Evaluation of losmapimod in patients with chronic obstructive pulmonary disease (COPD) with systemic inflammation stratified using fibrinogen (‘EVOLUTION’): Rationale and protocol
Joint first authors.
- DOI
- 10.1016/j.artres.2013.10.380How to use a DOI?
- Keywords
- COPD; Losmapimod; p38 MAPK inhibitor; Fibrinogen; Inflammation; 18F-FDG PET/CT
- Abstract
Introduction: p38 mitogen-activated protein kinases (MAPK) are key signalling molecules in cellular responses to external stresses, regulate pro-inflammatory cytokine expression and are implicated in the inflammatory pathogenesis of Chronic Obstructive Pulmonary Disease (COPD) and atherosclerosis. The EVOLUTION trial is a randomised, double-blind, placebo-controlled, Phase 2a trial recruiting from two UK centres that aims to evaluate the effects of Losmapimod (GW856553), a potent oral p38α/β MAPK inhibitor in COPD patients stratified by a fibrinogen level >2.8 g/L.
Methods: Patients are randomised to 7.5 mg losmapimod or matching placebo tablets twice daily for four months. Vascular and pulmonary inflammation is assessed by 18F-FDG PET/CT at baseline and following treatment. Other endpoints including flow-mediated dilatation, aortic pulse wave velocity, spirometry, six-minute walk distance and blood biomarkers of inflammation, are evaluated pre- and post-treatment.
Results: The primary endpoints following 16 weeks treatment include, 1) change in arterial inflammation measured by 18F-FDG PET/CT signal in the aorta and carotid arteries, 2) change in atheromatous plaque and aortic wall characterisation evaluated by 18F-FDG PET/CT and MRI, and 3) change in flow-mediated dilatation.
Key secondary endpoints include change in 1) pulmonary inflammation evaluated by 18F-FDG PET/CT, 2) change in respiratory and physical function indices, 3) arterial stiffness, and 4) measures of systemic biomarkers. Safety endpoints include serious and non-serious adverse events, clinical laboratory results and ECGs.
Discussion: Data gained from the EVOLUTION trial will provide novel information on pulmonary and extra-pulmonary effects of losmapimod in inflammatory disease, in a COPD population with evidence of systemic inflammation.
- Copyright
- © 2013 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is the third leading cause of death globally and is associated with substantial morbidity and healthcare utilisation.1–3 Although COPD is by definition, a pulmonary condition, it is associated with extra-pulmonary features, which include systemic inflammation, cardiovascular disease and muscle weakness. Cardiovascular disease accounts for half of all deaths in patients with mild to moderate COPD, and airflow limitation is inversely associated with cardiovascular mortality.4–6 Due to shared risk factors of COPD and cardiovascular disease; it is unclear whether a causal association between COPD and the coexistence of cardiovascular disease exists. However, recent evidence does suggest that COPD may indeed be an independent risk factor.7 This in part may be due to low-grade chronic systemic inflammation seen in COPD, since atherosclerosis is thought to be an inflammatory driven condition.8,9
Elevated levels of circulating biomarkers and cytokines, indicating systemic inflammation, are associated with COPD and cardiovascular disease. Raised fibrinogen levels are associated with impaired lung function, hospitalisation due to COPD, exacerbations and cardiovascular comorbidity and are also associated with cardiovascular risk in the general population.10–15 Of note, plasma fibrinogen is currently undergoing a regulatory qualification process as an enrichment biomarker for clinical trials in COPD.16
Inhibition of p38 mitogen-activated protein kinase (MAPK) is an attractive therapeutic target for both COPD and cardiovascular disease, because the p38 MAPK cascade is believed to have an important role in the initiation and progression of inflammatory disease. p38 MAP kinases are key signalling molecules in cellular responses to external stresses such as tobacco smoke and regulate pro-inflammatory cytokines.17 Such pro-inflammatory cytokines are implicated in the inflammatory pathogenesis of COPD and atherosclerosis,18–20 (Figs. 1 and 2).
