Adiponectin negatively correlated with carotid arterial structure in the leptin-resistant Zucker diabetic fatty rat
- DOI
- 10.1016/j.artres.2011.08.001How to use a DOI?
- Keywords
- Leptin; Adiponectin; Luminal cross-sectional area; Medial cross-sectional area; Wall stress; Zucker diabetic fatty rats
- Abstract
Background: Despite adipocytokines are implicated in arterial hemodynamic and stiffness, their effects on arterial histomorphometry remain poorly explored. The aim of the present study was to evaluate, in Zucker Diabetic Fatty (ZDF) rats, a model of type 2 diabetes with leptin resistance, carotid arterial structural changes and their determinants, with special focus on adiponectin and leptin.
Methods: Proximal aortic blood pressure (BP) was measured in conscious ZDF rats (n = 6–8) and their Lean controls (n = 6–8) at 6, 12 and 24 weeks. The contralateral carotid was harvested and fixed at the mean BP for histomorphometric quantification.
Results: Mean BP was similar in both strains and increased with age (p < 0.001). Medial thickness, luminal cross-sectional area (LCSA), medial cross-sectional area (MCSA) and wall stress (WS) increased with age (p < 0.001). LCSA and WS were higher in Lean than in ZDF rats (p < 0.001 for both). Leptin levels were higher in ZDF than in Lean rats (p < 0.001) but remained unchanged during development in ZDF rats. Adiponectin levels decreased with age in ZDF rats (p < 0.001) but remained unchanged in Lean rats. In all rats, adiponectin negatively correlated with medial thickness (r = −0.50, p < 0.01), LCSA (r = −0.64, p < 0.001), MCSA (r = −0.59, p < 0.001) and WS (r = −0.43, p < 0.05). These correlations were significant (p < 0.001) in ZDF rats considered separately (r = −0.73, r = −0.87, r = −0.83 and r = −0.79, respectively) but not in Lean rats; independently of mean BP and age after stepwise regression analyses.
Conclusion: These associations suggest a protective role for adiponectin against arterial wall thickening and wall stress. However for causal relation, further investigation is needed.
- Copyright
- © 2011 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Type 2 diabetes is associated with a high cardiovascular morbidity and mortality related to accelerated atherosclerosis. Several factors contribute to the increase of cardiovascular events, including long-term hyperglycemia, insulin resistance, dyslipidemia, hypertension, changes in clotting factors, vago-sympathetic imbalance and arterial wall structure.1–3
The role of adipocytokines has been extensively highlighted in the recent years.4–7 In particular, leptin level is increased in type 2 diabetic patients, with a relative leptin resistance in this population.4 Inversely, decreased levels of adiponectin have been shown in insulin resistance states.2,5 Both leptin and adiponectin are likely to play a role in arterial hemodynamics.2,4,8 Arterial stiffness and high leptin levels predict cardiovascular events.4,7,9–12
However the relationships between arterial structure and adipocytokines are poorly known. Matsuda et al. have demonstrated that adiponectin-deficient mice showed severe neointimal thickening in mechanically injured arteries, whereas adenovirus-mediated supplement of adiponectin attenuated neointimal proliferation.13 In humans, adiponectin but not leptin levels have been shown to be negatively associated with intima-media thickness (IMT) in middle-aged healthy white subjects, in type 2 diabetes and in 64 year-old women whatever their glycemic status.14–16 In another report, the negative association between IMT and adiponectin observed in men disappeared after adjustment for HbA1c and insulin resistance index.17 Störk et al. have recently shown in post-menopausal non-diabetic women that low levels of adiponectin were associated with adverse changes in morphology and function of central arteries over a 12-month period, independently of other cardiovascular risk factors18 No association was observed for leptin,18 and some authors suggest to consider leptin/adiponectin ratio as a better atherosclerotic marker.19 Furthermore, low adiponectin levels have been associated with increased plaque volume, lipid-rich plaque and pathological intimal thickening.20,21
To our knowledge the putative association between adipocytokines and arterial wall structure has never been studied in animals. Zucker Diabetic Fatty (ZDF) rats develop with time obesity, insulin resistance, diabetes and dyslipidemia, with controversial data regarding arterial blood pressure (BP).22 In addition, ZDF rats are leptin-resistant because of homozygous mutation of the leptin receptor and are therefore of interest to explore the role of adipocytokines, especially adiponectin, in arterial structural changes.
The aim of the present study was then to evaluate, during development in ZDF rats, carotid wall structural changes, and to explore their determinants, with a special focus on adiponectin and leptin.
