Aortic calcification, arterial stiffness and bone mineral density in patients with COPD☆
The work was carried out at Wales Heart Research Institute, School of Medicine, Cardiff University, Cardiff, UK.
- DOI
- 10.1016/j.artres.2011.01.001How to use a DOI?
- Keywords
- Aortic stiffness; Pulse wave velocity; Osteoporosis; Lung function
- Abstract
Background: Increased arterial stiffness, using aortic pulse wave velocity (PWV) has been demonstrated in patients with COPD. However, mechanisms underlying this remain unclear. We explored the contribution of aortic calcification to large artery haemodynamics and its association to bone mineral density (BMD) in patients with confirmed COPD.
Methods: Patients with COPD, free of maintenance oral corticosteroids, renal disease, diabetes or known cardiovascular disease (n = 45), 27 male, mean (SD) age 66(7) years underwent unenhanced thoraco-abdominal computed tomography to determine quantitative aortic calcium content using a volume scoring method. Aortic PWV was measured. A subgroup (n = 29) had BMD determined.
Results: All patients had some evidence of aortic calcification. Aortic PWV was related to log10 calcification in abdominal aorta (r = 0.34, p = 0.025) and to semi-quantitative assessment in the ascending and descending thoracic aorta (r = 0.47 and r = 0.39, both p < 0.01). Log10 calcium was inversely related to BMD hip (r = −0.43), p = 0.02. Both aortic PWV and log10 calcium were related to age, which on multiple regression was the independent variable.
Conclusions: Aortic calcification is related to aortic stiffness, an independent predictor of cardiovascular mortality and morbidity, and inversely to BMD in patients with COPD. Given, both cardiovascular disease and osteoporosis are common in patients with COPD, determining underlying mechanisms are essential as potential therapeutic targets.
- Copyright
- © 2011 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Cardiovascular disease is a major cause of the excess mortality reported in patients with chronic obstructive pulmonary disease (COPD).1,2 Increasing epidemiological evidence of an association between cardiovascular (CV) disease and impaired lung function has stimulated research into the possible pathophysiological mechanisms involved.3,4 Previously, we have reported that aortic stiffness, an independent predictor of future cardiovascular events, is increased in patients with COPD compared to age and gender matched smoking controls and inversely related to their lung function.5,6 These findings have been supported by a similar study in which the severity of emphysema on CT scanning in subjects with COPD was related to brachial PWV.7 A number of mechanisms may explain these findings and require further investigation.
Of particular recent interest has been the relationship between increased aortic PWV and calcification of the aorta in a number of different patient groups including hypertensive’s, subjects with chronic kidney disease and those with osteoporosis.8,9,10 Although aortic calcification is associated with the ageing process, being particularly manifest after the age of 60 yrs, it occurs earlier in subjects with increased CV risk. In our previous study showing increased aortic stiffness in COPD we also confirmed previous reports of increased aortic PWV in those with osteoporosis,6 COPD having a persistently heightened systemic inflammatory state. However, the relationship between aortic stiffness and arterial calcification in patients with COPD has not previously been studied. In the current study we have therefore investigated the relationship between aortic PWV, aortic calcification, bone mineral density (BMD) and systemic inflammation in a new cohort of patients with COPD.
Method
Patients
Patients with confirmed COPD were recruited and studied when clinically stable.11 All patients gave written informed consent and the study had Local Research Ethics Committee approval. Exclusion criteria included known ischaemic heart disease, diabetes mellitus, chronic renal failure, malignancy or maintenance oral corticosteroids, warfarin, bisphosphonates or calcium supplements. Women were post-menopausal and all participants were over the age of 50 years old (given the radiation dose) to meet ethical approval.