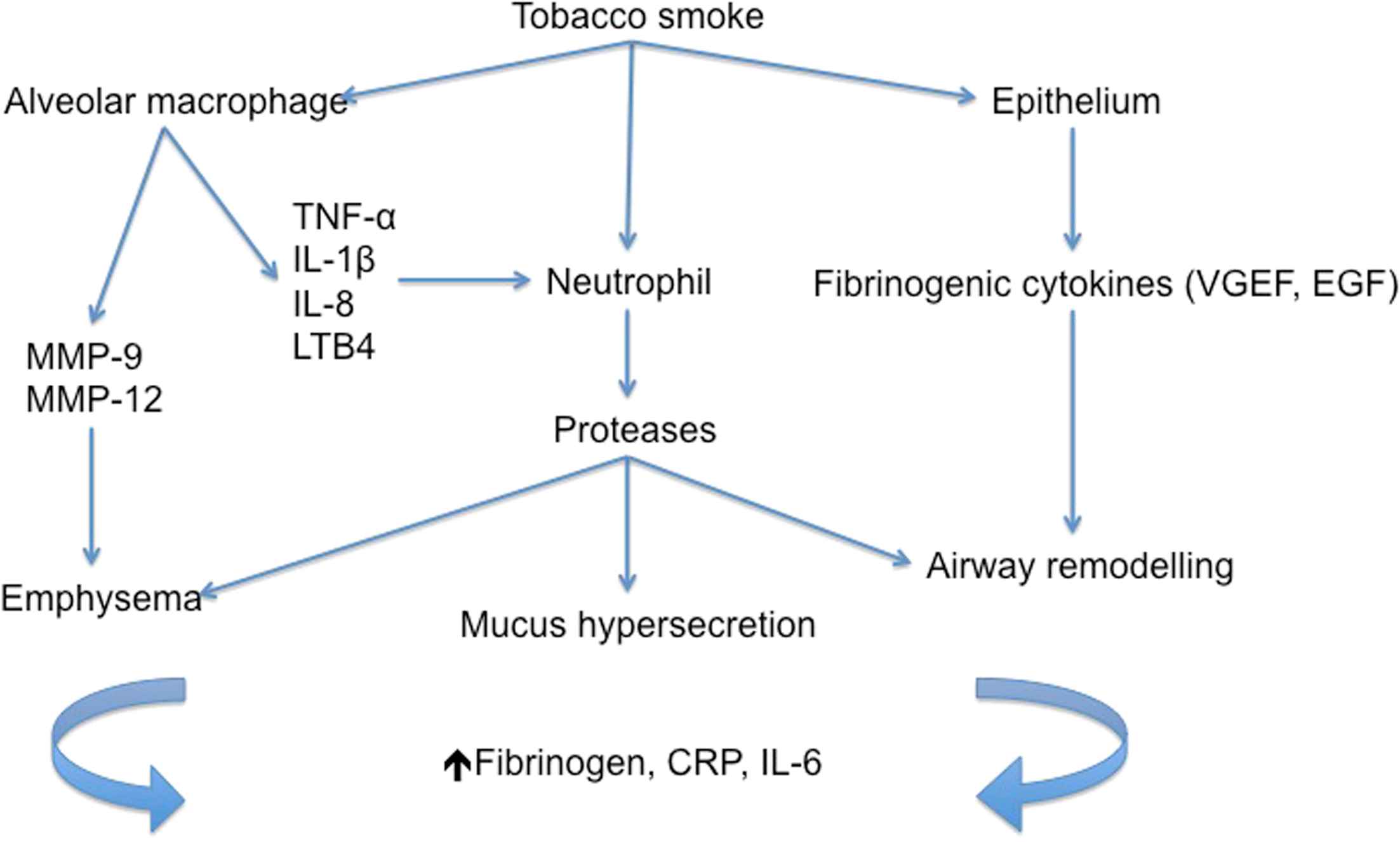
Inflammatory pathogenesis of COPD. The diagram demonstrates current understanding of inflammatory mediators and processes that lead to COPD.