Methods
Animals
Male ZDF rats (Gmi-fa/fa, n = 22) and their age-matched male controls (Lean (?/fa); n = 20) were obtained from Charles River France (L’Arbresle, France) at 5 or 6 weeks of age and were acclimatized for at least one week before the experiments. The animals were maintained at 22–24 °C with light on from 0600 to 1800. They were fed A04 (UAR) with tap water ad libitum. The study was performed at the 6th, 12th, and 24th week of age, after an overnight fasting. The protocol was approved by the Animal Ethic Committee of Institut National de la Santé et de la Recherche Médicale, Paris, France. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Blood pressure recording
The technique for BP measurement in rats has been recently described in details.23 Briefly, the rats were anaesthetized with pentobarbital sodium (60 mg/kg, ip). A polyethylene catheter, filled with heparinised 0.9% NaCl (50 U/ml), was inserted into the descending aorta, through the right common carotid artery, to measure proximal (central) aortic BP. The catheter was tunneled subcutaneously under the skin of the back to exit between the scapulae and was plugged with a short piece of stainless steel wire. The rats were allowed to recover during 24 h in individual cages. Then, in unanesthetised unrestrained rats, the catheter was connected to a signal processor (MacLab 8, AD Instruments, Oxfordshire, UK) via a pressure transducer (BP-T, EMKA Technologies, Paris, France). Aortic BP signals were recorded during at least 1 h on-line at a sampling rate of 1000 points/sec (Chart version 5.2, AD Instruments) and stored on a microcomputer (PowerMac 4400/200, Apple). Further, a stationary 60 s recording, selected at the end of the recording was analyzed beat-to-beat by means of Chart version 5.2 software. This software automatically detected the minimal value of proximal BP (diastolic BP), maximal value of proximal BP (systolic BP) and calculated mean BP (MBP) and heart rate (HR).
Biochemical assays
Blood samples were taken just after BP recording and before the rats were euthanized. Plasma glucose was analyzed using the Infinity glucose test (Thermotrace, Melbourne, Australia). Plasma insulin concentration was assayed with an ELISA kit (ELIT) obtained from Eurobio (Les Ulis, France). Serum triglycerides and total cholesterol were determined using the IL Test (Instrumentation Laboratory, Milano, Italy) and serum free fatty acids were measured by spectrometric method using the Wako NEFA C Test (Wako Chemicals, Neuss, Germany). The index of insulin resistance was appreciated by the homeostatic model assessment (HOMA). Serum leptin and total adiponectin concentrations were assessed with mouse leptin and adiponectin RIA kits, respectively (Linco Research CAT # HZDP-61HK, St. Charles, MO, USA).
Carotid wall histomorphometry
