Non-invasive ultrasound-based assessment of ventricular–arterial interaction in vascular Ehlers–Danlos syndrome patients
- DOI
- 10.1016/j.artres.2010.11.003How to use a DOI?
- Keywords
- Arterial rupture; Collagen; Pulse pressure; Radiofrequency; Vascular ultrasound; Ventricular-arterial coupling; Waveform analysis; Systolic time intervals
- Abstract
The role of ventricular–arterial hemodynamic interaction in the occurrence of arterial dissection and rupture in vascular Ehlers–Danlos syndrome (vEDS) is unknown. We recently introduced an ultrasound-based method to extract, from common carotid artery (CCA) diameter waveforms, central arterial properties and left ventricular (LV) systolic time intervals. We obtained CCA diameters, compliance and distensibility coefficients, and LV isovolumic contraction and ejection periods (ICP and EP) of 19 vEDS patients (aged 27–65 yrs) and 19 age-matched healthy controls. CCA distension and compliance tended to be lower in vEDS subjects (p = 0.062 and p = 0.073), especially in younger patients. ICP (−12 ms) and EP (−24 ms) were shorter (p < 0.001), while heart rate was increased (+10 bpm; p < 0.001) in vEDS. The ICP/EP ratio and estimated isovolumic dPLV/dt indicated increased LV contractility in vEDS. In conclusion, vascular EDS patients tend to have a lower CCA compliance but a normal pulse pressure, most likely reflecting reduced physical fitness.
- Copyright
- © 2010 Published by Elsevier B.V. on behalf of Association for Research into Arterial Structure and Physiology.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Vascular type (IV) Ehlers–Danlos syndrome (vEDS) is a genetic disorder (mutations in COL3A1), leading to abnormalities in type III procollagen which affect connective tissue development and wound healing. Affected patients suffer from rupture and dissection of large arteries due to fragility of arterial wall structure and increased wall stress.1–3 However, the role of ventricular–arterial interaction in the occurrence of vascular complications of vEDS is still unknown. A normal stroke volume ejected into a less compliant arterial system will produce a greater pulse pressure; similarly, an increased stroke volume into a normal compliant load will also increase pulsatile load on the arterial wall.
We recently developed a method to derive both central arterial and LV function parameters from non-invasively recorded carotid artery diameter waveforms by means of ultrasonography,4,5 giving the opportunity to study ventricular and arterial function, and their interaction. We retrospectively analysed existing M-mode ultrasound recordings of 19 vEDS patients and 19 age-matched controls. We hypothesized that in vEDS arterial distensibility may be lower than normal, because stiff structures are more prone to disintegrate than elastic structures when put under stress. Furthermore, we expected to observe normal LV function because, to the best of our knowledge, cardiac events are rather uncommon in vEDS.3
Methods
Study population
Vascular Ehlers–Danlos syndrome (vEDS) patients were diagnosed as reported previously1 and following the guidelines defined in the nosology for Ehlers–Danlos syndromes.3,6 The patients were apparently in good health at the time of measurement. None of them were taking any anti-hypertensive drugs and none of the patients had a history of diabetes or hypercholesterolemia. An age-matched control group was randomly selected from an existing ultrasound recording database of normal healthy subjects. None of the controls had a history of cardiovascular disease, diabetes or hypercholesterolemia, and all of them were normotensive and not taking any medication affecting cardiovascular function. The medical ethical committees of Saint-Germain-en-Laye (France) and Maastricht University Medical Centre (the Netherlands) approved the study. All subjects gave written informed consent prior to enrolment.
Measurement protocol
Ultrasound and blood pressure measurements were performed under controlled conditions, as described previously.1,7 Briefly, measurements were done in a quiet and temperature-controlled room (20–22 °C), after subjects were allowed to acclimatize for 10 min in supine position. Patients remained in this position during subsequent measurements. Brachial artery blood pressures were measured by automated oscillometry (Dinamap model 845, Critikon) in all subjects. Per subject, a total of six measurements of systolic (SBP) and diastolic blood pressures (DBP) were taken before, in between, and after the ultrasound M-mode recordings and averaged subsequently. After localizing the right common carotid artery (CCA) with a medical ultrasound scanner in B-mode, M-mode recordings were obtained (7.5 MHz linear array, P350 system, Esaote Europe, Maastricht, the Netherlands). The ultrasound probe was positioned with the M-line intersecting the CCA perpendicularly, 2–3 cm proximal to the carotid artery bifurcation. A minimum of three (range 3–7) repeated measurements was obtained per subject. Recording length was 5 s and thus each measurement covered 4–7 consecutive heartbeats. Radiofrequency data were directly recorded on a dedicated acquisition system, with a single ECG tracing (lead II) acquired simultaneously as a time reference.