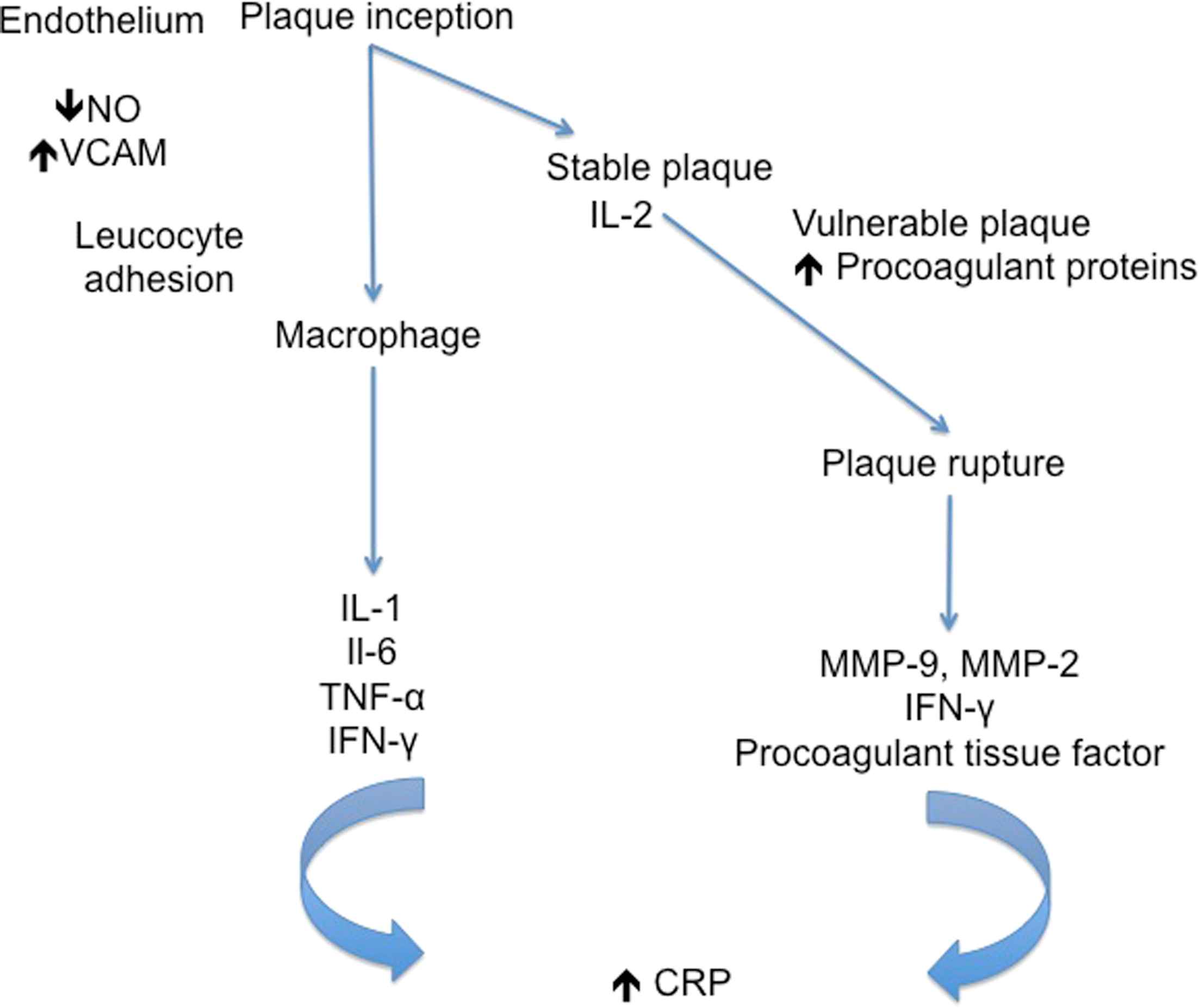
The role of inflammatory mediators in different stages of atherosclerosis.
Losmapimod (GW856553, GlaxoSmithKline [GSK], Brentford, UK) is a novel, potent, selective inhibitor that competes with adenosine triphosphate (ATP) for binding to p38 MAPKα/β. Losmapimod has been shown to improve endothelial dysfunction and vasoregulation in hypercholesterolaemics, and reduce arterial inflammation in patients with stable atherosclerosis.21,22 In COPD patients, a Phase 2a clinical trial with losmapimod showed a significant reduction in plasma fibrinogen and improvement in hyperinflation relative to placebo.23
The EVOLUTION trial is an exploratory, stratified medicine study (registered on ClinicalTrials.gov, NCT01541852) designed to evaluate the hypothesis that losmapimod will reduce arterial inflammation in an enriched COPD population. The trial is unique in using fibrinogen as an enrichment biomarker entry criterion. We hypothesize that subjects enrolled in the trial are more likely to have systemic inflammation, with the overall aim to improve future evaluation of anti-inflammatory medicines in COPD. The effects of orally administered losmapimod on arterial (aorta and carotid arteries) inflammation, and pulmonary inflammation will be assessed by 18F-Fluorodexoyglucose positron emission tomography with computed tomography co-registration (18F-FDG PET/CT). A magnetic resonance imaging (MRI) scan of the aorta in a subset of eligible patients will further enhance analysis of aortic wall and plaque response to losmapimod. The safety of losmapimod and effect on lung function and blood biomarkers in inflamed COPD patients will also be assessed.
