukapheresis from peripheral blood, and from umbilical cord blood [1]. To harvest an adequate number of HPCs to achieve engraftment from peripheral blood, granulocyte colony-stimulating factors (G-CSFs) must be used to mobilize HPCs from the bone marrow microenvironment into the peripheral bloodstream [3].
Biologic medications are typically large, complex molecules which can be difficult to characterize and are easily influenced by the process of manufacturing [4,5]. G-CSFs exhibit their mechanism of action by binding to cell surface receptors to increase hematopoietic cell proliferation, differentiation, and other cell functions [6]. One Food and Drug Administration (FDA)-approved G-CSF medication for use in mobilization for stem cell collection is filgrastim (Neupogen®) [6]. Although biologics, like filgrastim, can provide novel and sometimes life-saving treatment modalities, they have a significant cost burden on healthcare facilities [4].
In response to the phenomenon of specialty medications and their high costs, a new drug class of “biosimilars” has emerged. Similar to brand-generic small molecule medications, biosimilars mimic original biologic medications and are defined as being highly similar and having no clinically meaningful differences from an existing reference product [4]. In 2009, the U.S. FDA-approved the Biologics Price Competition and Innovation Act of 2009 (BPCI Act) to allow biosimilar products an expedited approval process resulting in more prompt and affordable medications for patients [4]. In 2012, the FDA approved the biologic tbo-filgrastim (Granix®) similar to the reference product filgrastim (Neupogen®) [7]. Like its reference product, tbo-filgrastim is a myeloid growth factor that is FDA approved to reduce the duration of severe neutropenia in patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically significant incidence of febrile neutropenia [7].
Currently, the American Society for Blood and Marrow Transplantation guidelines do not address the use of biosimilar versions of G-CSFs for HPC mobilization, and recognize filgrastim as the preferred agent for this indication [8]. The National Comprehensive Cancer Network guidelines discuss the use of biosimilar G-CSFs for a variety of indications. They recommend these products as part of supportive care following HSCT, but do not provide strong recommendations regarding G-CSFs use for mobilization of HPCs prior to HSCT. The guidelines suggest it is reasonable to substitute filgrastim with biosimilar drugs for mobilization in the autologous HSCT setting and discuss the possible use for mobilization of HPCs in the allogeneic HSCT population. However, they recognize there is minimal, lower-level evidence available to support these recommendations [9]. Currently, tbo-filgrastim is not FDA-approved for the mobilization of HPCs in HSCT, though it has been used off-label at the Tennessee Valley Healthcare System (TVHS) since 2015 for this indication [7].
While many studies have compared the use of biosimilar products to filgrastim for reducing neutropenic duration after myelosuppressive chemotherapy, few have focused on their use in HPC mobilization. Recent studies examining the use of filgrastim-sndz or tbo-filgrastim in the mobilization setting lack long-term outcomes and often include small population sizes. In a 2011 prospective trial by Lefrere et al., 40 patients were given biosimilar filgrastim and compared to 41 historical patients with matching characteristics. While researchers found no clinical differences between the two products, the study was limited by the size of the population [10]. Similar results were found in a 2013 retrospective study by Publicover et al. and a 2015 retrospective study by Elayan et al. While these studies included larger populations, 154 and 185 patients respectively, only patients undergoing autologous HSCT were examined [11,12].
More recently, a 2019 study published by Potter et al. included 71 autologous patients and found no significant differences in CD34+ counts during stem cell mobilization or neutrophil engraftment between filgrastim and biosimilar filgrastim [13]. Additionally, an abstract published in 2019 by Anders et al. examined 186 patients undergoing autologous transplantation and compared filgrastim to the biosimilar filgrastim-sndz. This study found no significant difference in the number of CD34+ cells collected [14]. Both of these recently published retrospective studies were again limited in population size and to the autologous population. The purpose of our study is to compare the use of filgrastim to the biologic product tbo-filgrastim for HSCT in both autologous and allogeneic donor populations.
2. MATERIALS AND METHODS
This was a single-center, retrospective, observational cohort study reviewed by the institutional review board. Study participants included patients or healthy related donors if they were ≥18 years of age and received filgrastim or tbo-filgrastim for HPC mobilization between September 1, 2012 and August 31, 2018 at the TVHS HSCT clinic. Patients were excluded if they received any other biotherapeutic G-CSF, if they underwent chemotherapy mobilization, or had undergone a previous autologous HSCT. Patients must have had a CD34+ count measured on day 4 of filgrastim or tbo-filgrastim administration. Additionally, patients were excluded if they were pregnant or breastfeeding, deceased before completion of apheresis or had a known hypersensitivity to filgrastim, plerixafor, or E. coli-derived products.