Radiofrequency processing and timing analysis
The radiofrequency signals from the anterior and posterior artery walls were tracked automatically to obtain the diameter waveform,8 utilizing spatial and temporal estimation windows of 600 μm and 10 ms, respectively. Temporal estimation windows were half-overlapping, resulting in an effective sample interval of 5 ms for the diameter waveforms. Left ventricular (LV) isovolumic contraction (ICP) and ejection periods (EP), and CCA diastolic diameter (Dd), and distension (ΔD; systolic minus diastolic diameter) were obtained as previously described.4,8 Second-derivative and post-processing filters had cut-off frequencies of 60 Hz and 30 Hz, respectively. These enable detection of time intervals down to 20 ms with less phase distortion than the previously employed 40 and 20 Hz cut-offs.4 Figure 1 (left panel) illustrates extraction of ICP and EP from the CCA distension waveform on the basis of the second time-derivative/acceleration waveform.4,5
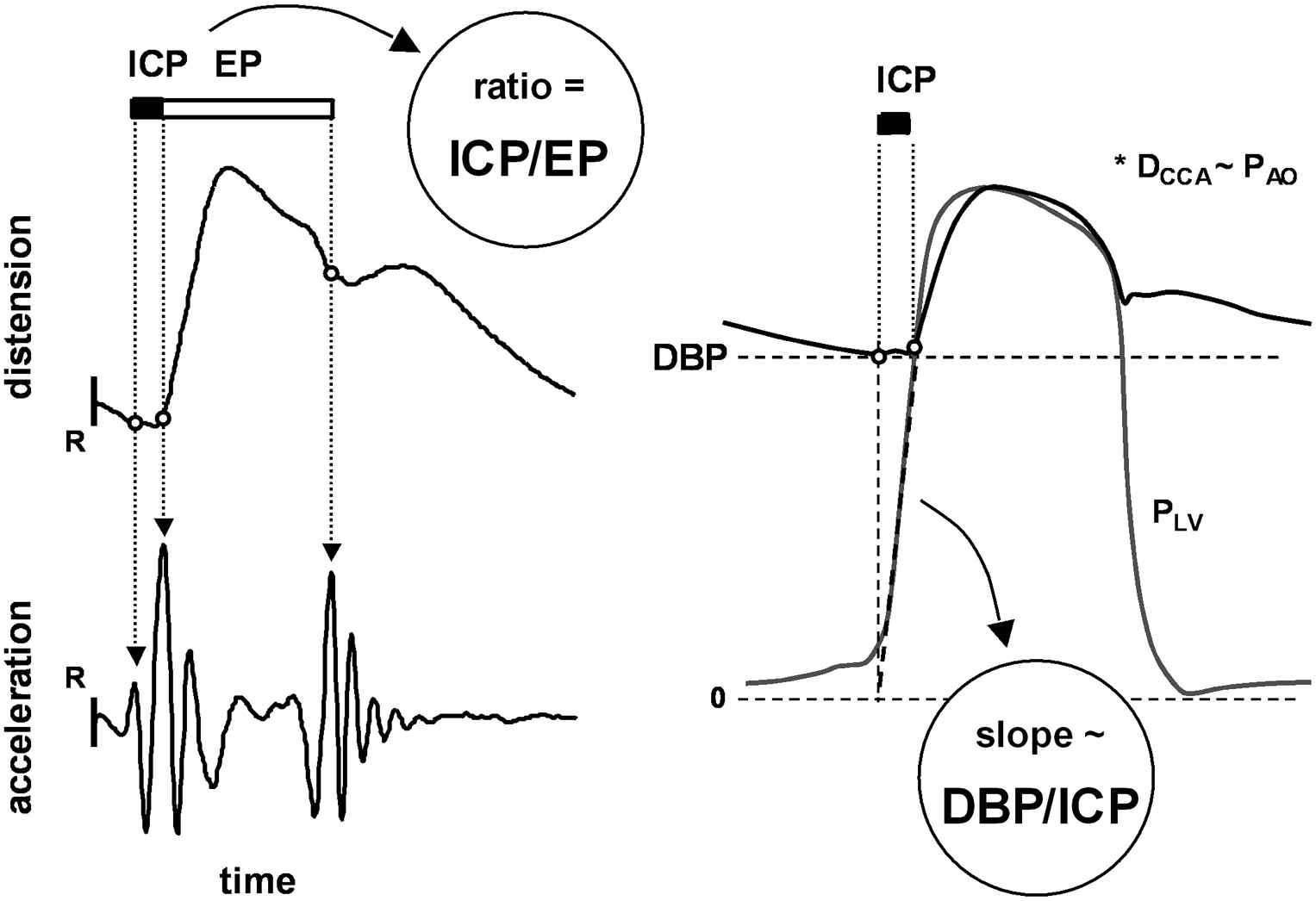
Left: Extraction of left ventricular isovolumic contraction period (ICP) and ejection period (EP) from the common carotid artery (CCA) diameter waveform, enabling calculation of ICP-to-EP ratio. R indicates the ECG R-wave. Right: Approximation of left ventricular isovolumic dP/dt by the DBP/ICP index. PLV, left ventricular pressure. *It is assumed that CCA diameter (DCCA) and aortic pressure (PAO) waveforms are similar in terms of relative timing.
Calculations
Figure 1 shows how the ICP-to-EP ratio (ICP/EP) and the DBP-to-ICP ratio (DBP/ICP) are obtained. Both indices reflect LV contractile performance. A shorter ICP at a given DBP causes DBP/ICP to rise, reflecting increased average isovolumic left ventricular dP/dt.9 A longer EP at a given ICP causes ICP/EP to decrease, similarly