Methods
Trial design
EVOLUTION is a prospective, randomised, double-blind placebo-controlled, parallel-group multicentre trial recruiting from two UK centres (Cambridge and London). Randomisation to active drug or placebo is performed at each site. Blinded study medication and regulatory support is provided by GlaxoSmithKline.
Role of funding source
This is a collaborative study, which is a component (work package 2) of the ERICA (Evaluation of the Role of Inflammation in Chronic Airways Disease) Consortium.24 Funding for this study has been awarded by the Technology Strategy Board (9157-61188). The funding source had no involvement in the study design, and has no role in the collection, analysis and interpretation of data, report writing or decision to submit this article for publication. An educational fellowship award from GlaxoSmithKline is also acknowledged in contributing to financial support for staffing for the trial.
The trial is jointly sponsored by the Cambridge University Hospitals NHS Foundation Trust (Cambridge Clinical Trials Unit) and the University of Cambridge. The sponsors’ role includes trial implementation, data handling and analysis, audit and medical governance.
Study organisation
GlaxoSmithKline is a partner in this collaborative academic-industry research initiative into stratified medicines. The trial steering committee which developed the original design and concept of the trial consists of ten academic physicians and representatives from GlaxoSmithKline, and is led by an independent chair from the University of Cardiff who also acts as the Medical Monitor. The study is being conducted in accordance with the Declaration of Helsinki and Good Clinical Practice standards. The two participating sites received favourable opinion from the local ethics committee as well as ARSAC (Administration of Radioactive Substances Advisory Committee) approval. All patients provide informed written consent before enrolment in accordance with the Declaration of Helsinki.
Patient population
The study includes COPD subjects as defined by GOLD (Global Initiative for Chronic Obstructive Pulmonary Disease),25 as well as a subset of GOLD-Unclassified (GOLD-U)26 aged 50–85 years, and entry to the trial is based on a fibrinogen level >2.8 g/L at screening.
Key exclusion criteria are related to the diagnosis of other respiratory disorders, inflammatory conditions, participation in prior research studies with radiation exposure, or the use of systemic steroids one month prior to baseline assessment and visit 2 (18F-FDG PET/CT scan). The full inclusion and exclusion criteria are listed in Tables 1 and 2.
A participant will be eligible for inclusion only if all of the following criteria apply:
|
Inclusion criteria of the EVOLUTION trial.
The presence of any of the following will preclude patient inclusion:
|
Exclusion criteria for EVOLUTION trial.
Randomisation & trial treatments
Randomisation to either losmapimod 7.5 mg (GW856553) or matching placebo twice daily is via an online coded programme (TENALEA data management, Holland). Blinded investigational medicinal products are supplied by GlaxoSmithKline, Harlow, UK.
Losmapimod 7.5 mg BD or placebo is provided for a treatment period of 16 weeks. This dose was selected based on pharmacodynamic evidence obtained previously in relevant populations. For example, in COPD, this dose led to a rapid and sustained reduction in systemic inflammation markers, especially plasma fibrinogen23 and in stable atherosclerosis, this dose led to reduction in vascular inflammation in the most inflamed regions (area with highest 18F-FDG measured uptake), as well as observed reduction in serum inflammatory biomarkers and 18F-FDG visceral fat uptake.22
Trial visits schedule
Details of trial visits, including measures performed at each visit, are provided in Table 3 and a trial flow chart is shown in Fig. 3. Participants are entered into the trial for a period of approximately six months, with ten scheduled outpatient visits. Blood and urine samples, vital signs, adverse events and a concomitant medication check are performed at all visits. Aortic pulse wave velocity (to evaluate large artery stiffness) and flow-mediated dilatation (to assess endothelial function) are performed at visits three and nine and are described in further detail below.