The protocol for HPC mobilization at this institution involves administration of a G-CSF agent for a minimum of four consecutive days. This process remained unchanged during the study period. The dosing of these agents is weight based (10 mcg/kg) and is given in a single daily dose. On the fourth day of the mobilization process, a peripheral blood CD34+ count is measured. While awaiting CD34+ lab results for clearance to proceed with collection, patients receive the fourth dose of G-CSF. All CD34+ counts were calculated by the same method throughout the study period. If insufficient cell numbers were measured, patients received one dose of plerixafor on the evening of the fourth day with the intention to undergo HPC collection on day 5. For patients to be eligible for apheresis on day four of G-CSF administration, a CD34+ count of ≥15 × 103 cells/uL is needed.
The primary endpoints of this study were to compare the rates of patients and donors eligible to undergo apheresis on day 4 of HPC mobilization who received the biosimilar tbo-filgrastim versus the biotherapeutic reference product filgrastim for HPC mobilization in both the autologous and allogeneic HSCT settings. The secondary objective was to compare the rates of patients who required plerixafor use in the autologous transplantation setting. For both primary objective populations, eligibility was defined using the surrogate lab marker CD34+. Patients were deemed eligible for apheresis if the CD34+ count was ≥15 × 103 cells/uL on day 4 of G-CSF administration.
2.1. Statistical Analysis
The primary endpoint, eligibility for collection on day 4 of GCSF administration based on CD34+ count, has not been previously examined. However, in an article by Murata et al., it was found that approximately 70% of patients were eligible for collection on day 4 of mobilization [15]. Additionally, the FDA guidance for industry on demonstrating interchangeability of biosimilars with a reference product states that the acceptable difference of biosimilars to the reference product for pharmacokinetic parameters (including AUC and Cmax) should be between 80% and 125% [16]. Therefore, using these two points with an alpha set at 0.05 and beta set at 0.20, it was calculated that a sample size of 103 patients in each arm was needed to provide adequate power.
Patient demographics are presented using descriptive statistics to compare baseline characteristics of the tbo-filgrastim and filgrastim groups in both the autologous and allogeneic HSCT settings. Continuous variables were analyzed using t-tests and categorical variables were analyzed using two-tailed Fishers exact tests.
3. RESULTS
From September 2012 through August 2018, a total of 534 patients were identified as having received tbo-filgrastim or filgrastim for HSCT mobilization. Of those, 65 met the exclusion criteria, resulting in the final population size of 469. Reasons for exclusion included patients undergoing chemotherapy mobilization (46/534), mobilization with an agent other than filgrastim or tbo-filgrastim (1/534), those who did not receive at least 4 days of G-CSF therapy during the mobilization process or did not have a CD34+ count measured on day 4 (13/534), and those who had undergone a previous autologous transplantation (5/534). Of the 469 patients included in the study, 367 underwent mobilization for autologous HSCT and 102 for allogeneic HSCT donation. The patient inclusion and exclusion process is outlined in Figure 1. When comparing the two groups, the baseline characteristics were found to be similar (Table 1).