reflecting an increase in LV contractility.4,10 Arterial pulse pressure (PP) was calculated from the brachial average SBP and DBP values: PP = SBP − DBP. Mean blood pressure (MBP) was estimated by the empirical formula MBP = DBP + 0.4·PP.11 Relative distension (strain) was calculated as the CCA distension-to-diameter ratio (ΔD/Dd, in %). CCA compliance (CC) and distensibility coefficients (DC) were calculated from: CC = π/4·((Dd + ΔD)2 − Dd2)/PP, in mm2/kPa, and DC = ((Dd + ΔD)2 − Dd2)/(Dd2·PP), in 1/MPa.
Statistical analysis
Values are given as mean (SD). For each parameter the group mean and standard deviation, and intra-subject variability (reflecting reproducibility) were calculated. Intra-subject variability is defined as the standard deviation over all subjects of the differences between individual measurements (mean per repeated measurement) and the ensemble average (mean per subject).
For each variable, statistical differences between vEDS and control groups were tested by two-tailed two-sample Student t-test, assuming equal variance between groups (GraphPad Prism 4, GraphPad Software Inc, San Diego, CA). Associations between parameters were visualized by scatter plots and quantified by the Pearson correlation coefficient. p-values below 0.05 were considered statistically significant. Differences in associations between groups were evaluated by F-test (GraphPad Prism 4).
Results
The intra-subject variability coefficients were comparable for both groups and were: ICP < 10%, EP < 2%, ICP/EP < 10%, R–R interval < 4%, Dd < 3% and ΔD < 8%. The corresponding intra-subject standard deviations (ICP < 4.5 ms, EP < 1.4 ms, ICP/EP < 1.4%, R–R int < 38 ms and ΔD < 45 μm) are smaller than the observed differences between the groups (see Table 1), justifying discrimination between the groups.