Cardiovascular measurements
Blood pressure was measured in duplicate in the seated position (HEM-705CP, Omron). Radial artery waveforms from the wrist of the same arm were recorded using a high-fidelity micromanometer (SPC-301; Millar Instruments). Pulse wave analysis (SphygmoCor, AtCor Medical, Australia) was used to generate a central waveform using a generalised and validated transfer function. Augmentation index (AIx), a composite measure of arterial stiffness and wave reflection was ascertained. The PWV was calculated by sequentially recorded ECG-gated carotid–femoral (aortic) and carotid–radial (brachial) waveforms and divided by the distance between the 2 sites.5,6
Clinical measures
Height and weight (Seca; Vogel and Halke, Hamburg, Germany) were measured. Blood was taken in the fasting state into plain tubes. They were centrifuged at 4 °C (4000 rpm for 20 min) and frozen at −80 °C for subsequent analysis. Fasting electrolytes and creatinine, lipids, C-reactive protein (CRP) and parathyroid hormone were analysed in an accredited NHS laboratory according to their standard operating procedure and subject to their standard quality control for clinical tests. Estimated GFR (eGFR) was determined using the Cockcroft–Gault formula.12 In addition, osteoprotegerin (OPG), a soluble decoy for RANKL and hence an indirect inhibitor of osteoclastogenesis was measured by ELISA (Quidel, San Diego, CA), with a limit of detection of 0.13 pmol/L, and coefficients of variation of 2.1–3.5% within run and of 4.2–6.1% between run. All subjects performed spirometry - forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and FEV1/FVC ratio, (Vitalograph Ltd Bucks UK) having withheld short acting bronchodilators and long acting bronchodilators for six and 12 h respectively, to ensure haemodynamic measures were made free of the effect of these agents.6
Computed tomography
Aortic calcification
Unenhanced computed tomography (CT) of the aorta from arch to bifurcation was performed with 1.25 mm slice thickness (16-slice Siemens Medical solutions system, DE). Calcium content was determined in 3 predefined sections: ascending aorta, descending thoracic aorta and a defined section of abdominal aorta by two researchers blind to the clinical status of the patients. For ascending aorta and descending thoracic aorta, a semi-quantitative method was used: no calcium present, flecks, moderate or confluent calcification. For the abdominal aorta, a volume scoring method was used. The number of voxels ≥ 130 Hounsfield Units within the aortic wall along a 5 cm length just proximal to the bifurcation was calculated and converted to cm3. This has been validated and has excellent within and between observer repeatability.13
Emphysema index
Unenhanced CT images of the chest, from apex to base, were reconstructed at 1.25 mm slice thickness with a 1 mm slice interval. Emphysema was assessed by the percentage of whole lung with attenuation below the thresholds of −950 and −910 Hounsfield Units using the Pulmonary Workstation 2.0 software (VIDA diagnostics, Iowa city, IA, USA).14
Dual energy X-ray absorptiometry (DXA)
A subset of the patients (n = 29) had agreed to DXA scanning (Hologic Discovery). The BMD was measured at the lumbar spine (total), hip (total) as well as the 3 separate hip subregions: femoral neck, trochanter and intertrochanteric region. The methodology is as described previously.15 Osteoporosis was defined as a T score < −2.5 at the hip (total hip or its subregions) or the lumbar region; osteopenia was defined as a T score < −1 but >−2.5.16
Statistics
Statistical analysis was performed using SPSS version 16 (SPSS Inc., Chicago, Illinois). Data is presented as the arithmetic mean and standard deviation (SD). Log10 transformations were performed on CRP, eGFR, PTH, OPG and calcium deposition (cm3) because of the positively skewed distribution and here, the geometric mean (SD) of the log values is presented. Differences between groups were determined by independent t test. Multiple regressions included independent variables that were shown to relate to the dependent in univariate analysis at the 10% level or less or which are known to relate in larger studies. A p-value < 0.05 was considered statistically significant.