The left carotid artery was carefully excised. A 1-cm long sample was perfused in vitro with 10% formol containing phosphate-buffered saline at its individual peripheral MBP to provide the tissue fixation closest to the physiological in situ state of the vessel. To that purpose, the carotid segment was mounted in a specially designed organ chamber and connected to a perfusion line both containing 10% formol phosphate-buffered saline. The carotid segment was submitted constantly to the MBP of the animal for 20 min. The sample was secondarily dehydrated and then embedded in paraffin. Two sections (6 μm thickness) were cut and stained with orceine for double-blind measurement of carotid external (EP) and internal (IP) perimeters, and medial thickness (h, the distance between the external and the internal elastic laminae was measured 4 times and the average was calculated; μm). All the measurements (Fig. 1), including luminal cross-sectional area (LCSA = IP2/4π), were performed with a computer-directed colour analysis (Quant’image software, Talence, France). Mean internal diameter (Di = IP/π), medial cross-sectional area (MCSA = (EP2/4 π)-LCSA), wall stress (WS = MBP × Di/2h; Lamé’s equation) were also calculated. The radius/medial thickness ratio (R/h with R = Di/2) was calculated. Elastin content was quantified according to the analysis of optic density.
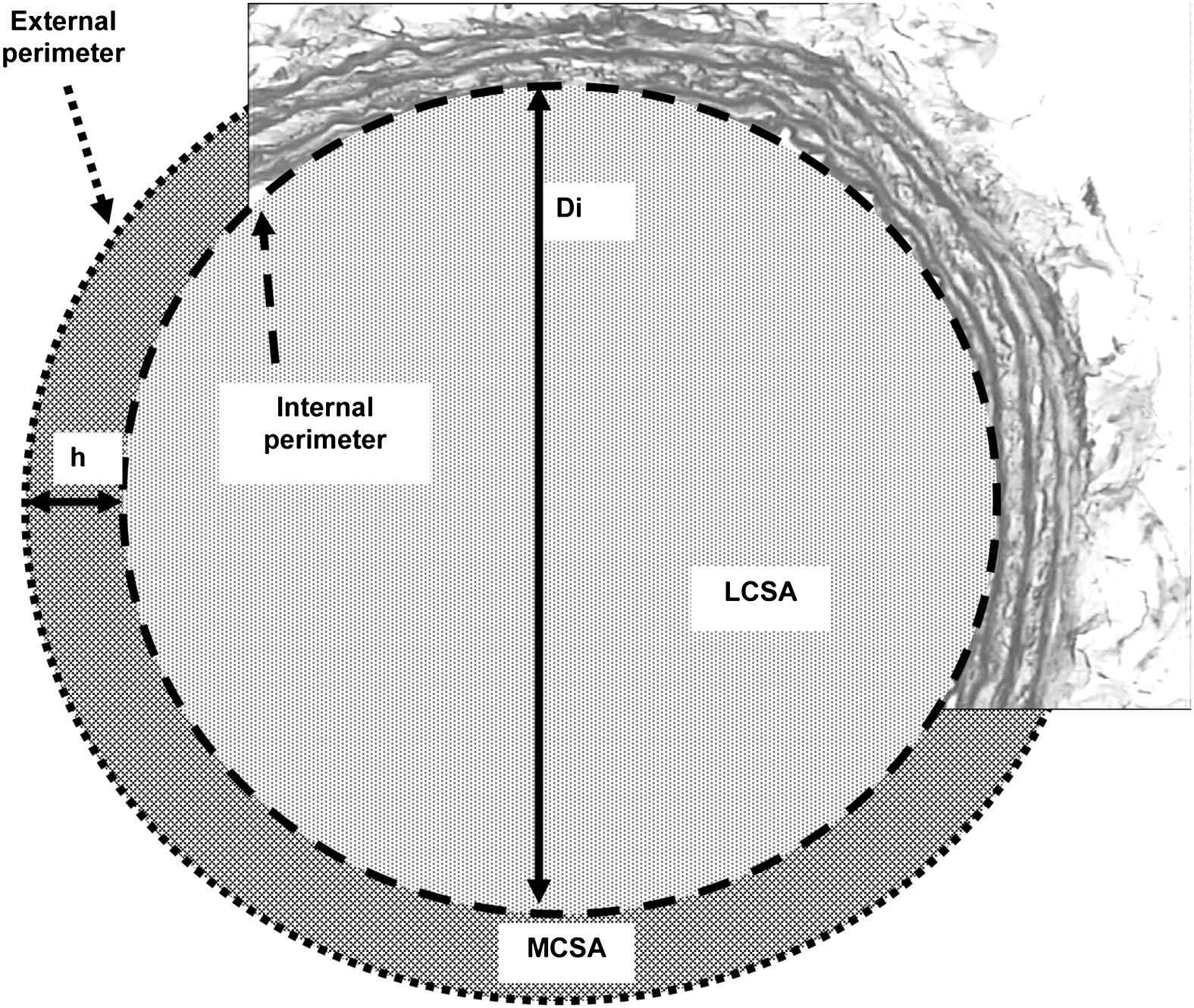
Schematic representation of a section of carotid artery for the determination of histomorphometric parameters: h, medial thickness; Di, mean internal diameter; LCSA, luminal cross-sectional area; MCSA, medial cross-sectional area.
Statistical analyses
Results are given as means ± SEM. Two ways ANOVA followed by a Fischer Protected Least Significant Difference (PLSD) tests for multiple comparisons were used to assess the significance of the results. To evaluate the major determinants of medial thickness, MCSA, LCSA and wall stress, robust stepwise regression analysis was used. Variables entered in the model were body weight, age, MBP (except for wall stress) and adipocytokines (adiponectin for ZDF rats, leptin for Lean rats). The statistical analysis was performed with NCSS 6.0 package software (Hintze JL, Kaysville, Utah, USA). A p value < 0.05 was considered as statistically significant.
Results
Body weight and biochemical parameters
Table 1 shows the changes in body weight and biochemical parameters with age in the two strains. ZDF rats were overweight compared with Lean control rats at wks 6 and 12, but underweight at wk 24. They were diabetic at wk 12 and 24 but not at wk 6, with hyperinsulinemia at 6 and 12 wks but not at wk 24. Plasma lipid levels were higher in ZDF rats as compared with Lean rats at every age. The index of insulin resistance, HOMA, was higher in ZDF rats compared to Lean rats, whatever the age, but was significantly reduced at wk 24 of age as compared to wk 12 of age. Adiponectin levels significantly and progressively decreased with age in the ZDF rats, whereas it did not change with age in the Lean control rats. The significant interaction shows that in ZDF rats, adiponectin was significantly higher at wk 6, but lower at wk 12 and 24 as compared to their controls. Leptin levels were higher in ZDF rats than in Lean rats at wks 6 and 12. Leptin/adiponectin ratio was higher at every age in ZDF rats than in Lean rats.