| vEDS | Controls | *p-value | ||
|---|---|---|---|---|
| N | 19 | 19 | ||
| Sex | Male/female | 4/15 | 6/13 | 0.71# |
| Age | yrs | 42 (10) | 44 (9) | 0.72 |
| Age range | yrs | 27–65 | 25–63 | |
| SBP | mm Hg | 115 (13) | 112 (8) | 0.51 |
| MBP | mm Hg | 87 (10) | 85 (7) | 0.42 |
| DBP | mm Hg | 68 (10) | 66 (7) | 0.42 |
| PP | mm Hg | 47 (9) | 47 (7) | 0.99 |
| Dd | mm | 6.79 (0.70) | 6.92 (0.60) | 0.55 |
| ΔD | mm | 0.44 (0.14) | 0.53 (0.15) | 0.061 |
| ΔD/Dd | % | 6.6 (2.2) | 7.7 (2.1) | 0.13 |
| CC | mm2/kPa | 0.8 (0.3) | 1.0 (0.4) | 0.073 |
| DC | 1/MPa | 23 (9) | 26 (8) | 0.24 |
| HR | 1/min | 67 (9) | 57 (6) | 0.0003 |
| R–R int | ms | 910 (129) | 1066 (113) | 0.0003 |
| ICP | ms | 36 (8) | 48 (7) | <0.0001 |
| EP | ms | 294 (23) | 318 (13) | 0.0004 |
| ICP/EP | % | 12 (3) | 15 (2) | 0.001 |
| DBP/ICP | mm Hg/s | 1974 (428) | 1396 (229) | <0.0001 |
Values are presented as mean (SD).
Two-sample equal-variance t-test;
Fisher exact test. vEDS, vascular Ehlers–Danlos syndrome; SBP, systolic blood pressure; MBP, mean blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; Dd, diastolic diameter; ΔD, distension; ΔD/Dd, relative distension (strain); CC, compliance coefficient; DC, distensibility coefficient; HR, heart rate; ICP, isovolumic contraction period; EP, ejection period.
Carotid artery and left ventricular function parameters.
Between vEDS and age-matched healthy control groups, there were no significant differences in sex, blood pressures and CCA diastolic diameter. CCA distension and the compliance coefficient tended to be lower in the vEDS group (p = 0.061 and p = 0.073, respectively), but relative distension (strain) and the distensibility coefficient were not different between the groups.
Left ventricular ICP and EP were significantly shorter in the vEDS group (−12 ms and −24 ms, respectively; p < 0.001) while heart rate was higher (+10/min, p = 0.0003). Both the heart rate independent ICP/EP index and DBP/ICP, a non-invasive estimate of isovolumic dP/dt, reflected increased LV contractile performance (−3% and +577 mm Hg/s, respectively; p < 0.001).