| Assessments | Treatment days | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Screening V1 | Day −14 to −1 Imaging visit V2 | Day 1 V3 | Day 14 (Days 10–18) V4 | Day 28 (Days 24–32) V5 | Day 56 (Days 52–60) V6 | Day 84 (Days 80–88) V7 | Day 107 (Days 105–111) imaging visit V8 | Day 112 (Days 109–112) V9 | Day 120–126 follow-up V10 | |
| Attend unit | * | * | * | * | * | * | * | * | * | * |
| Medical history | * | |||||||||
| Demography | * | |||||||||
| Physical examination | * | * | ||||||||
| Standard supine 12-lead ECG (triplicate) | * | * | * | |||||||
| Blood: haematology (FBC, LFT Chemistry (Urea, Uric acid), urinalysis | * | * | * | * | * | * | * | * | ||
| Hep B, Hep C and HIV Test, HbA1C | * | |||||||||
| Biomarkers (plasma fibrinogen, hsCRP) | * | * | * | * | * | * | * | * | ||
| Bloods for imaging (glucose & radiation levels) | * | * | ||||||||
| Lipid profile, serum store | * | * | ||||||||
| FDG-PET/CT | * | * | ||||||||
| MRI (optional) | * | * | ||||||||
| Adverse event check | * | * | * | * | * | * | * | |||
| Concomitant medication check | * | * | * | * | * | * | * | |||
| Arterial haemodynamics | * | * | ||||||||
| Flow mediated dilatation | * | * | ||||||||
| Muscle assessment (SNIP) | * | * | ||||||||
| Spirometry | * | * | * | |||||||
| 6 Minute walk test | * | * | ||||||||
| Randomisation | * | |||||||||
| Vital signs | * | * | * | * | * | * | * | * | ||
| Dispensing of IMP | * | * | * | * | ||||||
Trial visits schedule & events. The table highlights the various tests performed at different visits of the trial.
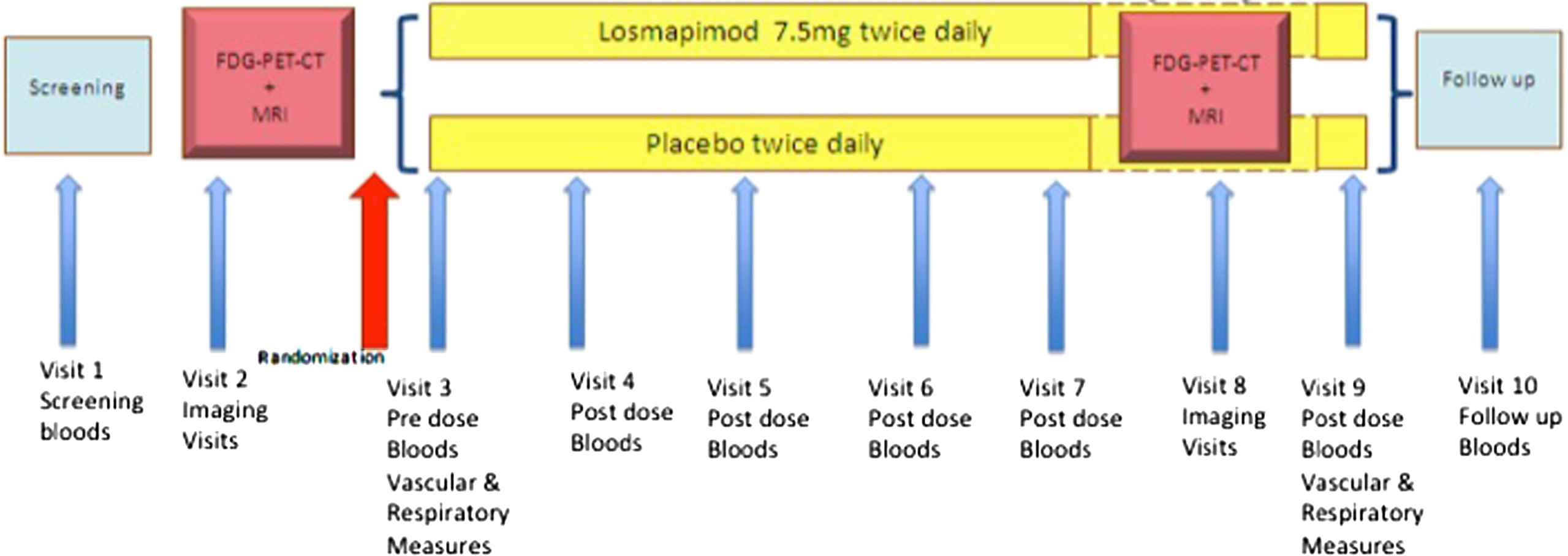
Trial flow chart.
Arterial stiffness measurements
Aortic pulse wave velocity (APWV) is performed before and on completion of 16 weeks of losmapimod versus placebo therapy. All haemodynamic studies are conducted in a quiet, temperature controlled room. Seated brachial blood pressure is first recorded using a validated oscillometric sphygmomanometer (OMRON-705CP, OMRON Corp, Japan). Then, using a high-fidelity micromanometer (SPC-301, Millar Instruments, Houston, Texas), radial artery waveforms are recorded and a corresponding central waveform generated using a validated transfer function27,28 (SphygmoCor, AtCor Medical, Sydney, Australia) as described previously. The augmentation index (AIx), which is a composite measure of arterial stiffness, wave-reflection amplitude and heart rate, is calculated using the integral software. Aortic PWV is measured by sequential recording of ECG-gated carotid and femoral pulse waveforms, as described previously.29 Pulse wave velocity is calculated by dividing the surface distance between the recording sites by the wave transit time.
