The Impact of First Complete Remission by PET-CT and Time to Next Treatment on Survival of Follicular Lymphoma Patients
- DOI
- 10.2991/chi.d.190528.001How to use a DOI?
- Keywords
- Follicular lymphoma; PET-CT response; Time to next treatment (TTNT)
- Abstract
We retrospectively analyzed the impact of initial positron emission tomography and computed tomography (PET-CT) complete remission (CR) and time to next treatment (TTNT) on patient outcome in follicular lymphoma. Between 2002 and 2014, 150 patients could be evaluated for treatment response and long-term outcome. The CR after first line treatment with either rituximab-cyclophosphamide, oncovin, and prednisolone (R-COP) or rituximab-cyclophosphamide, doxorubicin, oncovin, and prednisolone (R-CHOP) was 89% and partial response (PR) was 7%. The 5- and 10-year survival rates were 86.0% and 62.6%, respectively. In five years, 11% of patients had died of lymphoma and 3% from other causes. Forty-seven patients (31%) underwent a second line of treatment comprising 19 (40%) with a TTNT shorter than 24 months and 28 (60%) longer than 24 months. There was no difference in overall survival (OS) between R-COP (86%) and R-CHOP (77%) at 5 years, but there were more next treatment events in the R-COP compared with the R-CHOP group on longer follow-up (60% versus 35% at 8 years). For PET-CT response, there was a significant OS difference between initial CR and PR patients (88% versus 70%, p < 0.01), and a longer TTNT was seen in initial CR patients. Patients with a TTNT longer than 24 months had better OS compared with patients with a shorter TTNT (93% versus 54% at 5 years, p < 0.01). In conclusion, patients with initial PET-CT CR and TTNT longer than 24 months had better OS compared with those achieving only PR and shorter TTNT. PET-CT CR should be considered the treatment goal during initial treatment, and more aggressive treatment should be considered for patients with a TTNT of less than 24 months.
- Copyright
- © 2019 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Follicular lymphoma (FL) is an indolent hematologic malignancy with longer survival as compared with aggressive lymphoma [1], albeit with a remittent and relapsing course that is often difficult to cure. Patients can be observed for extended periods of time without treatment until they develop symptoms fulfilling the criteria of Groupe d'Etude des Lymphomes Folliculaires (GELF) [2]. Therefore, achievement of durable remission with hopes of translating to longer progression free survival (PFS) and even overall survival (OS), is the goal of treatment. Currently, chemo-immunotherapy with or without maintenance immunotherapy, for example, rituximab, is the treatment of choice [3]. Some studies have shown a better outcome in initial positron emission tomography-computed tomography (PET-CT) complete remission (CR) patients who were persistently progression-free over 24 months as compared with those who had progression of disease (POD) within 24 months (POD24) [4]. This study is a retrospective analysis of the outcome of our FL patients according to initial PET-CT response and time to next treatment (TTNT) longer or shorter than 24 months (TTNT24). We chose TTNT24 over PFS24 or POD24 because we know that radiographic relapse of FL is not an absolute indication to treat, unless symptoms occur or the GELF criteria are fulfilled. Therefore, TTNT24 provides a more accurate measurement of a treatment free period.
2. PATIENTS AND METHOD
Between 2002 and 2014, 174 newly diagnosed FL patients were treated at our institute. Of these, only 150 could be evaluated for treatment response and long-term outcome. All patients underwent histopathology diagnosis or confirmation at our hospital. The initial staging workup encompassed complete blood counts, biochemistry including LDH, HBsAg (HBV-DNA by PCR if HBsAg positive), anti-HBs, anti-HBc, anti-HCV, HIV combo antibody, plain film chest x-ray, PET-CT, and bilateral bone marrow biopsy. We gave entecavir prophylaxis to patients who were positive for HBsAg or anti-HBc.