Common carotid artery distension, relative distension (strain), CC and DC were significantly and negatively correlated to age in our study populations (Fig. 2). ICP/EP and DBP/ICP were not associated with age. Furthermore, there were no significant differences in regression slope between the groups (Fig. 2).
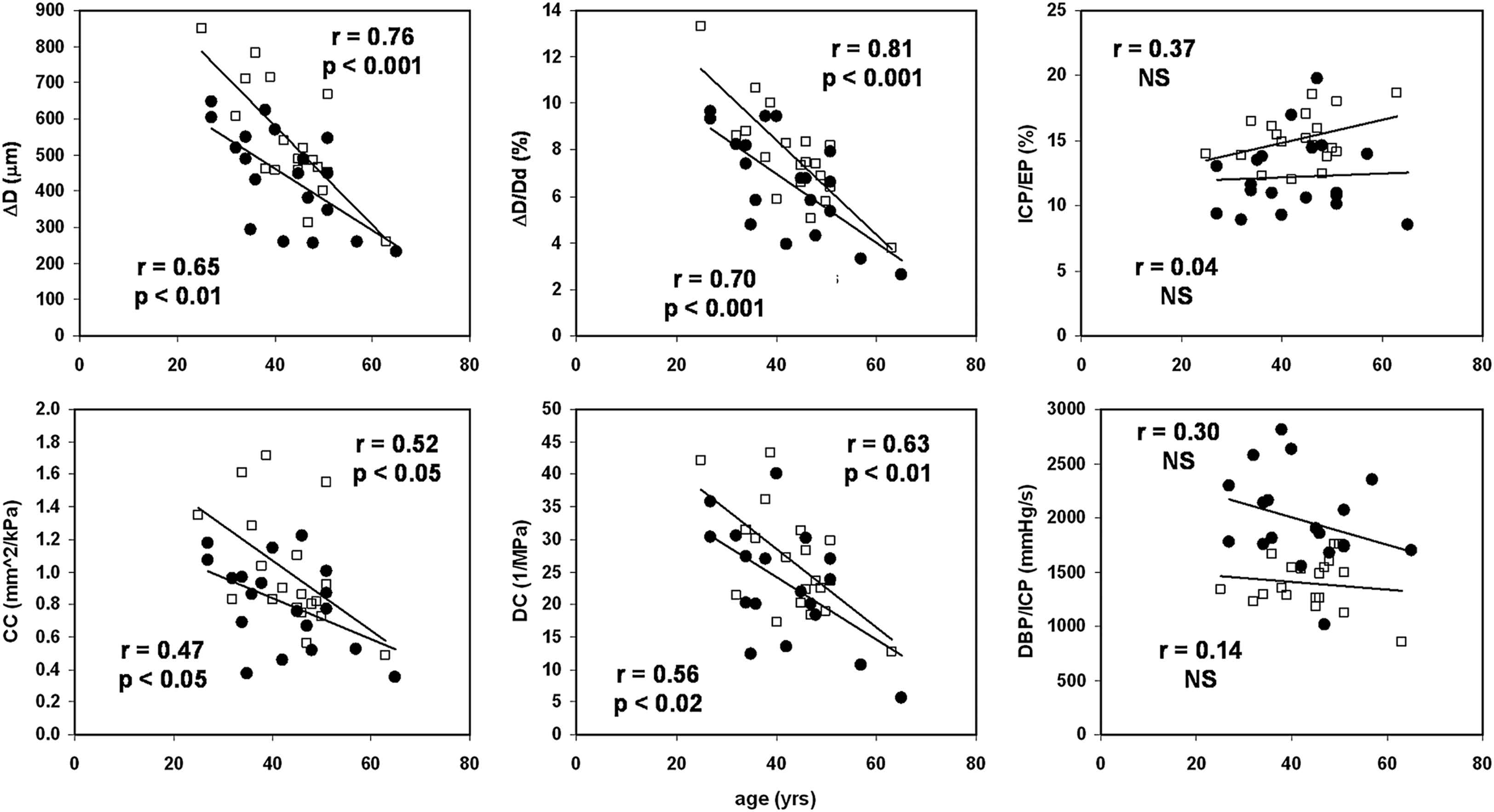
Age-related stiffening of the common carotid artery appears less pronounced in vascular Ehlers–Danlos syndrome (●) compared to healthy control subjects (□), but none of the differences in slope are statistically significant. Left ventricular systolic time index (ICP/EP) and non-invasive estimate of isovolumic dP/dt (DBP/ICP) do not change with age in these groups. ΔD indicates distension; ΔD/Dd, relative distension; CC, compliance coefficient; DC, distensibility coefficient; ICP, isovolumic contraction period; EP, ejection period; NS, non significant.
There were no significant associations between age, pulse pressure and heart rate (not shown). EP was inversely associated with heart rate (p < 0.001), while the composite index ICP/EP was heart rate and blood pressure independent (not shown). DBP/ICP, however, was not independent of heart rate (r = 0.6, p < 0.02). There were no significant within-group associations between any of the arterial function parameters (ΔD, ΔD/Dd, DC, and CC) and either ICP/EP, DBP/ICP or heart rate (not shown).
Biomechanically, artery distension and ejection duration are directly related to cardiac stroke volume. Given the observation that diastolic diameter, DBP, PP and the DC were not different between the groups, stroke volume, as reflected by the ΔD·EP product, is seen to be lower (on average) in the vEDS group (Fig. 3). When ΔD·EP is multiplied by heart rate (significantly higher in vEDS) the difference between the groups disappears (Fig. 3). In younger vEDS patients heart rate tended to be higher than in older patients (not shown).