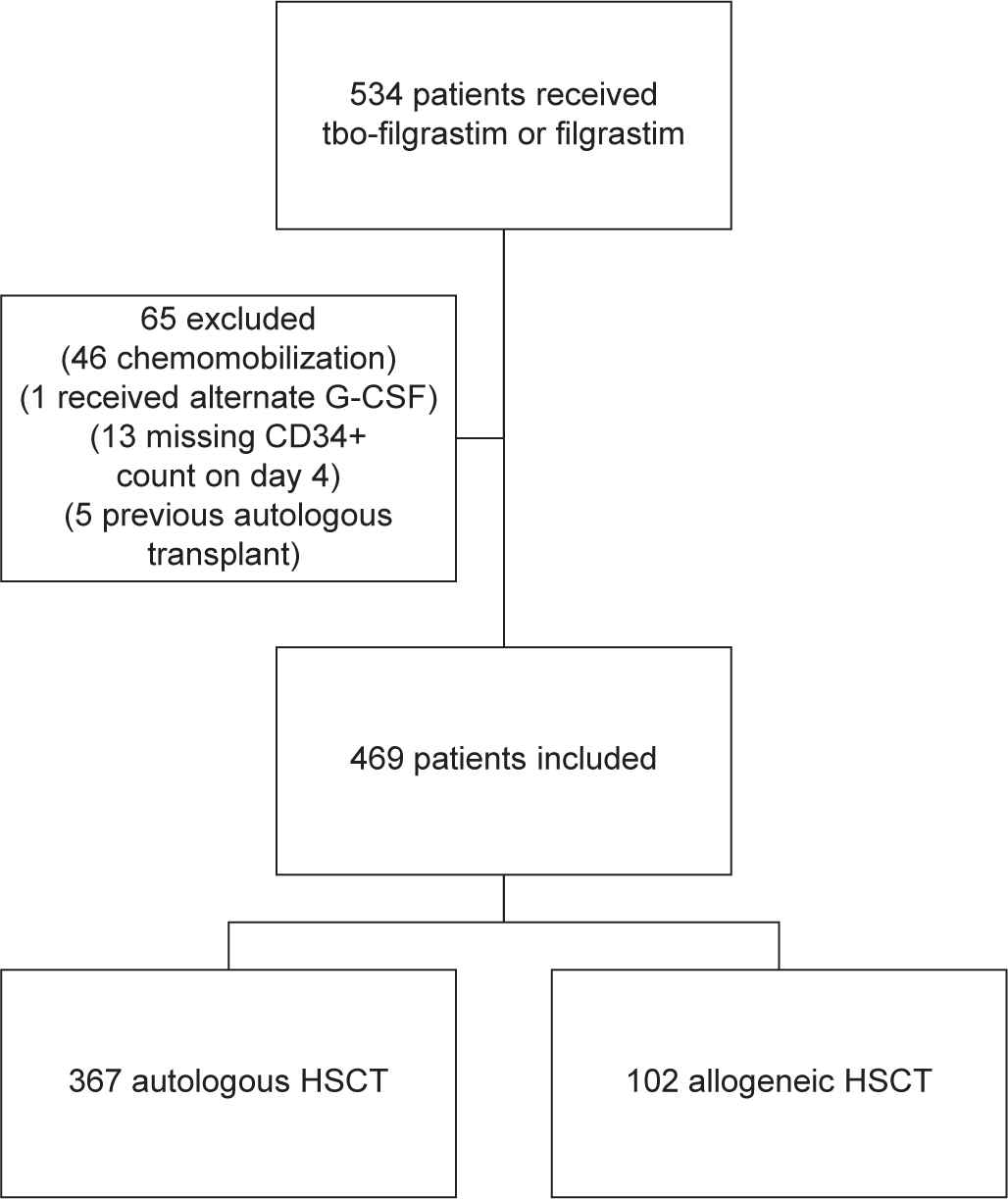
Patient inclusion and exclusion process.
| Autologous Hematopoietic Stem Cell Transplantation |
Allogeneic Hematopoietic Stem Cell Transplantation |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Filgrastim (N = 160) | Tbo-Filgrastim (N = 207) | p-Value | Filgrastim (N = 42) | Tbo-Filgrastim (N = 60) | p-Value |
| Age-years | 61 ± 8.4 | 61 ± 8.5 | 0.54 | 53 ± 12.0 | 48 ± 14.1 | 0.06 |
| Male gender | 153 (95.6%) | 190 (91.8%) | 0.20 | 23 (54.8%) | 35 (58.3%) | 0.84 |
| Weight (kg) | 98 ± 18.1 | 99 ± 21.9 | 0.52 | 90 ± 21.0 | 90.12 ± 18.1 | 0.97 |
| Race | ||||||
| White | 107 (66.9%) | 128 (61.9%) | 0.33 | 26 (61.9%) | 11 (18.3%) | 0.0001 |
| African American | 42 (26.2%) | 57 (27.5%) | 0.81 | 3 (7.1%) | 13 (21.7%) | 0.0561 |
| Other | 5 (3.1%) | 1 (0.5%) | 0.09 | 1 (2.4%) | 1 (1.7%) | 1.0 |
| Unknown | 6 (3.8%) | 21 (10.1%) | 0.03 | 12 (28.6%) | 35 (58.3%) | 0.0045 |
| Transplant indication | ||||||
| Multiple myeloma | 104 (65%) | 147 (71%) | 0.26 | N/A | N/A | N/A |
| Non-Hodgkin's lymphoma | 43 (26.9%) | 40 (19.3%) | 0.10 | |||
| Hodgkin's lymphoma | 8 (5%) | 12 (5.8%) | 0.82 | |||
| Other | 5 (3.1%) | 8 (3.9%) | 0.78 | |||
| Estimated GFR | 80.6 ± 22.7 | 80.8 ± 27.4 | 0.92 | 83.3 ± 18.3 | 88.3 ± 18.1 | 0.1747 |
| Average G-CSF dose administered | 1046.6 ± 121.3 | 1062.9 ± 171.3 | 0.29 | 997.1 ± 117.8 | 1019.3 ± 100.0 | 0.3224 |
GFR = Glomerular filtration rate.