| Strain | Age (weeks) | p value (ANOVA) | |||||
|---|---|---|---|---|---|---|---|
| 6 | 12 | 24 | Strain | Age | Interaction | ||
| Number of rats | ZDF | 7 | 7 | 8 | |||
| Lean | 7 | 7 | 6 | ||||
| Body weight (g) | ZDF | 140 ± 6 | 326 ± 4b | 357 ± 8b | 0.165 | <0.001 | <0.001 |
| Lean | 120 ± 4a | 290 ± 6a,b | 392 ± 8a,b | ||||
| Plasma glucose level (mmol/l) | ZDF | 7.79 ± 0.19 | 21.7 ± 1.5b | 30.1 ± 1.7b | <0.001 | <0.001 | <0.001 |
| Lean | 7.02 ± 0.32 | 7.64 ± 0.18a | 9.05 ± 0.35a,b | ||||
| Plasma insulin level (pmol/l) | ZDF | 337 ± 38 | 672 ± 102b | 287 ± 45 | <0.001 | <0.001 | <0.001 |
| Lean | 42±5a | 130 ± 18a,b | 243 ± 15b | ||||
| Total cholesterol (mmol/l) | ZDF | 2.60 ± 0.10 | 3.24 ± 0.17b | 5.07 ± 0.35b | <0.001 | <0.001 | <0.001 |
| Lean | 1.91 ± 0.10a | 1.83 ± 0.05a | 2.56 ± 0.07a,b | ||||
| Triglycerides (mmol/l) | ZDF | 0.47 ± 0.08 | 2.58 ± 0.56b | 0.83 ± 0.18b | <0.001 | <0.001 | <0.001 |
| Lean | 0.27 ± 0.04a | 0.29 ± 0.05a | 0.27 ± 0.03a | ||||
| Free fatty acids (mmol/l) | ZDF | 1.36 ± 0.16 | 3.13 ± 0.32b | 1.92 ± 0.18b | <0.001 | <0.001 | <0.001 |
| Lean | 0.84 ± 0.08a | 0.95 ± 0.06a | 0.97 ± 0.06a | ||||
| HOMA | ZDF | 16.29 ± 8.19 | 89.19 ± 8.19b | 55.10 ± 7.66b | <0.001 | <0.001 | <0.001 |
| Lean | 1.81 ± 8.19a | 5.72 ± 7.22a,b | 13.47 ± 8.84a,b | ||||
| Leptin (ng/ml) | ZDF | 10.20 ± 1.22 | 9.66 ± 0.34 | 8.89 ± 0.93 | <0.001 | <0.01 | <0.001 |
| Lean | 1.31 ± 0.18a | 2.91 ± 0.41a | 7.67 ± 0.84 | ||||
| Adiponectin (μg/ml) | ZDF | 18.71 ± 1.43 | 6.00 ± 0.62b | 3.21 ± 0.45b | 0.095 | <0.001 | <0.001 |
| Lean | 8.24 ± 0.96a | 8.40 ± 1.26a | 7.15 ± 0.85a | ||||
| Leptin/adiponectin ratio | ZDF | 0.57 ± 0.09 | 1.76 ± 0.26b | 2.93 ± 0.35b | <0.001 | <0.001 | <0.001 |
| Lean | 0.17 ± 0.02a | 0.37 ± 0.06a,b | 1.17 ± 0.19a,b | ||||
Data are mean ± SEM.
HOMA, homeostatic model assessment.
p < 0.05 versus ZDF at the same age.
p < 0.05 versus week 6 for the same strain.
Weight and biochemical characteristics of ZDF rats and their Lean controls.
Blood pressure, carotid histomorphometric parameters and wall stress