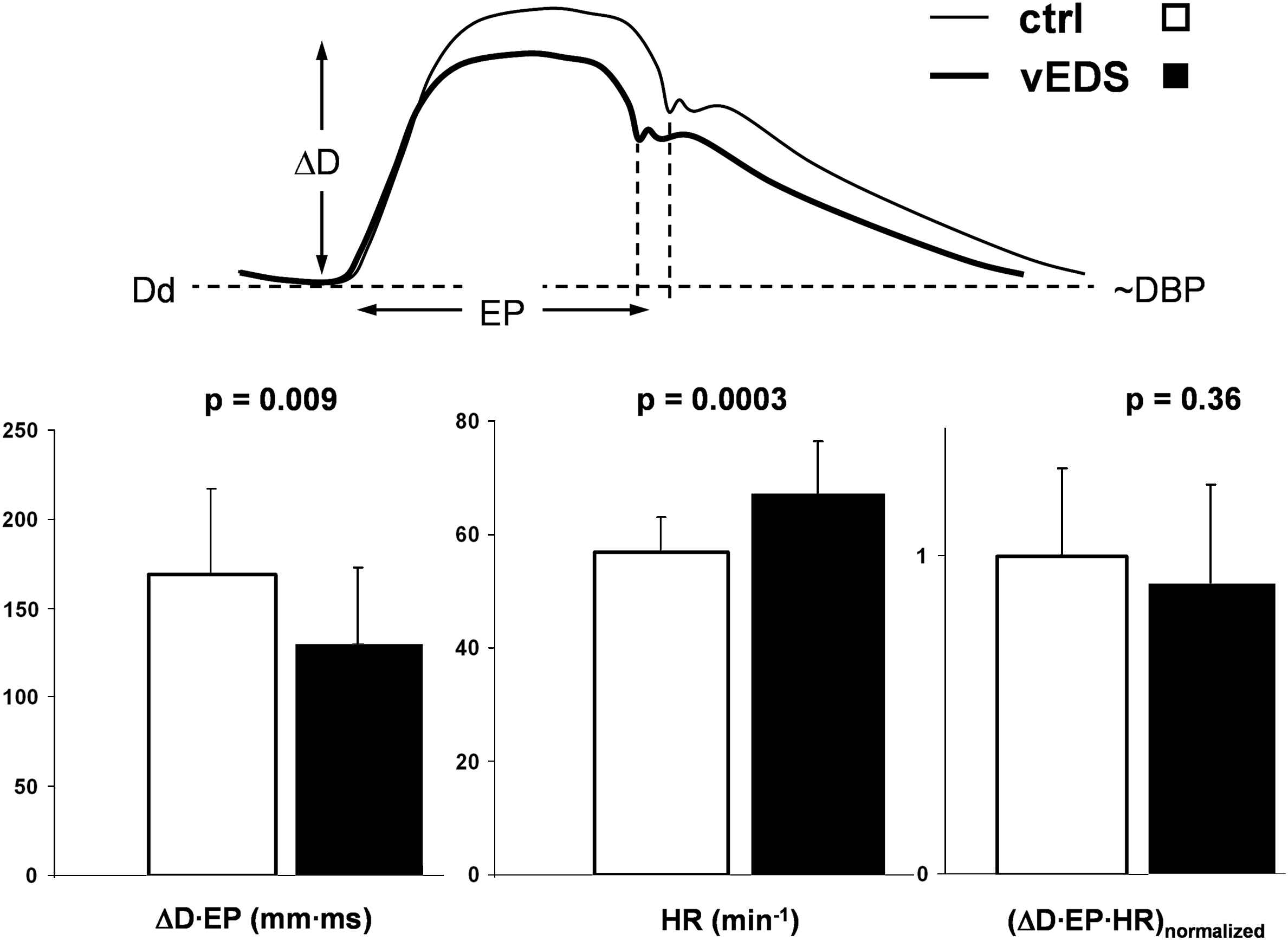
Lower ΔD·EP product reflecting smaller stroke volume in vascular Ehlers–Danlos syndrome (vEDS, N = 19). When corrected for heart rate (ΔD·EP·HR) the difference with controls (N = 19) disappears, suggesting normal resting cardiac output in vEDS. ΔD indicates common carotid artery distension; EP, ejection period; Dd and DBP, diastolic diameter and blood pressure, respectively.
Discussion
In the present study, we obtained non-invasive parameters of both carotid artery and left ventricular function from carotid artery diameter waveforms, as obtained in vascular Ehlers–Danlos syndrome patients and healthy controls. Although carotid artery distension and compliance tended to be lower in the vEDS group, we did not observe significant differences regarding arterial geometry, strain and distensibility, comparing vEDS with normotensive, healthy subjects. We did find that resting heart rate was significantly higher in the vEDS group. This observation and the measured left ventricular systolic time intervals and derived indices support the notion of an elevated sympathetic drive in these patients. Two aspects could explain our findings.
