Relationship between QRS characteristics and delayed-enhancement cardiac magnetic resonance in patients with ischemic cardiomyopathy
- DOI
- 10.1016/j.artres.2014.04.002How to use a DOI?
- Keywords
- Myocardial infarction; Non-invasive imaging; Fibrosis; Depolarization; Q wave; Fragmentation
- Abstract
Background: We explored the relationship between QRS characteristics and myocardial phenotype by delayed-enhancement cardiac magnetic resonance (DE-CMR) in patients with coronary heart disease (CHD).
Methods and results: Eighty five consecutive patients with CHD that were referred for DE-CMR evaluation constituted the study population. Of a total of 1445 left ventricular (LV) segments evaluated, 346 (23.9%) segments had fibrosis.
Compared to patients without pathological Q waves, patients with pathological Q waves showed a higher number of segments with fibrosis (5.9 ± 3.1 vs. 2.7 ± 2.8, p < 0.001), and lower left ventricular ejection fraction (LVEF) (42.9 ± 13.6% vs. 51.8 ± 18.3, p = 0.01); whereas no significant differences were observed regarding LV size.
When discriminated in according to the QRS duration tertiles, no significant differences were observed regarding the number of segments with fibrosis (p = 0.34), whereas the highest QRS tertile was related to the presence of a low LVEF (p = 0.005) and larger LV size (p = 0.01). QRS fragmentation (fQRS), defined as the presence of an R′ or notching in the nadir of the R wave or the S wave, or the presence of >1 R′ in 2 contiguous leads, was significantly related to LV size (LV end diastolic volume 153.6 ± 81.6 ml, vs. 111.5 ± 41.4 ml, p = 0.003), function (LVEF 43.2 ± 15.9% vs. 53.6 ± 16.3%, p = 0.005), and extent of fibrosis (5.1 ± 3.4 segments vs. 3.2 ± 3.1 segments, p = 0.01).
Conclusions: In the present study, fQRS was the only QRS-derived variable systematically and more closely related to LV size, LV systolic function, and to the presence and extent of fibrosis.
- Copyright
- © 2014 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
A recent prospective population-based study identified that prolonged QRS duration on resting electrocardiogram (ECG), even if moderate (>110 ms), was related to a higher risk of sudden cardiac death (SCD).1 Furthermore, a sub-study of the Oregon Sudden Unexpected Death Study that analyzed the subset of patients with SCD and associated coronary heart disease (CHD), identified prolonged QRS duration as an independent predictor of SCD, irrespective of the presence of prolonged ventricular repolarization.2 In parallel, fragmented QRS (fQRS) has been shown to be a better predictor of perfusion defects and functional abnormalities than the presence of pathological Q waves, and has been identified as an independent predictor of events.3–6
During the past decade, cardiac magnetic resonance (CMR) has become the gold standard to evaluate left ventricular (LV) morphology and function. Several studies have established delayed-enhancement CMR (DE-CMR) as a validated technique to identify and quantify irreversible myocardial damage. LV ejection fraction (LVEF), aside from age, has consistently been identified as one of the most powerful predictors of survival among patients with CHD.7–9 The extent of areas of irreversible myocardial damage detected by DE-CMR has been established as a strong predictor of all-cause mortality or cardiac transplantation, independently from traditional risk factors.10
The relationship between QRS characteristics and myocardial phenotype (LV size, systolic function, and extent of fibrosis) in patients with CHD is not fully understood. We therefore sought to explore this association by means of DE-CMR.
Methods
Study population
The present was an investigator-driven prospective study aimed to explore the relationship between QRS duration on resting ECG and LV characteristics (dimensions, systolic function, and extent of fibrosis) using DE-CMR in patients with CHD.
To that end, consecutive patients with a history of CHD, defined as previous MI (>3 months), previous coronary revascularization, and/or chronic stable angina that were referred for CMR evaluation to evaluate the extent and distribution of fibrosis and/or the presence of myocardial viability constituted the study population. Patients with moderate to severe valvular heart disease were excluded, as well as patients with pericardial disease and non-ischemic cardiomyopathy. A resting 12-lead ECG was obtained from all patients prior to examination. A single independent investigator blinded for clinical and CMR information evaluated all ECGs performed. Parameters evaluated included QRS duration, presence of fQRS, and presence of pathological Q waves. fQRS was defined as QRS complexes with the presence of an additional R wave (R′) or notching in the nadir of the R wave or the S wave, or the presence of >1 R′ (fragmentation) in 2 contiguous leads, corresponding to a major coronary territory.11 Typical bundle branch block pattern (QRS ≥ 120 ms) and incomplete right and left bundle branch block were excluded from the analysis.