Table 2 and Fig. 2 show the changes in systolic BP and carotid arterial structure with ageing in each strain. Systolic BP was similar in ZDF and Lean rats at wks 6, 12 and 24, with an increase between wks 6 and 12, and stability between wks 12 and 24. The same profile was observed for diastolic BP and MBP (results not shown). Wall stress was lower in ZDF rats and increased with age, especially in Lean rats. R/h ratio was lower in ZDF than in Lean rats (strain effect <0.001) whereas elastin content was similar and stable with ageing in both strains (Table 2). Medial thickness was higher in ZDF rats than in Lean rats only at wk 12, and increased significantly (p < 0.001) with age in both strains. LCSA was significantly lower in ZDF rats than in Lean rats (strain effect <0.001) at wks 6 and 12. MCSA was similar in the 2 strains and significantly increased with age in both strains (age effect <0.001) (Fig. 2).
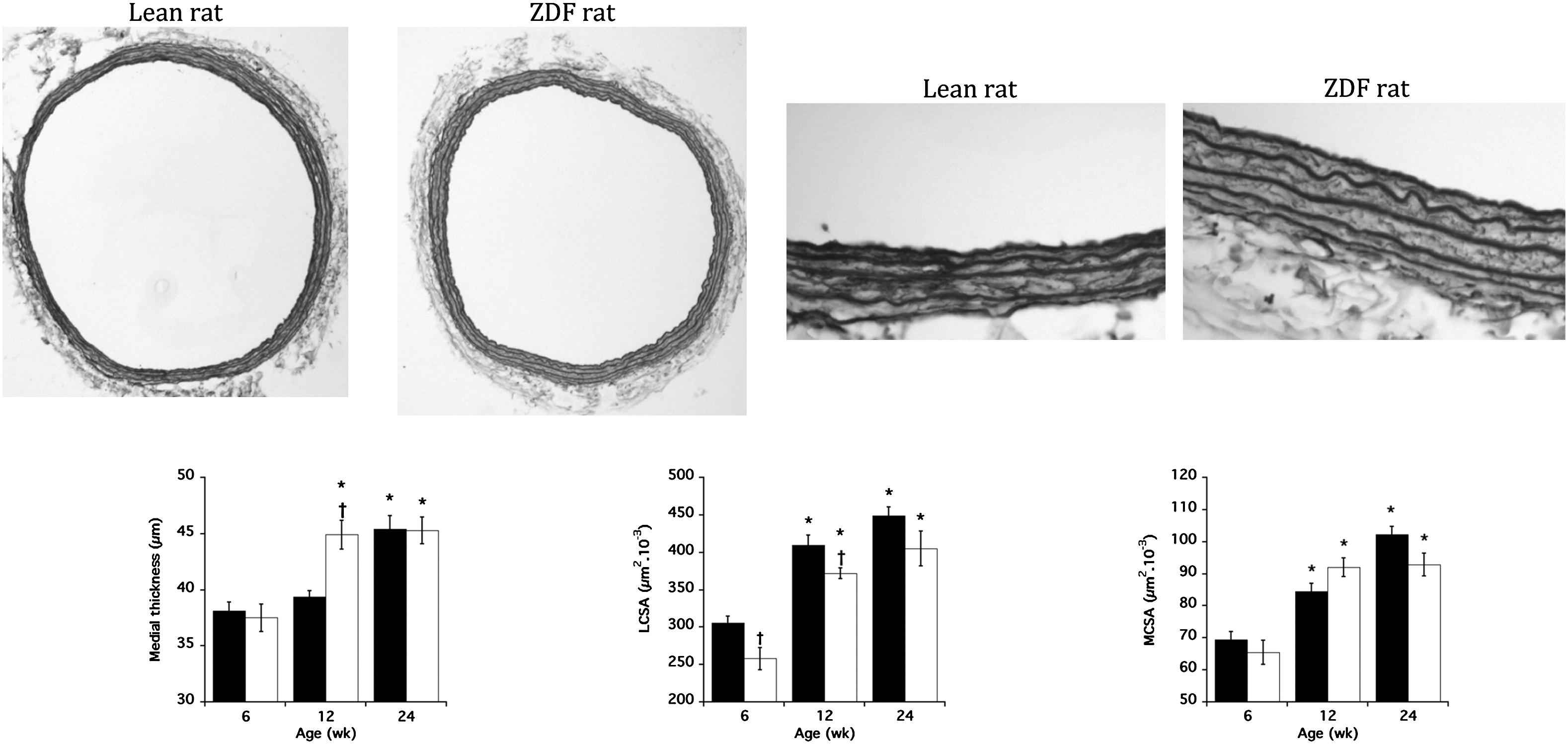
Representative histological images of carotid arteries of a Lean and a ZDF rat at 12 weeks old (orceine staining; x10 and x40) showing significant decrease in luminal cross-sectional area (LCSA) and increase in medial thickness in ZDF rats. The changes of the mean values of medial thickness, LCSA and media cross-sectional area (MCSA) are presented at the bottom. Each bar is the mean ± SEM of 6–8 rats. Full bars, Lean rats; open bars, ZDF rats. *p < 0.001 compared to 6 weeks for the same strain; †p < 0.05 compared to Lean rats at the same age.
| Strain | Age (weeks) | p value (ANOVA) | |||||
|---|---|---|---|---|---|---|---|
| 6 | 12 | 24 | Strain | Age | Interaction | ||
| Number of rats | ZDF | 7 | 7 | 8 | |||
| Lean | 7 | 7 | 6 | ||||
| Systolic blood pressure (mmHg) | ZDF | 121 ± 3 | 131 ± 5b | 136 ± 3b | 0.069 | <0.001 | 0.916 |
| Lean | 125 ± 4 | 138 ± 3b | 140 ± 3b | ||||
| Lean | 69.5 ± 2.5 | 84.5 ± 2.5b | 102.2 ± 2.5b | ||||
| Wall stress (mmHg) | ZDF | 750 ± 27 | 882 ± 21b | 926 ± 41b | <0.001 | <0.001 | 0.168 |
| Lean | 834 ± 49 | 1082 ± 28a,b | 993 ± 40b | ||||
| Radius/medial thickness ratio | ZDF | 7.66 ± 0.31 | 7.69 ± 0.22 | 7.95 ± 0.29 | <0.001 | 0.158 | 0.089 |
| Lean | 8.20 ± 0.20 | 9.16 ± 0.17a | 8.37 ± 0.34 | ||||
| Elastin content (%) | ZDF | 23 ± 1 | 24 ± 2 | 21 ± 3 | 0.837 | 0.177 | 0.716 |
| Lean | 21 ± 3 | 25 ± 1 | 21 ± 1 | ||||
Data are mean ± SEM.
p < 0.05 versus ZDF at the same age.
p < 0.05 versus week 6 for the same strain.
Blood pressure, carotid histomorphometry and wall stress in ZDF rats and their Lean controls.