Endothelial function measurements
Endothelial function is evaluated in the brachial artery with the technique of flow-mediated dilatation.30 Using a high-resolution vascular ultrasound (Acuson 128XP/10; Siemens AG, Germany) with a 5–20 MHz linear-array transducer, vessel diameter is measured continuously for 1 min at baseline and for a further 5 min, following release of a cuff inflated to 200 mm Hg, placed distal to the ultrasound probe. After returning to baseline, vessel diameter is again measured continuously for 5 min after administration of 25 μg of sublingual glycerl trinitrate (GTN). FMD is defined as the maximum percentage increase in vessel diameter during reactive hyperaemia; GTN-mediated dilatation is defined as the maximum percentage increase in vessel diameter after sublingual administration. This is a standard technique described in previous studies.
Imaging protocols
Scanning protocol information for the 18F-FDG PET/CT (which includes a high resolution computed tomography scan (HRCT) at baseline only) and an optional MRI scan, are provided in the Supplementary Appendix. The effective radiation dose is estimated to be 21 mSv for the entire study. To compare this with health risks of daily living, this radiation dose is equivalent to having two diagnostic CT scans of the body, and is below the 50 mSv threshold considered to be the radiation limit for research purposes by ARSAC.31
Trial endpoints
This is an exploratory study and has a range of endpoints centred around the effects of losmapimod on vascular and pulmonary inflammation in COPD patients selected based on an enrichment biomarker (plasma fibrinogen). The endpoints of the trial are summarised in Table 4. Participant withdrawal and dose stopping criteria are provided in the Supplementary Appendix.
Primary endpoints
|
Secondary endpoints
|
EVOLUTION trial Primary and Secondary Endpoints.
Trial size and data analysis
Sample size estimation for this exploratory trial is based on feasibility and a formal power calculation was inappropriate for such an experimental trial. However, data from our previous 18-F-FDG PET study in stable atherosclerosis which evaluated the effects of losmapimod on vascular inflammation in atherosclerosis determined a feasible number per arm (approximately 30 per arm) to provide a 90% chance of achieving a reasonable conclusion (SD of 0.3 for TBR).22 TBR is a measure of tissue 18F-FDG uptake corrected for blood 18FDG uptake.
The methodology for evaluating vessel wall tracer uptake is established from previous PET/CT vascular imaging studies.22,32,33 From co-registered PET/CT images, a Standard Uptake Value (SUV) and a Target to Background Ratio (TBR) are calculated using dedicated software (OsiriX 5.52, OsiriX Imaging Software, Geneva, Switzerland). The maximum standardized uptake values (SUV) of 18F-FDG within each region of interest defined on scan slices, containing the arterial wall and the lumen, will be recorded and divided by blood 18F-FDG concentration (average of 5 slices) in the superior vena cava or jugular vein (for carotids) to yield an arterial TBR ratio.
Assessment of pulmonary tissue 18F-FDG uptake will involve quantification of tracer uptake in pulmonary regions of interest using a voxel-based 3-dimensional Patlak map analysis. This enables calculation of the ratio of lung tissue PET signal (SUV level) to plasma radioactivity normalised to the Patlak determined intercept and visualisation of uptake. Plasma 18F-FDG activity will be measured by venous blood sampling performed during the dynamic lung acquisition scan. Glucose samples are also taken to evaluate serum glucose levels during this acquisition scan.
For all derived variables and endpoints to be evaluated in the trial, summary statistics and change from baseline will be reported and assessed by time-point and treatment group.
All participants who receive at least one dose of trial drug will be included in the safety population data. Participants in the safety population will be classified according to the treatment received.