Results
The demographics and clinical parameters of these patients are presented in Table 1. There were more males than females patients with an age from 50 to 83 years. The group with a DXA scan available was not different from those without in terms of age and gender; however, more patients who had a DXA scan were current smokers and had milder severity airways obstruction, Table 1.
| All Patients n = 45 | Patients with DXA n = 29 | Patients with no DXA n = 16 | P value | |
|---|---|---|---|---|
| Gender (male) n(%) | 27 (60%) | 16 (55%) | 11 (68%) | 0.37 |
| Age (years) | 66 (7) | 66 (8) | 66 (7) | 0.69 |
| FEV1 (l) | 1.47 (0.69) | 1.57 (0.64) | 1.28 (0.75) | 0.18 |
| FEV1 % predicted | 56 (20) | 61 (19) | 47 (22) | 0.03 |
| Emphysema index | ||||
| –Mean HU both lungs | −847 (27) | −839 (25) | −862 (25) | 0.004 |
| –% below 950 HU | 16.2 (14.7) | 11.5 (11.7) | 24.1 (16.0) | 0.005 |
| –% below 910 HU | 32.7 (15.5) | 27.7 (14.8) | 41.2 (12.9) | 0.011 |
| BMI (kg/m2) | 29.0 (4.4) | 29.1 (4.6) | 28.7 (4.1) | 0.80 |
| Smoking (Current/ex/never) | 13/32/0 | 12/17 | 1/15 | 0.025 |
| Smoking (pack years) | 45 (23) | 46 (17) | 43 (33) | 0.71 |
| Hypertension | 17 (38%) | 8 (28%) | 9 (56%) | 0.06 |
| Medications | ||||
| –Statins | 9 | 3 | 6 | 0.03 |
| –ACE inhibitors | 10 | 7 | 3 | 0.68 |
| –HRT | 1 | 1 | 0 | |
Abbreviations: ACE, angiotensin concerting enzyme; BMI, body mass index; DXA, dual energy X-ray absorptiometry; FEV1, forced expiratory volume in 1 s; HRT, hormone replacement therapy; HU, Hounsfield units.
P value refers to the difference between those with and without DXA.
Patient characteristics.
There were 17 (38%) patients with a prior diagnosis of hypertension and 10 were taking ACE inhibitors. None were taking calcium channel blockers. Of the 29 patients, four had a new diagnosis of osteoporosis and a further 12 had osteopenia at either the hip or lumbar spine.