CMR acquisition
All CMR exams were performed using the same system (Achieva 3.0 Tesla, Philips Healthcare, Cleveland, OH). A six-element cardiac phased-array coil was used for signal reception and cardiac synchronization was performed using a vector ECG. Cine-CMR images were acquired in 8–10 contiguous short-axis slices from the level of the mitral valve annulus through the LV apex using a commercially available steady-state free precession pulse sequence. For detection of the presence, extent and location of infarcted myocardium, a breath-hold, T1-weighted, contrast-enhanced inversion-recovery segmented gradient echo sequence (TR/TE (ms) 4.8/2.3; flip angle 25°; section thickness 10 mm; matrix 184 × 154; field of view 320 mm; voxel size 1.75 × 1.95 mm; minimum inversion time delay 79.3 ms) was used. These DE images were acquired 10 min after intravenous administration of 0.2 mmol/kg of a commercially available gadolinium chelate of diethylenetriamine pentaacetic acid bismethoxyethylamide (gadoversetamide, Mallinckrodt, St. Louis, USA), using identical long- and short-axis planes to the cine images, except for the most apical short-axis slice, which was excluded because it can be affected by partial-volume effects.
Image analysis
All MR imaging studies were analyzed offline in a dedicated workstation (Viewforum; Philips Healthcare) by an observer blinded to the clinical history. LV end diastolic volume (EDV) and end systolic chamber volume (ESV) were calculated using the Simpson method and LVEF was calculated as [EDV − ESV]/EDV × 100. Basal image position was defined as the basal-most image encompassing at least 50% circumferential myocardium. Myocardial mass was obtained on the basis of end diastolic endocardial and epicardial contours, and calculated as the product of myocardial volume and specific density of myocardial tissue (1.05 g/mL).
At DE imaging, myocardial fibrosis was considered present if signal intensity of the infarcted myocardium exceeded two standard deviations of that of the remote myocardium, and MI size was defined using a 17-segment LV model.12 The extent of DE (defined as the percentage of the myocardial thickness involved) within the myocardium was quantified visually according to the method described by Kim et al.13 Furthermore, we calculated the scar index proposed by Cheong et al., a modified scoring system based on the DE data: no hyperenhancement (score 1), 1%–25% myocardial hyperenhancement (thin subendocardial scar, score 2), 26%–50% myocardial hyperenhancement (dense subendocardial scar, score 3), 51%–75% myocardial hyperenhancement (near transmural scar, score 4), and 76%–100% myocardial hyperenhancement (transmural scar, score 5). A scar index was computed for each patient as the sum of the scar scores of all 17 segments divided by 17. A patient with no evidence of DE would therefore have a scar index of 1.10 In parallel, among patients with evidence of fibrosis, a binary classification was performed to discriminate between patients with transmural (≥2 segments with >50% myocardial hyperenhancement) or subendocardial fibrosis.
The study was approved by our institution’s ethics committee, and all the patients enrolled gave their written informed consent.
Statistical analysis
Discrete variables are presented as counts and percentages. Continuous variables are presented as mean ± SD or median (25th, 75th percentile) whenever appropriate. Comparisons among groups were performed using independent Student t tests, Fisher’s exact tests, or one way analysis of variance, as indicated. Pearson’s correlation coefficient was used to detect any association between variables. Finally, as secondary analyses, receiver-operating characteristic (ROC) curve analyses were also performed to evaluate the diagnostic performance of the three QRS parameters (pathological Q waves, QRS > 110 ms, and fQRS) to detect fibrosis using specific software for ROC analysis. Pairwise comparison between ROC curves were tested using binomial exact confidence interval for the area under the curve. A two-sided p value of less than 0.05 indicated statistical significance. Statistical analyses were performed with use of SPSS software, version 13.0 (Chicago, Illinois, USA) and MedCalc Software (Ostend, Belgium).
Results
A total of 85 patients with CHD who had completed CMR investigation and resting ECG immediately prior to the scan constituted the study population, whereas 2 patients with typical LBBB were prospectively excluded from the study. The mean age was 66.6 ± 10.1 years, 67 (79%) were male, 19 (22%) had diabetes mellitus, 56 (66%) had history of prior myocardial infarction (MI), 58 (68%) had hypertension, and 52 (61%) had hypercholesterolemia.