Discussion
To our knowledge, this is the first trial in which fibrinogen has been used as an entry criterion to identify patients with high vascular inflammation risk. Given that this biomarker has been selected by the COPD Biomarker Qualification Consortium (CBQC) for regulatory qualification as a drug development tool,16 it is anticipated that this trial will provide some novel data on the use of targeted anti-inflammatory therapies in COPD patients with high baseline measures of systemic inflammation.
p38 MAPK in COPD: pulmonary and non-pulmonary manifestations
Studies have shown increased p38 MAPK in alveolar walls of COPD subjects compared to smoking and non-smoking controls and a preclinical study of a selective p38 MAPKα/β inhibitor in a murine model of tobacco smoke-induced inflammation showed markedly reduced inflammatory responses, whereas oral dexamethasone was ineffective.34,35 This demonstrates that p38 MAPK inhibitors and steroids mediate their effects on different anti-inflammatory pathways. p38 MAPK inhibitors have been shown in vitro to reduce cytokine production in different lung cells of COPD subjects, including alveolar macrophage bronchial epithelial cells, and lymphocytes.36,37 Early clinical trials of different p38 MAPK inhibitor compounds have shown improvement in both fibrinogen levels and trough FEV1.23,38
p38 MAPK inhibitors are also currently being evaluated in cardiovascular disease and early clinical studies have shown improved nitric-oxide mediated vasodilatation in hypercholesterolaemia and a reduction in vascular and systemic inflammation in stable atherosclerosis.21,22 The results of the SOLSTICE (Study of Losmapimod treatment in inflammation and infarct size) trial evaluating the effect of losmapimod in ACS (Acute Coronary Syndrome) have been encouraging from a cardiovascular viewpoint.39,40
Fibrinogen stratification
Fibrinogen has proven to be one of the most promising biomarkers in COPD in prognosis and profiling of patients both at baseline and repeated measurement. It does not show the variability observed in repeated CRP (C-reactive protein) measurement, and is a widely available cost-effective clinical laboratory test.11 The use of biomarkers beyond improving delineation of a population to stratifying treatment is however the most clinically effective way to improve patient outcomes. Evidence based guidelines for statin therapy use, glycosylated haemoglobin (Hb1ac) for diabetic control and troponin in the acute coronary syndrome demonstrate how biomarker stratification can direct targeted treatments.
The recruitment of subjects with plasma fibrinogen >2.8 g/L was selected to include patients approximately in the upper and middle tertiles of the general population. Dahl showed in a cross sectional Danish population that fibrinogen values of 2.7–3.3 g/L and >3.3 g/L compared to <2.7 g/L in smokers and non-smokers were associated with worse FEV1 and increased risk of hospitalisation due to COPD.41 In addition, epidemiological studies from the United States have shown the mean fibrinogen level is approximately 3 g/L.42 Hence an enriched cohort of COPD subjects with evidence of systemic inflammation is likely the most relevant patient group to benefit from therapy in a small trial with a novel anti-inflammatory medication. A post-hoc analysis centred on response to treatment compared to fibrinogen level within the trial group will also be of value; particularly as studies of p38 MAPK inhibitors in non-stratified COPD patients have also shown preliminary evidence of benefit.23,38
18F-FDG PET/CT
The use of 18F-FDG PET/CT to assess vascular inflammation has been evaluated in previous clinical studies assessing arterial wall inflammation in rheumatoid arthritis, atherosclerosis, and in a small pilot study involving COPD patients.32,33,22,43 Aortic inflammation identified by 18F-FDG PET/CT in rheumatoid arthritis, is thought to account for arterial stiffening and endothelial dysfunction observed in this condition.32 Indeed, the increased cardiovascular (CV) risk in rheumatoid arthritis is independent of traditional CV risk factors and postulated to be due to systemic inflammation.44,45 In atherosclerosis, tracer uptake reflects plaque inflammation, which has been validated by histology as correlating with macrophage rich areas of plaque.46 Vascular inflammation quantified by 18F-FDG PET/CT has also been observed in COPD subjects in comparison to healthy ex-smokers as controls.43
Additionally, 18F-FDG PET/CT has been used to evaluate pulmonary parenchymal inflammation in a range of respiratory conditions including cystic fibrosis, pneumonia and pulmonary fibrosis.47,48 Though 18F-FDG is a non-specific tracer, relying on cellular uptake of glucose, it enables assessment of all types of metabolically active inflammatory cells. In COPD, where a host of inflammatory cells are implicated in pulmonary inflammation and coexistent atherosclerosis is a likely finding, it is an ideal tracer to use to evaluate the objectives of this study.49,50
Aortic stiffness and endothelial function
Aortic pulse wave velocity (PWV) (carotid to femoral PVW) is a robust predictor of cardiovascular risk.51 We have previously demonstrated a strong association between PWV and inflammation (defined by serum CRP levels) in health and disease.52–54 Others have similarly shown a positive association between PWV and circulating biomarkers including interleukin-6 (IL-6) and fibrinogen.55,56 We have also shown that intervention with anti-inflammatory agents reduces aortic PWV as well as direct vascular inflammation using 18F-FDG PET/CT, suggesting that inflammation may have a role to play in this process.57,32
Aortic PWV is elevated in COPD patients compared to matched controls adjusted for cofounding variables, and systemic inflammation present in COPD may be a contributing factor.58 The hypothesis is that systemic inflammation drives atherosclerosis, which may account for the increased cardiovascular risk in COPD, by impairing endothelial function and promoting premature arterial stiffening, both of which predispose to atheroma development.