The autonomic nervous system in part controls sympathetic drive to the heart via the baroreflex. Roughly half of the baroreceptors are located in the walls of the carotid arteries. Therefore, changes in elastic wall properties or strain may influence baroreceptor function. A decreased wall strain will lead to lower baroreceptor stretch and, thereby, a lower efferent firing frequency, which will elicit from the cardiovascular centre of the medulla a compensatory response (to increase heart rate) to normalize blood pressure and, thereby, baroreceptor stretch. In the present study, however, no differences in carotid artery strain and distensibility, or blood pressure were observed, making this explanation rather unlikely. Moreover, this explanation would imply that the neural part of the baroreflex loop does not adjust to long term changes, which does not comply with the current insights.12
Another explanation could be that the elevated resting heart rate reflects a lower physical fitness level. These patients most likely have a reduced exercise capacity, because they are discouraged to perform heavy exercise and contact sports,2,13 and they are generally prudent in everyday activity not to acquire bruises, etc. Given the fact that blood pressures and distensibility were not significantly different between vEDS patients and healthy controls, the shorter ejection period and trend towards lower arterial distension suggest a lower stroke volume in vEDS. Biomechanically, ejection duration and arterial distension are directly related to stroke volume. Assuming same compliance, a higher stroke volume will cause a greater arterial distension and requires a longer ejection period. We showed that when the ΔD·EP product is corrected for heart rate, the difference in ‘stroke volume’ disappears (Fig. 3). To the best of our knowledge, vEDS is not associated with ventricular pump failure or insufficient system perfusion, so resting cardiac output might indeed be normal in these patients.
The question remains why pulse pressure is normal in vEDS patients while stroke volume appears lower. This is only possible if arterial compliance is lower in vEDS. Indeed our results show that arterial compliance tends to be lower in the vEDS group, especially in the younger patients (Fig. 2). In other words, in these particular subjects a higher heart rate permits a lower stroke volume, which, in the presence of lower arterial compliance, produces a normal pulse pressure.
Although we were able to study a substantial sample of vEDS patients, our study appears to lack some statistical power to substantiate reduced carotid artery distension and compliance in vEDS. We could not detect the hypothesized lower arterial distensibility in these patients. It is important to note that we assessed carotid artery distensibility under resting conditions. It might be that with exercise distensibility is lower in vEDS patients due to pressure dependent elastic behavior of the arteries.14
While cardiac action appears different in vEDS patients, our study did not show functional LV abnormalities. Rather, the parallel elevation in heart rate and LV contractile performance in vEDS patients likely reflects a normal force–frequency relationship, also known as the Bowditch phenomenon.15 Unfortunately, we did not have echocardiography data available. Therefore, we could not evaluate structural abnormalities of the left ventricle in our study population.
In future studies, changes in heart rate, blood pressure and arterial strain in response to pharmacological intervention or to an orthostatic challenge and (mild) exercise should be investigated, to further identify the biomechanical risk factors for debilitating events in vEDS. A non-invasive and integrated ventricular-arterial function assessment, as presented in this study, could be a very useful tool for this purpose. Currently, there exists no preventive treatment. Our findings and the non-invasive method we used may play a role in developing optimal pharmacological treatment for vascular Ehlers–Danlos syndrome patients.
We conclude that vascular Ehlers–Danlos syndrome patients have normal carotid artery distensibility and pulse pressure but an elevated heart rate, most likely reflecting reduced physical fitness. Our analyses imply that the higher heart rate is linked to maintenance of a normal arterial pulse pressure, especially in the younger patients. Carotid artery ultrasonography and distension waveform analysis enable non-invasive, simultaneous assessment of left ventricular performance and central arterial properties.
Funding
This study was supported by grant
Conflict of interest
None.
Disclosure
None.
References
Cite this article
TY - JOUR AU - Koen D. Reesink AU - Evelien Hermeling AU - Kim-Thanh Ong AU - Martijn C.G.J. Brouwers AU - Robert S. Reneman AU - Pierre Boutouyrie AU - Arnold P.G. Hoeks PY - 2010 DA - 2010/12/04 TI - Non-invasive ultrasound-based assessment of ventricular–arterial interaction in vascular Ehlers–Danlos syndrome patients JO - Artery Research SP - 24 EP - 29 VL - 5 IS - 1 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2010.11.003 DO - 10.1016/j.artres.2010.11.003 ID - Reesink2010 ER -