2.1. Study Design and Treatment
Patients received four to six cycles of R-CHOP or R-COP with or without involved field irradiation. For relapsed patients, retreatment with R-CHOP or R-COP, bendamustine, fludarabine, or additional salvage therapy including high dose chemotherapy (HDC) and autologous stem cell transplantation (ASCT), and any post-ASCT consolidation therapies were performed at the discretion of the treating physicians according to institutional practices.
2.2. Study Assessment
We performed PET-CT before the start and at the end of treatment (EOT), which occurred 21 to 28 days after the last dose of drugs. Responses were assessed by the investigator per the Revised Response Criteria for Malignant Lymphoma [5]. The PET scan metabolic uptake was graded using the Deauville 5-point scale with a score of 1–3 considered a complete metabolic response (CMR) [6,7].
2.3. Statistical Analysis
The primary efficacy end points were OR/CR rate and TTNT after the completion of treatment. The secondary end point was OS. The CR rate was defined as the proportion of patients with CR at EOT. OR was defined as the proportion of patients with CR or partial response (PR) at EOT. The TTNT was defined as the interval between the end of frontline and the start of salvage treatment. The Kaplan–Meier method and log-rank test were used to compare OS rates of either PET-CT CR or PR, treatment groups of R-CHOP or R-COP, and TTNT groups of longer or shorter than 24 months. We also compared the probability of next treatment in different groups of patients and initial PET-CT responses. All p values < 0.05 were considered significant.
3. RESULTS
One hundred and fifty patients were available for evaluation (Table 1). Their median age was 53.4 years and 47% were male. Low, intermediate, and high FL international prognostic index (FLIPI) scores of patients were 26.0%, 32.7%, and 41.3%, respectively. The most common first line treatment was R-CHOP or R-COP (78%), starting between 1 and 3 months (range, 0.5–137 months) after diagnosis. Initial PET-CT responses were CR (87%), PR (7%), no response (4%), and not yet treated at time of evaluation (2%). Forty-seven patients (31%) had disease relapse and needed salvage treatment, of which 19 (40%) started the next treatment within 24 months, and 28 (60%) started later than 24 months. The censored 5-year OS was 83%, and 14% of patients died, including 11% from lymphoma and 3% from other causes.
| Follicular Lymphoma | Total | N 150 |
(%) 100% |
|---|---|---|---|
| Age: Years (Mean ± S.D.) | 53.4 ± 11 | ||
| Gender | |||
| F | 79 | 53 | |
| M | 71 | 47 | |
| FLIPI score | |||
| 0, 1 | 39 | 26 | |
| 2 | 49 | 33 | |
| 3, 4, 5 | 62 | 41 | |
| First treatment | |||
| R-CHOP/R-CEOP | 61 | 41 | |
| R-COP/R-CP | 55 | 37 | |
| Others | 31 | 21 | |
| Not yet treated | 3 | 2 | |
| R Maintenance | 35 | 23 | |
| Months to first treatment | |||
| Median | 0.5 | ||
| (Min-Max) | (0.5,137) | ||
| (P75–P90) | (1, 3) | ||
| First PET-CT response | |||
| CR | 130 | 87 | |
| PR | 10 | 7 | |
| No response | 7 | 5 | |
| Not yet treated | 3 | 2 | |
| Next treatment | 47 | 31 | |
| Time to next treatment (TTNT) | |||
| ≤24 m | 19 | 40 | |
| >24 m | 28 | 60 |
R-CHOP: rituximab-cyclophosphamide, doxorubicin, oncovin, and prednisolone; R-CEOP: rituximab-cyclophosphamide, epirubicin, oncovin, and prednisolone; R-COP: rutiximab-cyclophosphamide, oncovin, and prednisolone; R-CP: rituximab-cyclophosphamide, and prednisolone; CR: complete response; PR: partial response.
Patients' characteristics.
There were no significant differences in OS and TTNT between patients who underwent R-CHOP or R-COP treatment induction therapy. However, there were more next-treatment events in the R-COP group compared with the R-CHOP group on longer follow-up (60% versus 35% at 8 years) (Figure 1).