Correlations between arterial structural parameters and adipocytokines
Table 3 shows the univariate correlations in the overall series of rats and in ZDF rats and Lean rats considered separately. Medial thickness correlated positively with age, body weight and MBP (except in Lean rats taken separately). Similar correlations were observed between LCSA or MCSA and age, body weight and MBP. Wall stress correlated with body weight and age (except in Lean rats considered separately) (Table 3).
| All rats | ZDF rats | Lean rats | ||||
|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | |
| Medial thickness | ||||||
| Age | 0.68 | < 0.001 | 0.59 | < 0.01 | 0.83 | < 0.001 |
| Body weight | 0.73 | < 0.001 | 0.78 | < 0.001 | 0.73 | < 0.001 |
| Mean blood pressure | 0.59 | < 0.001 | 0.86 | < 0.001 | 0.39 | 0.235 |
| Adiponectin | −0.50 | <0.01 | −0.73 | <0.001 | −0.13 | 0.611 |
| Leptin | 0.35 | <0.05 | −0.15 | 0.553 | 0.73 | <0.001 |
| Leptin/Adiponectin ratio | 0.56 | <0.001 | 0.56 | <0.05 | 0.58 | <0.01 |
| Luminal cross-sectional area | ||||||
| Age | 0.74 | < 0.001 | 0.75 | < 0.001 | 0.82 | < 0.001 |
| Body weight | 0.83 | < 0.001 | 0.87 | < 0.001 | 0.91 | < 0.001 |
| Mean blood pressure | 0.69 | < 0.001 | 0.69 | < 0.001 | 0.70 | < 0.001 |
| Adiponectin | −0.64 | <0.001 | −0.87 | <0.001 | −0.14 | 0.599 |
| Leptin | −0.01 | 0.964 | −0.35 | 0.160 | 0.75 | <0.001 |
| Leptin/Adiponectin ratio | 0.33 | <0.05 | 0.57 | <0.05 | 0.75 | <0.001 |
| Media cross-sectional area | ||||||
| Age | 0.77 | < 0.001 | 0.90 | < 0.001 | 0.64 | < 0.01 |
| Body weight | 0.88 | < 0.001 | 0.77 | < 0.001 | 0.91 | < 0.001 |
| Mean blood pressure | 0.68 | < 0.001 | 0.86 | < 0.001 | 0.60 | < 0.001 |
| Adiponectin | −0.59 | <0.001 | −0.83 | <0.001 | −0.15 | 0.551 |
| Leptin | 0.22 | 0.215 | −0.21 | 0.414 | 0.78 | <0.001 |
| Leptin/Adiponectin ratio | 0.49 | <0.01 | 0.57 | <0.05 | 0.76 | <0.001 |
| Wall stress | ||||||
| Age | 0.43 | < 0.01 | 0.65 | < 0.01 | 0.38 | 0.113 |
| Body weight | 0.57 | < 0.001 | 0.78 | < 0.001 | 0.58 | < 0.01 |
| Adiponectin | −0.43 | <0.05 | −0.79 | <0.001 | 0.12 | 0.658 |
| Leptin | −0.18 | 0.319 | −0.34 | 0.185 | 0.38 | 0.130 |
| Leptin/Adiponectin ratio | 0.12 | 0.496 | 0.48 | <0.05 | 0.40 | 0.113 |
Univariate correlations with carotid wall histomorphometric parameters.
Adiponectin significantly and negatively correlated to medial thickness, LCSA, MCSA and wall stress in the overall population and in particular in ZDF rats. There were no correlations in Lean rats considered separately (Table 3). In contrast, leptin significantly and positively correlated to medial thickness, LCSA and MCSA, only in Lean rats considered separately. No correlation was observed between wall stress and leptin (Table 3). The leptin/adiponectin ratio significantly and positively correlated to medial thickness, LCSA and MCSA, but not with wall stress, except in ZDF rats considered separately (Table 3).
Table 4 shows the results of stepwise regression analyses for the determinants of arterial structural parameters in ZDF and in Lean rats. In ZDF rats, MBP and body weight were the only determinants of medial thickness and MCSA, respectively whereas adiponectin was the only independent determinant of LCSA and wall stress. In Lean rats, age and body weight were significant determinants of medial thickness and, LCSA and MCSA, respectively. The two variables were independently associated to wall stress, explaining 34% and 17% of its variance, respectively. Adjustment to other cardiovascular risk factors as total cholesterol and HOMA did not change these results in both ZDF and Lean rats (not shown).