Thirty two patients (38%) had a normal QRS (absence of pathological Q waves or fQRS, and QRS duration ≤ 110 ms). Thirteen patients (41%) with normal QRS had presence of fibrosis (p = 0.01) and 5 (16%) had LV ejection fraction <40% (p = 0.002). Of a total of 1445 LV segments evaluated, 346 (23.9%) segments had evidence of fibrosis on DE; with a median scar index of 1.47 (interquartile range 1.03–2.29). Among patients with evidence of fibrosis at DE (n = 64), 37 (58%) had transmural fibrosis and 27 (42%) subendocardial fibrosis.
Myocardial phenotype according to the presence of pathological Q waves
A pathological Q wave was present in 40 patients (47%). The presence of pathological Q wave was not related to LV size, with no differences regarding LV end diastolic volume (143.8 ± 51.5 ml, vs. 128.5 ± 83.7 ml, p = 0.32) and diameter (53.8 ± 7.5 mm, vs. 51.8 ± 11.1 mm, p = 0.35); whereas LVEF was significantly lower in patients with pathological Q wave (42.9 ± 13.6% vs. 51.8 ± 18.3, p = 0.01). Patients with pathological Q waves showed a significantly higher number of segments with evidence of myocardial fibrosis on DE than patients without pathological Q waves (5.9 ± 3.1 vs. 2.7 ± 2.8, p < 0.001). Twenty six (70%) patients with transmural MI had pathological Q waves, whereas 17 (63%) patients with subendocardial MI had absence of pathological Q waves (p = 0.01).Table 1
| Pathological Q waves | |||
|---|---|---|---|
| Presence (n = 40) |
Absence (n = 45) |
p Value | |
| QRS duration (ms) | 106.0 ± 19.7 | 109.1 ± 29.6 | 0.58 |
| LV diastolic diameter (mm) | 53.8 ± 7.5 | 51.8 ± 11.1 | 0.35 |
| LV systolic diameter (mm) | 40.5 ± 9.9 | 36.7 ± 14.2 | 0.16 |
| LV diastolic volume (ml) | 143.8 ± 51.5 | 128.5 ± 83.7 | 0.32 |
| LV systolic volume (ml) | 87.1 ± 49.5 | 71.7 ± 81.2 | 0.31 |
| LV ejection fraction (%) | 42.9 ± 13.6 | 51.8 ± 18.3 | 0.01 |
| LV ejection fraction <40% | 20 (50%) | 11 (24%) | 0.02 |
| Left atrium area (cm2) | 23.2 ± 5.3 | 24.2 ± 6.6 | 0.43 |
| RV diastolic volume (ml) | 86.2 ± 17.5 | 90.7 ± 25.8 | 0.37 |
| RV systolic volume (ml) | 32.6 ± 11.0 | 37.7 ± 16.9 | 0.11 |
| RV ejection fraction (%) | 62.8 ± 7.9 | 60.0 ± 8.7 | 0.13 |
| LV mass (g) | 105.3 ± 26.1 | 101.9 ± 35.9 | 0.63 |
| Number of segments with DE | 5.9 ± 3.2 | 2.7 ± 2.8 | <0.001 |
| Scar index | 2.01 ± 0.7 | 1.42 ± 0.6 | <0.001 |
LV refers to left ventricular, RV to right ventricular, and DE to delayed-enhancement.
Relationship between the presence of pathological Q waves and both morphological and functional parameters.
Myocardial phenotype according to the QRS duration
The median QRS duration was 100 ms (interquartile range 90–110). When discriminated according to QRS duration tertiles, significant differences were observed regarding left ventricular size (Table 2). In particular, patients at the lowest tertile showed the smallest LV end systolic volumes (T1 = 55.9 ± 32.0 ml, vs. T2 = 76.9 ± 52.8 ml, vs. T3 = 113.4 ± 105.6 ml; p = 0.01) and diameters (T1 = 32.6 ± 6.6 mm, vs. T2 = 38.4 ± 10.6 mm, vs. T3 = 46.2 ± 16.5 mm; p < 0.001). Indeed, a significant, albeit weak, correlation was found between the QRS duration and the LV end systolic volume (r = 0.38, p < 0.001). In parallel, patients within the lowest tertile showed the smallest left atrium areas (T1 = 21 ± 5 cm2, vs. T2 = 25 ± 6 cm2, vs. T3 = 26 ± 6 cm2, p = 0.004). Right ventricular size and function were almost identical between groups (Table 2).