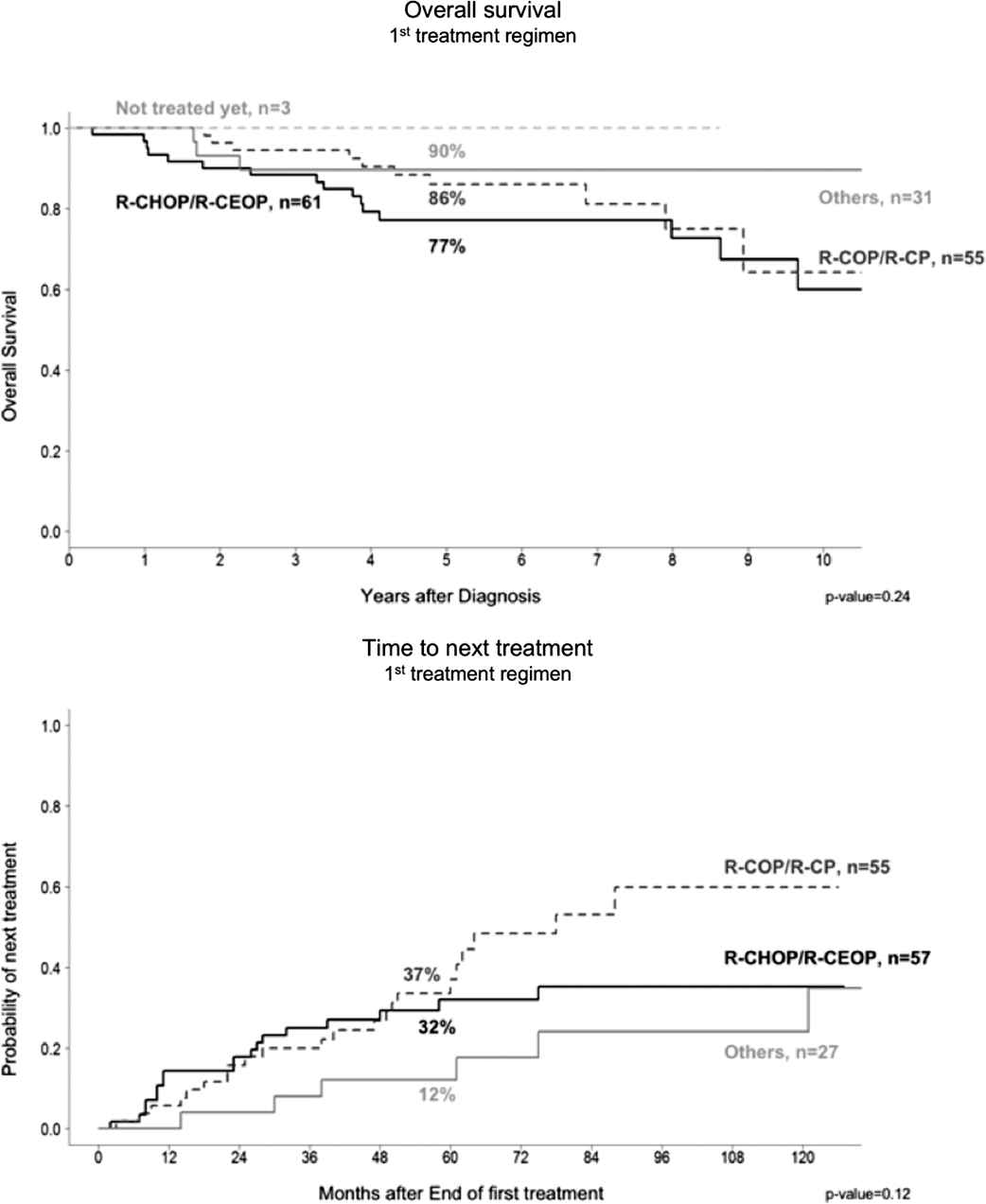
The choice of initial regimens of either R-CHOP or R-COP showed similar overall survival, however, there was a trend to a longer time to next treatment (TTNT) treatment after 60 months.