| Variable | ZDF rats | Lean rats | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| In | Standard coefficient | R2 increment | t value | p value | In | Standard coefficient | R2 increment | t value | p value | |
| Medial thickness | ||||||||||
| Mean blood pressure | Yes | 0.867 | 0.752 | 6.737 | <0.001 | No | 0.023 | 1.083 | 0.297 | |
| Body weight | No | 0.017 | 1.008 | 0.330 | No | 0.009 | 0.679 | 0.500 | ||
| Adiponectin | No | 0.013 | 0.879 | 0.394 | ||||||
| Leptin | No | 0.014 | 0.836 | 0.417 | ||||||
| Age | No | 0.007 | 0.628 | 0.540 | Yes | 0.840 | 0.706 | 6.002 | <0.001 | |
| Adjusted global R2 = 0.75 | Adjusted global R2 = 0.71 | |||||||||
| Luminal cross-sectional area | ||||||||||
| Mean blood pressure | No | 0.009 | 0.718 | 0.485 | No | 0.005 | 0.687 | 0.503 | ||
| Body weight | No | 0.010 | 0.776 | 0.450 | Yes | 0.915 | 0.838 | 8.803 | <0.001 | |
| Adiponectin | Yes | −0.871 | 0.759 | −0.868 | <0.001 | |||||
| Leptin | No | 0.000 | 0.102 | 0.920 | ||||||
| Age | No | 0.007 | 0.670 | 0.514 | No | 0.004 | 0.604 | 0.556 | ||
| Adjusted global R2 = 0.76 | Adjusted global R2 = 0.84 | |||||||||
| Media cross-sectional area | ||||||||||
| Mean blood pressure | No | 0.014 | 0.919 | 0.374 | No | 0.002 | 0.478 | 0.640 | ||
| Body weight | Yes | 0.865 | 0.749 | 6.684 | <0.001 | Yes | 0.928 | 0.862 | 9.667 | <0.001 |
| Adiponectin | No | 0.000 | 0.025 | 0.981 | ||||||
| Leptin | No | 0.000 | 0.127 | 0.901 | ||||||
| Age | No | 0.001 | 0.211 | 0.836 | No | 0.019 | 1.475 | 0.162 | ||
| Adjusted global R2 = 0.75 | Adjusted global R2 = 0.86 | |||||||||
| Wall stress | ||||||||||
| Body weight | No | 0.007 | 0.496 | 0.628 | Yes | 1.732 | 0.339 | 3.095 | <0.01 | |
| Adiponectin | Yes | −0.785 | 0.616 | −4.904 | <0.001 | |||||
| Leptin | No | 0.005 | 0.371 | 0.717 | ||||||
| Age | No | 0.005 | 0.415 | 0.684 | Yes | −1.224 | 0.169 | −2.187 | <0.05 | |
| Adjusted global R2 = 0.62 | Adjusted global R2 = 0.50 | |||||||||
In, entering or removal of variable after stepwise regression; Yes, variable entering in stepwise regression model; No, variable is removed after stepwise regression. Adiponectin was used only in ZDF and leptin only in Lean rats.
Stepwise regression analysis of the determinants of arterial structural parameters in ZDF rats and their Lean controls.
Discussion
The present study evaluated vascular wall structure and wall stress in ZDF rats and investigated the correlations with adipocytokines in this model of type 2 diabetes characterized by a leptin resistance. In contrast to their Lean controls, leptin levels were high but did not increase with ageing along with fat mass. Conversely, adiponectin levels decreased with ageing in ZDF rats, but did not change in Lean rats. Carotid histomorphometric analysis showed that LCSA was lower in ZDF rats compared to Lean rats and that medial thickness tended to increase earlier in ZDF rats than in Lean rats. Lower LCSA together with similar medial thickness may suggest that the intima was larger in ZDF rats than in Lean rats. Furthermore, the lower R/h ratio observed in ZDF rats than in Lean rats suggested a eutrophic internal remodeling in the formers. Simultaneously, BP was similar in both strains at every age as we previously reported.23 As a result, wall stress was lower in ZDF rats than in Lean rats. Finally, the major finding of our study was that adiponectin negatively correlated with medial thickness, LCSA, MCSA and wall stress. These correlations were especially observed in ZDF rats, independently of body weight, age and MBP. Conversely, independent positive correlations between leptin and LCSA and between leptin and MCSA were observed in Lean rats.
Compared to Lean rats, ZDF rats revealed to be heavier from 7 to 23 wks old. They had similar body weight as Lean rats between 20 and 31 wks old and became lighter than Lean rats between 28 and 36 wks old.24,25 Likewise, Szöcs et al. have recently shown that body weight gain in ZDF rats was linear and high from 7 to 12.5 weeks (0–150 g) but became very less between 15.5 and 18 weeks olds (15 and 5 g).26 Our results are therefore in accordance with the literature as our ZDF rats were heavier than Lean rats at 6 and 12 wks old but lighter than Lean rats at 24 wks old.
Body weight may play an important role in arterial structural parameters. Indeed, in obese subjects and type 2 diabetic patients, IMT is higher than in controls and is associated to body weight.15 In the present experiments, the lower LCSA together with similar medial thickness suggests that the intima was larger in ZDF rats than in Lean rats. As in humans, in Lean rats, body weight was associated to LCSA, MCSA and wall stress, independently of MBP, age or leptin. By contrast, in ZDF rat, body weight was associated only to MCSA, whereas adiponectin is the major determinant of LCSA and wall stress. These results suggest that in type 2 diabetes, adiponectin may play a major role in arterial structural changes whereas body weight, but not leptin may be the major determinant of arterial structure in obesity.
Adipose tissue is not only a simple energy pool, but also secretes metabolic hormones, enzymes, anti-fibrinolytic proteins, and inflammatory cytokines.6,27 In contrast to adiponectin, other adipocytokines, including leptin, are markedly up regulated in obesity. Despite higher concentrations, the inhibitory effect on food intake of leptin is not enhanced, suggesting a leptin resistance.2,4 The model of ZDF rats is therefore interesting since these rats genetically resist to leptin.22 As a result, we showed, as others, that leptin levels were high in this model and did not increase in parallel with adipose tissue growth.28 In agreement with the leptin resistance, leptin did not correlate in ZDF rats with histomorphometric parameters. In contrast, positive correlations were observed in Lean rats between leptin levels and medial thickness, LCSA and MCSA. The correlation with LCSA and MCSA disappeared in the stepwise regression analysis when body weight was introduced as an independent variable, suggesting that in Lean rats, body weight influences more arterial structure than leptin.