Baseline characteristics.
There was no significant difference in the number of autologous HSCT patients eligible for collection on day 4 between filgrastim and tbo-filgrastim. The primary endpoint was reached in 76/160 patients receiving filgrastim and 104/207 patients receiving tbo-filgrastim (47.5% vs. 50.2%; p = 0.67). Additionally, there was no difference in donors undergoing mobilization for allogenic HSCT. Of the 102 donors, 41/42 (97.6%) in the filgrastim group were eligible for collection on day 4 of G-CSF administration compared to 60/60 (100%) in the tbo-filgrastim group (p = 0.41) (Table 2).
| Group | Outcome | Filgrastim (160) | Tbo-Filgrastim (207) | p-Value |
|---|---|---|---|---|
| Autologous | CD34+ count of ≥15 × 103 cells/uL on day 4 of mobilization | 76/160 (47.5%) | 104/207 (50.24%) | p = 0.67 |
| Allogeneic | CD34+ count of ≥15 × 103 cells/uL on day 4 of mobilization | 41/42 (97.6%) | 60/60 (100%) | p = 0.41 |
Results primary outcomes.
When the primary outcome was further analyzed based on cancer type for patients undergoing autologous transplantation, no difference was seen in the filgrastim group versus the tbo-filgrastim group for patients eligible for apheresis on day 4 of HSCT mobilization (Table 3).
| Cancer type | Filgrastim (160) | Tbo-Filgrastim (207) | p-Value |
|---|---|---|---|
| Multiple myeloma | 64/104 (61.5%) | 84/147 (57.1%) | p = 0.52 |
| Non-Hodgkin's lymphoma | 8/43 (18.6%) | 12/40 (30%) | p = 0.31 |
| Hodgkin's lymphoma | 2/8 (25%) | 2/12 (16.7%) | p = 1.0 |
| Other | 2/5 (40%) | 6/8 (75%) | p = 0.29 |
Primary outcome for patients undergoing autologous HSCT by cancer type.
A secondary endpoint sought to evaluate the use of plerixafor in the autologous HSCT patient population. There was no difference between those who received either filgrastim or tbo-filgrastim (40% vs. 48.8%; p = 0.11) (Table 4).
| Outcome | Filgrastim | Tbo-Filgrastim | p-Value |
|---|---|---|---|
| Patients undergoing autologous HSCT mobilization requiring plerixafor | 64/160 (40%) | 101/207 (48.8%) | p = 0.11 |
Results secondary outcome.
In addition to these previously discussed endpoints, average cell dose collected during apheresis was assessed for autologous transplantations. The average cell dose collected for the tbo-filgrastim patients was 5.5 × 106 CD34+ cells/kg and 5.2 CD34+ cells/kg for the filgrastim group (p = 0.21). This information was not available for donors undergoing apheresis for allogeneic HSCT.
4. DISCUSSION
This study sought to ensure that equivalent care was provided to patients whether they received a reference product or biosimilar medication during HPC mobilization. The subjects undergoing autologous HSCT were representative of an elderly population as they were primarily older, white males that were being treated with HSCT for a variety of malignant conditions. Our findings showed no difference in the number of patients eligible for apheresis on day 4, demonstrating that tbo-filgrastim is equally efficacious in mobilizing HPCs into the peripheral blood as the reference biologic product. Tbo-filgrastim is a reasonable choice for mobilization of HPC for both autologous and allogeneic transplants at a reduced cost to the healthcare system.