All patients had evidence of aortic calcification to some degree in at least one of the regions, Table 2. The degree of calcification in one region was related to calcification in other area, all p < 0.03. Log10 calcium deposition in abdominal aorta was related to aortic PWV (r = 0.34, p = 0.025), (Figure 1). Similarly, ascending aorta (r = 0.47, p = 0.001) and descending aorta (r = 0.39, p = 0.009) were related to aortic PWV. However, aortic calcification at any site did not relate to brachial PWV or AIx.
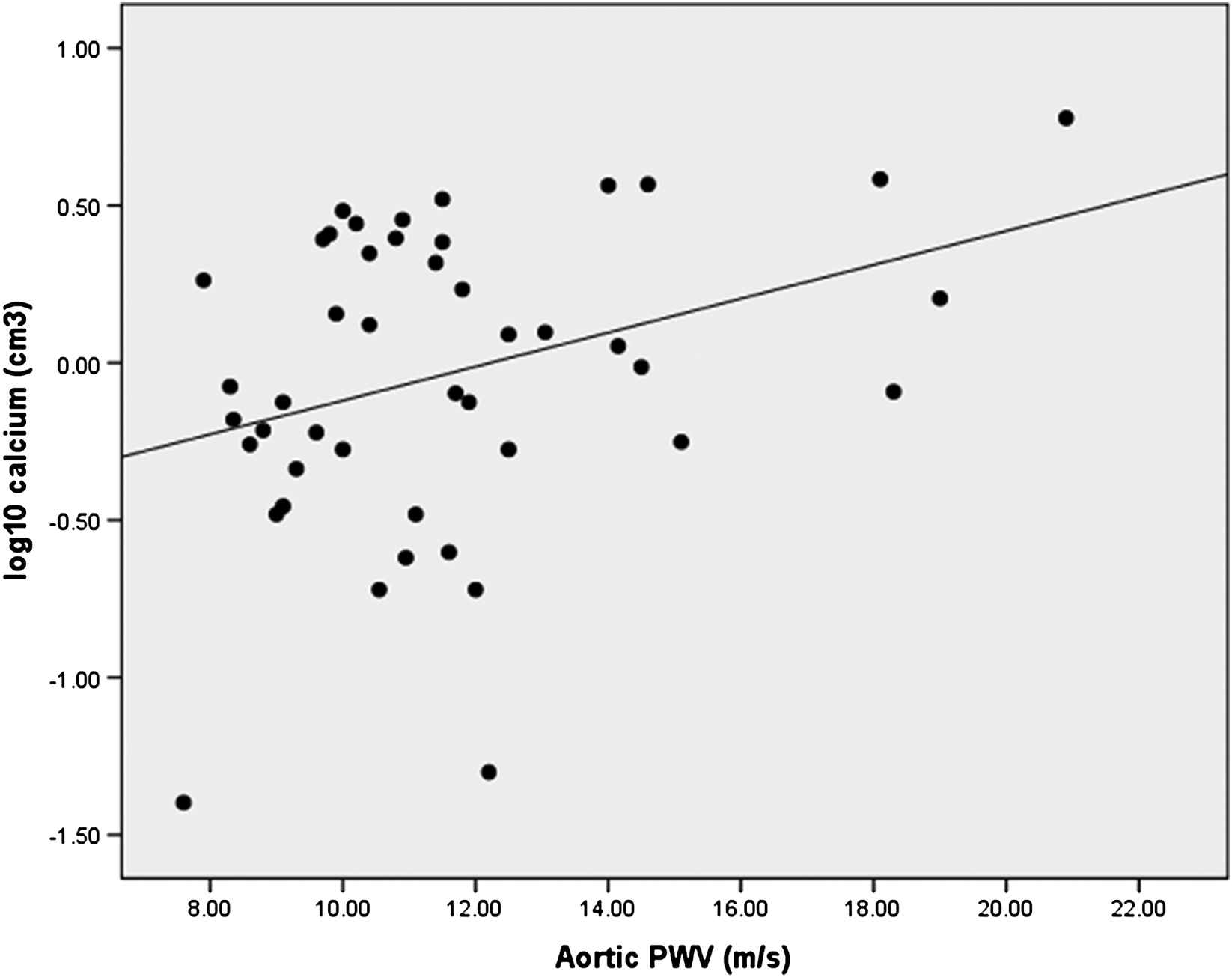
Association of aortic calcium volume to aortic pulse wave velocity (PWV).
| All patients n = 45 | Patients with DXA n = 29 | Patients without DXA n = 16 | |
|---|---|---|---|
| Peripheral Systolic BP (mmHg) | 153 (18) | 151 (14) | 155 (24) |
| Peripheral Diastolic BP (mmHg) | 88 (12) | 87 (12) | 88 (12) |
| Peripheral PP (mmHg) | 65 (16) | 64 (14) | 67 (19) |
| Peripheral MAP (mmHg) | 109 (12) | 108 (11) | 111 (14) |
| HR (bpm) | 65 (16) | 64 (14) | 67 (19) |
| Aortic PWV (m/s) | 11.6 (3.0) | 11.4 (2.6) | 12.0 (3.7) |
| Brachial PWV (m/s) | 9.4 (1.3) | 9.2 (1.1) | 9.7 (1.5) |
| AIx (%) | 23.6 (9.3) | 23.3 (7.9) | 24.1 (11.6) |
| Ascending aorta calcium (AU) | 0.4 (0.7) | 0.4 (0.7) | 0.4 (0.7) |
| Descending thoracic aorta calcium (AU) | 1.2 (0.8) | 1.2 (0.8) | 1.1 (0.9) |
| Abdominal aorta calcium (cm3)a | 0.93 (3.01) | 0.96 (2.95) | 0.87 (3.23) |
| BMD Total Lumbar spine (g/cm2) | 1.04 (0.19) | ||
| BMD Total Hip (g/cm2) | 0.94 (0.16) | ||
| BMD Femoral neck (g/cm2) | 0.75 (0.12) | ||
| BMD Intertrochanteric (g/cm2) | 1.10 (0.21) | ||
| BMD Trochanter (g/cm2) | 0.73 (0.15) |
None of the haemodynamic parameters were different between the patients who had and did not have a DXA.