Endothelial dysfunction is also an independent predictor of future cardiovascular events.59 In COPD patients, it is related to the severity of airflow limitation and extent of emphysema measured on CT scan.60,61 Losmapimod has been shown to improve endothelial function in stable hypercholesterolaemics21 and its effect on endothelial function in COPD patients will certainly be of interest.
This trial therefore provides an opportunity to test the hypothesis that using a targeted anti-inflammatory therapy in COPD patients may help reduce vascular inflammation, (as assessed by 18F-FDG PET/CT) and also evaluate its effects on aortic stiffness and endothelial function.
Losmapimod safety & tolerability
To date, losmapimod has been evaluated in a range of inflammatory conditions and in total over 2000 subjects have been exposed to the drug. Various trial dosing regimens and duration periods have been assessed including a 6 month Phase 2b COPD trial at doses of 2.5 mg and 7.5 mg and 15 mg BD, (ClinicalTrials.gov NCT01218126). No major adverse safety or tolerability concerns have been identified in any of the clinical studies involving losmapimod thus far.62,63
Conclusion
EVOLUTION is an academic-industry partnership trial of a novel, potent selective p38 MAPK inhibitor (losmapimod) in COPD subjects stratified by fibrinogen level. The trial will provide novel understanding of the potential for fibrinogen as a drug development tool in COPD and the effects of losmapimod on arterial inflammation and function, as well as pulmonary inflammation and function, in an enriched COPD population. EVOLUTION will also provide further data regarding safety and tolerability of losmapimod and results from this will help inform plans for future trials evaluating p38 MAPK inhibitors and other anti inflammatory medicines in COPD.
Disclosures
Dr Fisk and Professor Wilkinson receive an educational award from GlaxoSmithKline for an imaging fellowship. Dr Mohan is employed by the Royal Brompton and Harefield NHS Trust and her salary is part funded by a grant from the Technology Strategy Board. Dr. Yang receives funding from the Wellcome Trust Translational Medicine and Therapeutics Fellowship, and the Sackler Fellowship. Dr. Cheriyan is employed by Cambridge University Hospitals NHS Foundation Trust and is obligated to spend 50% of his time on GlaxoSmithKline clinical trial research; however, he receives no other benefits or compensation from GlaxoSmithKline. Dr Tal-Singer is an employee and shareholder of GlaxoSmithKline and a principle investigator of the ERICA Consortium. Professor Polkey has received fees paid to him and/or to his institution for consulting on COPD from GlaxoSmithKline, Lilly, Boehringer Ingelheim, Novartis, Chiesi and Astra Zeneca.
Acknowledgements
We would like to thank participating patients and to acknowledge all clinical study site personnel who contribute to the conduct of this trial. All authors contributed to this manuscript development, approved the submitted manuscript and assume responsibility for its content. We would also like to acknowledge the support we receive from the NIHR Biomedical Research Centres at Cambridge University Hospitals NHS Foundation Trust and the Royal Brompton & Harefield NHS Foundation Trust, as well as the Cambridge Clinical Trials Unit in its delivery and oversight of this important academic-industry collaboration. We acknowledge the contribution of Professor John Cockcroft, University of Cardiff as the independent steering committee chair and Medical Monitor.
Appendix A
Supplementary data
Supplementary data related to this article can be found at
References
Cite this article
TY - JOUR AU - M. Fisk AU - D. Mohan AU - J. Cheriyan AU - L. Yang AU - J. Fuld AU - C.M. McEniery AU - R. Tal-Singer AU - M.I. Polkey AU - I.B. Wilkinson PY - 2013 DA - 2013/11/08 TI - Evaluation of losmapimod in patients with chronic obstructive pulmonary disease (COPD) with systemic inflammation stratified using fibrinogen (‘EVOLUTION’): Rationale and protocol JO - Artery Research SP - 24 EP - 34 VL - 8 IS - 1 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2013.10.380 DO - 10.1016/j.artres.2013.10.380 ID - Fisk2013 ER -