Even though no difference between groups was noted, the study was adequately powered to detect a difference for the autologous population and is one of the largest projects comparing biologic medications for this indication. Furthermore, the use of tbo-filgrastim did not result in higher median drug doses being administered, nor did patients receiving tbo-filgrastim required more plerixafor use, both of which justify the formulary decision to use tbo-filgrastim over filgrastim from an efficacy and cost-saving standpoint at our healthcare system.
The primary endpoint of the proportion of patients or healthy related donors with a CD34+ count of ≥15 cells/uL on day 4 was utilized, as it is the most appropriate and accurate method to assess G-CSF's ability to mobilize stem cells out the marrow and into the peripheral blood. Outcomes assessed in previously published studies—including cell dose collected, number of hours on the apheresis machine, blood volume filtered, viability of collection, and even disease outcomes such as mortality and relapse rates—can be influenced by many other variables, including individual facility collection processes, apheresis procedures, and post-transplant care.
There are a few limitations of this study to consider. First, subjective data such as patient tolerability of the medication compared to the biotherapeutic reference product could not be assessed, due to the retrospective nature of the study. Second, this project was unable to assess variability of clinical judgment between BMT providers. While the current protocol recommends that patients should reach a CD34+ count ≥15 cells/uL prior to apheresis, individual providers may deviate from the protocol, if deemed clinically appropriate. When using this prerogative, there were instances when patients underwent apheresis, both successful and not, prior to reaching the target cell count. Therefore, the success or failure of clinical judgment and subsequent deviations from the protocol was not adequately reflected in the standardized primary endpoint of this study. Additionally, the study was not adequately powered to detect a difference in the allogeneic HSCT donor population due to a smaller sample size. Despite the inability to meet power for this group, the sample size was larger than most published studies in this population, and successful mobilization rates were extremely high in both groups.
This study supports the continued off-label use of tbo-filgrastim in HPC mobilization in both the autologous and allogeneic populations with no difference in efficacy. However, there is also a significant financial incentive for use of tbo-filgrastim in HSCT. TVHS performs an average of 120 HSCT annually. Of these, approximately two-thirds are autologous and one-third allogeneic. Using the average wholesale price of filgrastim and tbo-filgrastim, it is estimated that the annual cost savings for the healthcare system when using tbo-filgrastim in place of filgrastim for the HSCT mobilization is close to $90,000 per 120 patients. In addition to the potential cost-savings that biosimilar products can have, they also can present a potential solution for managing drug shortages of biologic reference products [17]. Future directions to investigate include the development of a multicenter, prospective trial to provide a more homogenous patient population. Such a trial would have the ability to assess adverse effects and other variables unavailable for collection in this retrospective study.
5. CONCLUSION
There was no significant difference between the use of filgrastim and the biologic tbo-filgrastim for mobilization of HPC for patients in the autologous HSCT or donors in the allogeneic HSCT setting. Additionally, there was no difference in the use of plerixafor in the autologous population.
CONFLICT OF INTEREST
None of the authors have any personal or financial conflicts of interest to report in relation to this manuscript.
AUTHORS' CONTRIBUTIONS
Dr. Eplin as the corresponding author was the primary investigator and the individual responsible for creation of the research question. Drs. Jackson and Smith were responsible for data collection, analysis, and development of the manuscript for publication. Dr. Salvig, along with those previously mentioned, worked closely to create the study protocol. All authors listed were responsible for final edits, evaluation, and preparation of the manuscript for publication.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr. Terry Hoffman and Dr. Regina Cassidy for their assistance in informatics and data collection and for identifying patients for study inclusion, as well as the members of the TVHS Resident Research Advisory Board for their contribution to the efforts of this project.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Dwight D. Eplin AU - Anna D. Jackson AU - Austin M. Smith AU - Brent Salvig AU - Wichai Chinratanalab AU - Bipin N. Savani PY - 2019 DA - 2019/10/14 TI - Use of Biosimilar Granulocyte Colony-Stimulating Factor for Mobilization in Autologous and Allogeneic Hematopoietic Stem Cell Transplantation JO - Clinical Hematology International SP - 229 EP - 233 VL - 1 IS - 4 SN - 2590-0048 UR - https://doi.org/10.2991/chi.d.191008.001 DO - 10.2991/chi.d.191008.001 ID - Eplin2019 ER -