In humans no correlation was found between leptin and IMT in post-menopausal women, in type 2 diabetic patients or in controls.15,18 Furthermore, leptin-deficient ob/ob mice develop only small lesion after arterial injury even if kept on atherogenic diet despite severe obesity and proatherogenic metabolic profile. Administration of leptin enhances lesion formation in wild type and ob/ob mice, despite reducing body weight and plasma lipids and improving insulin sensitivity.29 These experiments and others have suggested that leptin promotes remodeling and neointimal growth; with a protective effect due to leptin resistance30 and that adipocytokines may play a crucial role, which is expected to be emphasized in case of leptin resistance.
One of the most interesting features of adiponectin is that its adipose tissue expression and plasma concentration are reduced in overweight and obese subjects.31 In the present study, adiponectin decreased accordingly with age in ZDF rats as previously reported.32 However, despite a higher body weight than, and similar plasma glucose level as Lean rats, a high level of plasma adiponectin was observed in ZDF rats at 6 wks old. This seems unexpected. Nonetheless, Szöcs et al. have shown a negative association between plasma glucose and plasma adiponectin in ZDF rats and have suggested that the impact of metabolic deterioration on circulating adiponectin is by far superior to established direct influences of body weight.26 Therefore, the changes in biochemical parameters observed in the present experiments are in agreement with those reported by others.24,26
In the present study, in ZDF rats, adiponectin correlated with MCSA, LCSA and wall stress, independently of age and BP. In humans, a negative correlation was also observed between adiponectin and IMT in post-menopausal women whatever their glucose status,16,18 in middle-aged healthy white subjects,14 in patients with coronary arterial disease,33 in men17 and in people with34 or without diabetes.15 Such an association does not mean a causal relationship. Therefore, animal studies might further highlight the potential role of adiponectin on vessel wall structure as shown in adiponectin-deficient mice and in rabbits.13,35 In the latter, local transfer of adiponectin gene in aorta adventitia or intima reduced the development of atherosclerotic plaques.
The role played by adiponectin may be multifactorial. First, adiponectin has been shown to exhibit anti-proliferative properties. This effect may be exerted through inhibition of growth factors.36,37 Adiponectin has been reported to inhibit angiogenesis, endothelial cell proliferation and migration and to be tightly related to endothelial function.38–41 When the vascular endothelium is injured, adiponectin accumulates in the subintimal space of the arterial wall through its interaction with collagen in the vascular intima.42 Adiponectin inhibits tumor necrosis factor (TNF) α-induced monocyte adhesion and expression of endothelial-leucocyte adhesion molecule 1 (E-selectin), vascular cell adhesion molecule-1 (VCAM-1)43 and intracellular adhesion molecule-1 (ICAM-1) on the endothelium.44 Recently it has been shown that adiponectin exerts an NO-dependent vasodilatation in resistance arteries of Lean rats but not ZDF rats, and this may contribute to endothelium dysfunction in ZDF rats, leading to the changes observed in arterial structure.45 Adiponectin also suppresses lipid accumulation in monocyte-derived macrophages through the suppression of macrophage scavenger receptor expression.13 Finally, adiponectin may also play a beneficial role through a decrease in arterial stiffness,46 as suggested by a negative correlation between these parameters in patients with hypertension,9 metabolic syndrome47 and in Black asymptomatic young adults.48 Furthermore, the changes in arterial stiffness under glitazones or metformin correlate negatively with changes in plasma adiponectin levels.10
In conclusion, the different associations observed between arterial structure and adipocytokines in ZDF rats, a model of insulin resistance, although not providing a direct causal relationship, strongly suggest that adiponectin may play a protective role against arterial wall thickening and wall stress and that the balance between leptin and adiponectin may be crucial in obesity-related arterial structural changes. The data further suggest that modifying adiponectin levels may be beneficial against cardio-metabolic disease.3 In this respect, further experiments remained to be performed to draw a clear-cut conclusion.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
We thank Mrs Monique Herissé for assistance in the investigations.
Abbreviations
- BP
blood pressure
- Di
mean internal diameter
- EP
external perimeter
- h
medial thickness
- IMT
intima-media thickness
- IP
internal perimeter
- LCSA
luminal cross-sectional area
- MBP
mean BP
- MCSA
medial cross sectional area
- R/h
radius/medial thickness ratio
- WS
wall stress
- ZDF
Zucker Diabetic Fatty
References
Cite this article
TY - JOUR AU - Emmanuel Cosson AU - Paul Valensi AU - André Bado AU - Hubert Dabiré PY - 2011 DA - 2011/08/19 TI - Adiponectin negatively correlated with carotid arterial structure in the leptin-resistant Zucker diabetic fatty rat JO - Artery Research SP - 12 EP - 20 VL - 6 IS - 1 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2011.08.001 DO - 10.1016/j.artres.2011.08.001 ID - Cosson2011 ER -