Abbreviations: AIx, Augmentation index; AU, arbitrary units; BMD, bone mineral density; BP, blood pressure; DXA, dual energy X-ray absorptiometry; HR, heart rate; MAP, mean arterial pressure; PP, pulse pressure; PWV, pulse wave velocity.
Geometric mean (SD).
Vascular and bone parameters in patients.
Log10 calcium abdominal aorta was related to BMD hip (r = −0.43, p = 0.021), (Figure 2); as well the 3 hip subregions (neck r = −0.42, p = 0.025; intertrochanteric region r = −0.47, p = 0.01 and trochanter r = −0.38, p = 0.04) but not to BMD lumbar spine (r = −0.09, p = 0.66). Log10 calcium deposition was related to age (r = 0.55, p < 0.001).
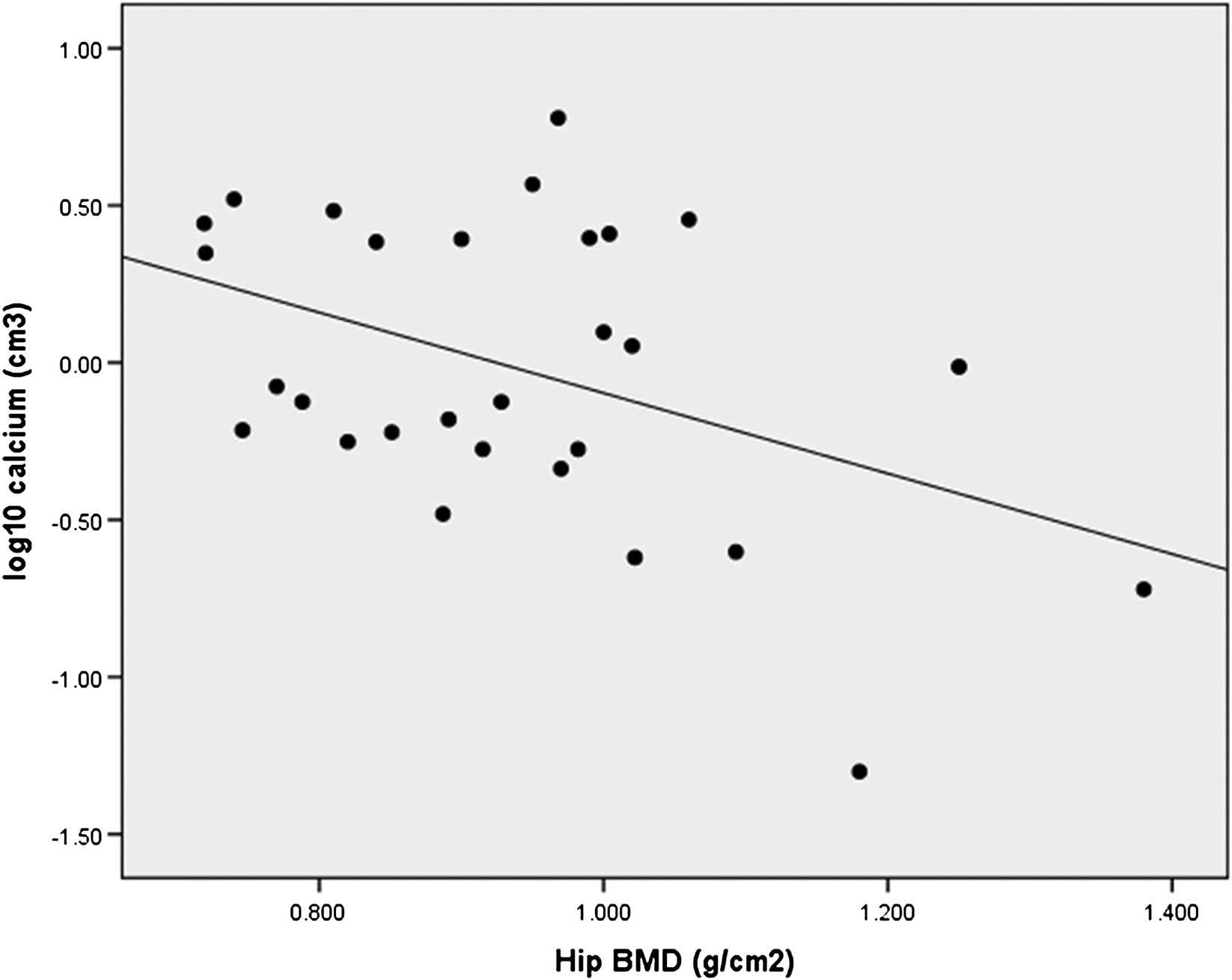
Association of aortic calcium volume to hip bone mineral density (BMD).