For PET-CT response, there was a significant OS difference between CR and PR patients (88% versus 70%, p < 0.01), and a longer TTNT was seen in patients achieving CR (Figure 2). Patients with a TTNT longer than 24 months had better OS as compared with those with a TTNT shorter than 24 months (93% versus 54% at 5 years, p < 0.01) (Figure 3).
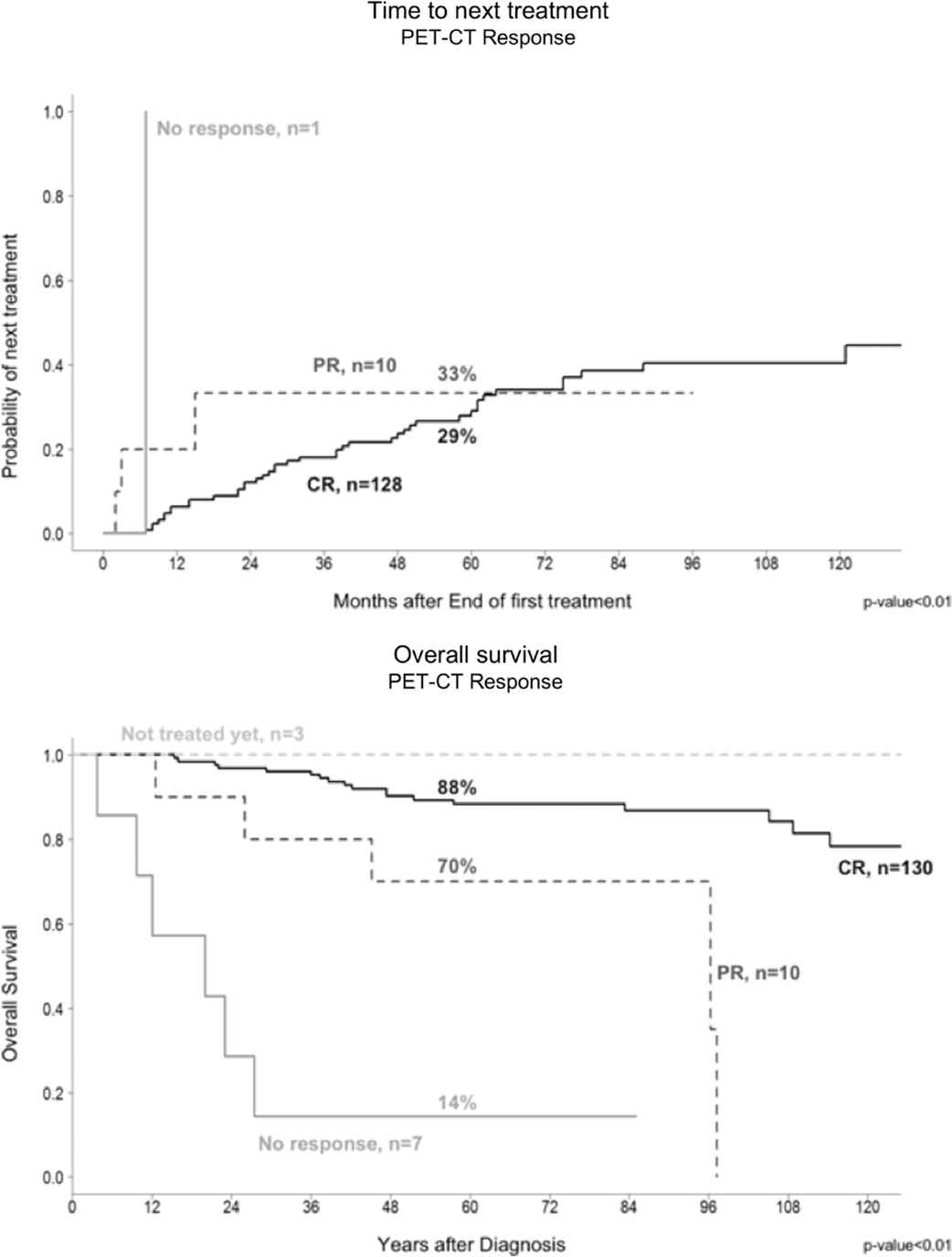
Initial PET-CT response showed significantly longer time to next treatment (TTNT) and better overall survival for complete remission (CR) patients compared with the non-CR group.