Biochemistry and bone chemistry results for all subjects are presented in Table 3. There were 3 patients with a eGFR < 60 mls/min.
| Patients | |
|---|---|
| Glucose (mmol/l) | 5.6 (1.1) |
| Total cholesterol (mmol/l) | 4.8 (1.1) |
| Triglycerides (mmol/l) | 1.6 (0.8) |
| Creatinine (μmol/l) | 71 (1) |
| eGFR (mls/min)a | 101.0 (28.8) |
| CRP (mg/L)a | 4.5 (2.1) |
| Corrected Calcium (mmol/l) | 2.2 (0.1) |
| Phosphate (mmol/l) | 1.1 (0.2) |
| PTH (pmol/mL)a | 2.8 (1.7) |
| OPG (pmol/L)a | 6.3 (1.3) |
Abbreviations: eGFR, estimated glomerular filtration rate; CRP, C-reactive protein; PTH, parathyroid hormone; OPG, osteoprotegerin.
Geometric (SD) mean.
Biochemistry and bone chemistry in all patients.
In univariate analysis, aortic PWV was related to age (r = 0.42, p = 0.004); log10CRP (r = 0.34, p = 0.026); log10eGFR (r = −0.32, p = 0.03), creatinine (r = 0.33, p = 0.03) but not MAP, FEV1 % predicted or measures of emphysema index. Aortic PWV was not related to hip or lumbar spine BMD, however was directly related to log10OPG (r = 0.38, p = 0.01). In this study, there was no difference in aortic PWV between those with (n = 4) and without osteoporosis. Interestingly, brachial PWV was not related to any of the parameters above, except for MAP (r = 0.42, p = 0.025) and emphysema index % < −910 HU (r = 0.36, p = 0.02) but not other emphysema index markers.
In multiple regressions, with aortic PWV as the dependent and the following variables entered: age, gender, log10 calcium and MAP; age (R2 = 0.14), MAP (R2 = 0.06) and gender (R2 = 0.06) were significant predictive variables.
With log10 calcium aortic deposition as the dependent and the following variables entered: age, gender, aortic PWV, the calcium phosphate product and MAP; only age (R2 = 0.29) was the significant predictive variable.
Discussion
Epidemiological studies have consistently demonstrated that subjects with impaired lung function are at increased cardiovascular risk even allowing for smoking. However, the pathophysiological mechanisms underlying this increased risk are unclear. Aortic stiffness is a major independent predictor of cardiovascular risk and we and others have previously demonstrated increased stiffness as manifest by an increased aortic PWV in subjects with impaired lung function as well as in subjects with COPD.6,7,17 The current study was therefore designed to investigate the relationship between aortic stiffness and vascular calcification in subjects with COPD utilising quantitative, high-resolution CT imaging and aortic PWV.5 Here we demonstrate, for the first time in patients with COPD, a direct relationship between a quantitative assessment of calcification in the lower abdominal aorta and aortic PWV. Supporting this, there was also a relationship of the semi-quantitative measures of ascending and descending thoracic aortic calcification with arterial stiffness. In those who underwent a DXA scan, aortic calcification was inversely related to the BMD at the hip.