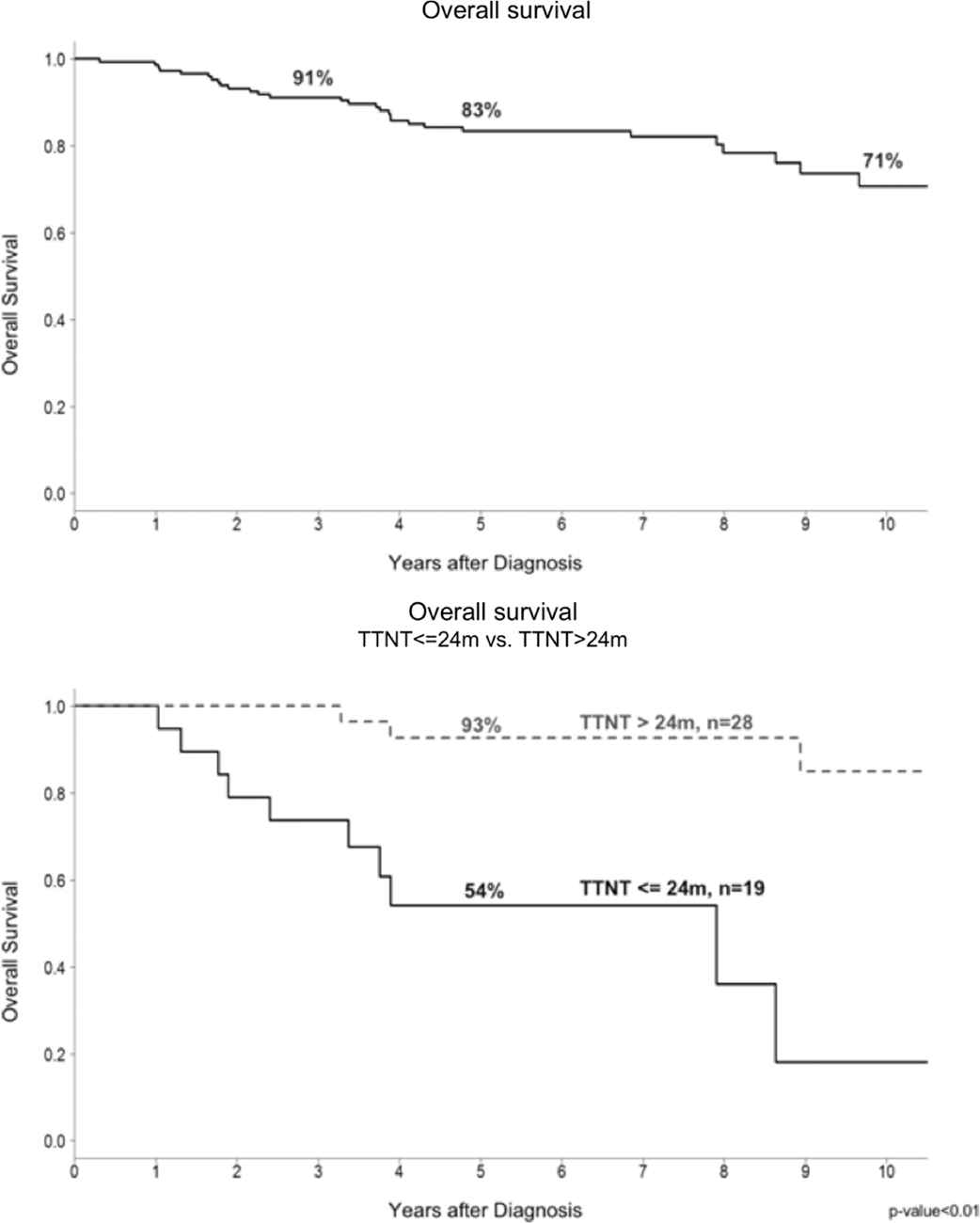
The censored overall survival was 83% and 71% at 5 years and 10 years, respectively. The time to next treatment (TTNT) longer than 24 months group had significantly better overall survival than TTNT shorter than 24 months of treated patients.
4. DISCUSSION
FL is the second most common subtype of non-Hodgkin lymphoma, characterized by an indolent clinical course and a continuous pattern of relapse. A retrospective analysis of long-term outcomes showed significant improvement in OS after the introduction of anthracycline, aggressive chemotherapy, purine analogues, and rituximab in the past 50 years [8]. At the present time, combination chemo-immunotherapy is the initial treatment of choice for patients with FL.
The median OS of most FL patients is between 14 and 20 years; however, about 20% of patients exhibit earlier progression and an even higher mortality. A multicenter study from the USA of 588 patients showed that 19% had POD in 24 months (POD 24) after induction with R-CHOP and the 5-year OS was lower in the early POD than in the reference group (50% versus 90%) [9].
In our study, we chose TTNT rather than PFS or POD as the end point because we did not have to periodically follow up image studies after the EOT, and did not necessarily immediately treat asymptomatic relapsed patients. TTNT is a good primary outcome measure in an indolent disease where patients need subsequent treatment, and also a more stringent measurement of status of disease relapse and treatment-free duration. We found that patients who achieved initial PET-CT-based CR have a longer TTNT and OS than patients achieving PR (88% versus 70% at 5 years, p < 0.01), similar to the result of the PRIMA trial participants [10]. Patients with TTNT longer than 24 months also had a better OS (93% versus 54% at 5 years, p < 0.01). The OS was similar whether the initial treatment was with R-CHOP or R-COP, but a trend for a longer TTNT was seen in the R-CHOP group.
Two issues were raised in our study: one is whether a more aggressive induction chemo-immunotherapy, for example, obinutuzumab plus CHOP, or other regimen could result in better initial PET-CT CR and, thus, better survival. The other question is whether it is necessary to give additional treatment for initial PET-CT PR patients until CR is achieved.
In the randomized phase III (StiL trial) which compared bendamustine plus rituximab (BR) with R-CHOP in 514 patients with advanced follicular, indolent, and mantle cell lymphomas, BR showed a superior median PFS (69.5 versus 31.2 months) for all histologic subtypes, except marginal zone lymphoma, and produced less toxicity. There was, however, no difference in OS (70% versus 66% at 10 years) [11,12]. In another international phase III BRIGHT trial, 447 previously untreated patients with advanced stage of follicular (n = 314), mantle cell (n = 74), or other indolent lymphoma were randomly assigned to six cycles of BR or to R-CHOP or R-COP. BR treatment resulted in a similar CR (31% versus 25%) and overall response rate (97% versus 91%). Preliminary data suggest that BR resulted in improved PFS (66% versus 56% at 5 years) for the group as a whole, a finding that lost statistical significance when the patients with mantle cell lymphoma were removed from the analysis and there was no difference in OS [13,14]. Another open-label, multicenter, randomized trial enrolled 534 patients with previously untreated stages II to IV FL and showed R-CHOP and rituximab-fludarabine and mitoxantrone (R-FM) to be superior to rituximab-cyclophosphamide, vincristine, and prednisone (R-CVP) in terms of three-year time to treatment failure and PFS; however, R-FM resulted in higher rates of toxicity and second malignancies. Therefore, no specific regimen is better than R-CHOP during induction chemotherapy [15] at the present time.