One of the key properties of the aorta is to absorb pulsatile energy to dampen the pressure extremes from ventricular ejection, as it propagates down the arterial tree and hence reduce the impact on the microvasculature, such as the retinal and renal microvascular beds,18,19 which in turn lead to augmentation of the reflective wave. Further, increasing arterial stiffness contributes to increased cardiac workload and thus leads to left ventricular hypertrophy and diastolic dysfunction as we have previously demonstrated in patients with COPD.20
The aorta contains a large proportion of elastic fibres compared to the more muscular peripheral arteries. There is thus a gradual stiffening of the vascular tree towards the peripheries, creating a gradient. The elastic fibres are made up predominantly of elastin and are susceptible to degradation, fatigue fracture, and calcification. Vascular calcification can manifest in different manners - calcification associated with atherosclerotic plaques has an uneven distribution and predominantly affects coronary and muscular arteries. However, medial calcification reflects deposition on elastin fibres; elastin degradation appearing to be a stimulus.11,21
Elastin degradation is an interesting consideration in COPD given one of the key pathological processes underlying the lung destruction is an imbalance in protease:antiprotease activity. This imbalance is likely to be more systemic than purely the lungs, as it is in alpha-1-antitrypsin deficiency.11,22 Indeed, Maclay and colleagues recently reported increased elastin degradation in skin biopsies in subjects with COPD as an indication of a systemic elastolytic process, which interestingly was related to aortic PWV.23 Animal models developed to study isolated systolic hypertension by inducing aortic calcification, demonstrate that it is predominantly the elastin fibres that calcify.24 However, it remains unclear as to whether elastin fragmentation is required to promote calcification or whether fragmentation of elastin is a result of the calcification process. Interestingly, a recent animal study suggested that the pro-inflammatory elastase, cathepsin S, induced medial elastin fragmentation predisposing to aortic calcification,25 with other studies supporting an inflammatory/elastolytic stimulus.26,27,28 A recent study in former or ex smokers reported thoracic calcification was associated with severity emphysema29 but did not measure haemodynamics such as PWV nor relate calcification to the subjects BMD. In addition, the exclusion of confounding states was not as robust. The concept of elastin degradation leading to the vascular calcification is an interesting one, albeit currently speculative and further work to identify a therapeutic window where disease modification could occur prior to the fixed vascular calcification remodelling is required.
This study demonstrated that age was the key variable affecting both aortic stiffness and calcium deposition. Whilst we had no age and gender matched control group to directly compare, the volume of aortic calcium in all regions and the aortic PWV were almost double compared to the healthy subjects participating in our previously reported study with the same methodology, who were of a similar age.8 This does not deviate from the current concept that COPD is an accelerated ageing process.30,31 The relationship between aortic stiffness and aortic calcification only explained 10% of variance and may have been stronger, had regional aortic stiffness been employed such as MRI techniques.32 A recent study suggested the distal aorta may stiffen preferentially with age, which may in part be due to calcification. We did not report an association of abdominal calcification with AIx, perhaps reflecting the non-linear relationship of this haemodynamic measure with age, which plateaus over the age of 50 years. Similarly, the brachial PWV did not relate, but this more muscular artery, is likely to be regulated by different mechanisms. These findings do not deflect from the association of calcification with aortic PWV, given that this is the optimal measure in this age range.5 Aside, the intriguing association of brachial PWV with emphysema index is in keeping with McAllister et al. and requires further consideration.7
The further link of aortic calcification to BMD in patients with COPD is in keeping with findings in renal disease where there is similarly an increased cardiovascular risk.9,33 Seminal to renal disease, but not COPD, is alteration to calcium homeostasis. However, recognition of a relationship between BMD and cardiovascular risk and vascular calcification has been identified in women without renal disease together with dynamic loss of BMD mirroring vascular calcification.34,35,36 The association of aortic PWV with both BMD and circulating OPG together with the reports that key bone regulators are expressed within the arterial wall of patients,10,37,38 gives a tantalising hint that classical osteoporosis treatments may not only ameliorate bone loss but also address the vascular element too.39
The BMD was determined using DXA at the hip and lumbar region. For lumbar spine, it utilises an AP view and thus includes any overlying calcium in other tissues such as osteophytes and, ironically, the aortic calcification itself, thus obscuring any actual BMD loss. This may account for the lack of association with aortic calcification at the lumbar spine.