In the Gallium study, an international, open-label, randomized phase III trial comparing rituximab-based chemotherapy with the anti-CD20 monoclonal antibody (obinutuzumab)-based chemotherapy showed similar estimated rates of overall response (89% versus 87%), CR (20% versus 24%), and OS (94% versus 92% at 3 years), and similar rates of histologic transformation in the two groups. Obinutuzumab was significantly superior to rituximab PFS (80% versus 73% at 3 years; HR 0.66, p = 0.001) [16].
HDC plus ASCT (HDC/ASCT) and reduced-intensity conditioning (RIC) allogeneic transplantation are options for relapsed refractory FL patients. A retrospective analysis from Spain showed that HDC/ASCT could induce durable PFS and OS in CR1 and CR2/3 patients [17]. Another NHLBI and NCI sponsored study showed comparatble outcomes between HDC/ASCT and RIC allogeneic transplant [18]. For the early progression of FL, a German study of low-grade lymphoma showed that for patients with POD24, a significant survival benefit was associated with ASCT [19]. Likewise, the Center for International Blood and Marrow Transplant Research reported that patients with high-risk FL and early progression, who underwent auto-HCT for FL had low nonrelapse mortality and a promising 5-year OS rate of 70% for ASCT or matched-sibling allogeneic transplantation, far better than for matched-unrelated transplant [20]. However, FL patients are relatively elderly and only a limited percentage of patients can be treated with stem cell transplantation.
The SWOG S0016 randomized phase III study compared R-CHOP with CHOP followed by consolidation with iodine-133-tositumomab radio-immunotherapy (CHOP-RIT) for previously untreated FL patients. Patients in the CHOP-RIT arm had significantly better 10-year PFS (56% versus 42%, p = 0.01); however, there was no difference in OS between these two arms (75% versus 81%, p = 0.13). The cumulative incidence of death resulting from secondary myelodysplastic syndrome or acute myeloid leukemia was higher in the CHOP-RIT arm compared with the R-CHOP arm (4% versus 0.9%; p = 0.02) [21]. A phase II trial of 40 FL patients showed that ibrutinib, a Bruton tyrosine kinase (BTK) inhibitor, had only modest activity in recurrent FL, with low response rates in rituximab-refractory patients. CARD11 mutations predicted a lack of response. Evaluation of BTK inhibitors in earlier lines of therapy may be warranted on the basis of improved response rates in rituximab-sensitive disease [22].
Achieving CR or PR seems not to be related to different kinds of treatment but rather to the selection of after-treatment. CR patients certainly have a better outcome than non-CR patients. It remains to be answered whether any additional treatment or HDC, plus autologous or even allogeneic stem cell transplant as consolidation for patients achieving PR after initial chemotherapy would lead to improved survival until further randomized trials are conducted [3,23].
CONFLICT OF INTEREST
The authors have no other affiliations or financial involvement with any organization or entity apart from those disclosed.
The authors have no financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
AUTHORS' CONTRIBUTIONS
Dr Tan as the first and corresponding author to take care of patients and Dr Chiou and Wu, MC take care part of patients as well; Dr Wu, JS to perform irradiation treatment; Dr Lee to make pathologic diagnosis; Dr Huang to perform PET-CT scan exam and interpretation; Dr Chen to perform radiation exam and interpretation.
ACKNOWLEDGMENTS
Special thanks to Mr Lu, Shao-Min and Ms Feng, An-Chen of our Department of Epidemiology and Biostatistics to help us make the statistics and survival figures.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Tran-Der Tan AU - Lun-Wei Chiou AU - Mau-Ching Wu AU - Jia-Shing Wu AU - Ming-Yuan Lee AU - Yu-Yi Huang AU - Shing-Su Chen PY - 2019 DA - 2019/09/01 TI - The Impact of First Complete Remission by PET-CT and Time to Next Treatment on Survival of Follicular Lymphoma Patients JO - Clinical Hematology International SP - 168 EP - 172 VL - 1 IS - 3 SN - 2590-0048 UR - https://doi.org/10.2991/chi.d.190528.001 DO - 10.2991/chi.d.190528.001 ID - Tan2019 ER -