We confirmed previous findings that systemic inflammation, using CRP, was related to aortic PWV, however failed to find an association with aortic calcification. This is perhaps not surprising, given that CRP is a non-specific mediator and just one of several inflammatory pathways.40 In addition, any inflammatory causality, if present, is likely to be within the aortic wall,41 with circulating levels a downstream effect. This would necessitate further novel imaging techniques or postmortem histopathology to determine.
Limitations
This study was cross-sectional and therefore causality cannot be demonstrated. The study represents a modest number of subjects with COPD but all were clinically phenotyped, withpatients with important confounders excluded. In contrast to previous studies, we found a relatively low prevalence of osteoporosis.6,17 Locally, previous research has lowered the threshold for clinical evaluation of BMD. A large proportion of patients were thus not eligible having previously commenced treatment for osteoporosis. This is inevitable if prior clinical research is implemented into wider clinical practice and assessment for osteoporosis encompassed more widely. The thoracic aorta was only evaluated semi-quantitatively. A number of reasons including the thoracic aorta curvature, motion artefact of breathing, prominent osteophytes adjacent to the descending aorta, and the variable extent of calcification at the site of the ligamentum arteriosum make quantitative assessment challenging in this area. However, reassuringly they were related to the quantitative assessment of the 5 cm portion of straight abdominal aorta which is reliable.15 Given the exploratory nature of this in COPD, no controls were studied, given the radiation exposure.
Conclusions
In conclusion, we present evidence of aortic calcification in patients with COPD that is related to aortic stiffness and inversely to bone mineral density. Given that both cardiovascular disease and osteoporosis are so prevalent in patients with COPD, mechanisms underlying such an association are imperative in order to identify potential therapeutic targets to attenuate these co-morbidities.
Author contribution
CEB, CMM, DJS, IBW and JRC designed the protocol; CEB, CMM, BJM, MM, RS, IBW, JRC performed the study; AKD (calcification), VR (emphysema) and NS (emphysema) performed CT analysis; CEB, CMM, IBW, DJS and JRC were involved in the analysis and interpretation of the study; CEB wrote the first draft of the manuscript; All authors contributed to the writing of the manuscript; JRC is the PI of the study.
Funding
CEB is supported by
Acknowledgements
Yasmin, Alvin So, Nichola Gale, Susannah Williams, James Duckers, Andrew Oram, Jaak Kals and Priit Kampus who all assisted with the clinical assessments; Rebecca Pettit and Wil Evans, Medical Physics who assisted with the DXA scans; Sri Aitken who assisted with the CT analysis; Gareth Dunseath who performed the OPG assays.
References
Cite this article
TY - JOUR AU - Charlotte E. Bolton AU - Carmel M. McEniery AU - Vimal Raj AU - Barry J. McDonnell AU - Adrian K. Dixon AU - Margaret Munnery AU - Ramsey Sabit AU - Nicholas Screaton AU - Michael Stone AU - Ian B. Wilkinson AU - Dennis J. Shale AU - John R. Cockcroft PY - 2011 DA - 2011/02/04 TI - Aortic calcification, arterial stiffness and bone mineral density in patients with COPD☆ JO - Artery Research SP - 30 EP - 36 VL - 5 IS - 1 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2011.01.001 DO - 10.1016/j.artres.2011.01.001 ID - Bolton2011 ER -