| Mean ± SD (ms) | QRS tertile | p Value | ||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| (86.0 ± 5.0) | (103.3 ± 4.8) | (143.6 ± 21.1) | ||
| LV diastolic diameter (mm) | 48.2 ± 5.0 | 53.3 ± 7.5 | 58.1 ± 13.7 | 0.001 |
| LV systolic diameter (mm) | 32.6 ± 6.6 | 38.4 ± 10.6 | 46.2 ± 16.5 | <0.001 |
| LV diastolic volume (ml) | 110.3 ± 31.4 | 135.4 ± 52.0 | 170.9 ± 110.2 | 0.08 |
| LV systolic volume (ml) | 55.9 ± 32.0 | 76.9 ± 52.8 | 113.4 ± 105.6 | 0.01 |
| LV ejection fraction (%) | 52.1 ± 14.2 | 47.5 ± 16.5 | 41.6 ± 19.1 | 0.08 |
| LV ejection fraction <40% | 6 (20%) | 11 (33%) | 14 (64%) | 0.005 |
| Left atrium area (cm2) | 20.9 ± 5.2 | 24.7 ± 5.8 | 26.1 ± 6.4 | 0.004 |
| RV diastolic volume (ml) | 88.0 ± 17.6 | 88.5 ± 23.9 | 89.5 ± 26.0 | 0.98 |
| RV systolic volume (ml) | 36.0 ± 12.8 | 35.2 ± 15.1 | 34.5 ± 16.5 | 0.95 |
| RV ejection fraction (%) | 60.2 ± 8.2 | 61.0 ± 8.2 | 63.4 ± 8.7 | 0.39 |
| LV mass (g) | 95.2 ± 25.5 | 105.2 ± 31.7 | 112.0 ± 36.7 | 0.17 |
| Number of segments with DE | 3.3 ± 3.2 | 4.5 ± 3.1 | 4.4 ± 4.1 | 0.34 |
| Scar index | 1.53 ± 0.6 | 1.81 ± 0.7 | 1.76 ± 0.8 | 0.26 |
LV refers to left ventricular, RV to right ventricular, and DE to delayed-enhancement.
Morphological, functional, and cardiac magnetic resonance characteristics according to the QRS duration at resting ECG.
Regarding LV function, patients within the lowest QRS duration tertile had the lowest proportion of patients with LVEF <40% (T1 = 20%, vs. T2 = 33%, vs. T3 = 64%, p = 0.005). Furthermore, the QRS duration was significantly longer in the subset of patients with LVEF ≤40% compared to patients with LVEF >40% (117.7 ± 26.3 ms vs. 101.9 ± 23.1 ms, p = 0.007).
No significant differences were observed regarding the number of segments with DE among the different QRS tertiles (T1 = 3.3 ± 3.2 segments, vs. T2 = 4.5 ± 3.1 segments, vs. T3 = 4.4 ± 4.1 segments, p = 0.34). In addition, no correlation was found between the QRS duration and the number of segments with DE-CMR (r = 0.07, p = 0.56) or the scar index (r = 0.06, p = 0.56). Among patients with presence of fibrosis at DE, QRS duration did not differ between patients with transmural or subendocardial fibrosis (107.6 ± 24.9 ms vs. 109.6 ± 25.0 ms, p = 0.75).
Myocardial phenotype according to the presence of fQRS
fQRS was present in 49 patients (58%). The presence of fQRS was related to LV size (Table 3). Indeed, patients with fQRS showed the largest LV end diastolic volumes (153.6 ± 81.6 ml, vs. 111.5 ± 41.4 ml, p = 0.003) and diameters (55.5 ± 10.5 mm, vs. 49.0 ± 6.7 mm, p = 0.002). In parallel, LV systolic function was related to the presence of fQRS, being the LVEF significantly lower in patients with fQRS (43.2 ± 15.9% vs. 53.6 ± 16.3%, p = 0.005). Finally, most 42 (86%) patients with fQRS had evidence of myocardial fibrosis at DE, as well as a significantly larger number of segments with fibrosis compared to patients without fQRS (5.1 ± 3.4 vs. 3.2 ± 3.1, p = 0.01). Indeed, the scar index was also significantly higher in patients with fQRS than patients without fQRS (1.9 ± 0.7 vs. 1.5 ± 0.6, p = 0.01). With respect to patients with presence of fibrosis at DE (Figs. 1–3), we found no significant relationship between the presence of fQRS and the radial extent (subendocardial or transmural) of fibrosis (p = 0.36).
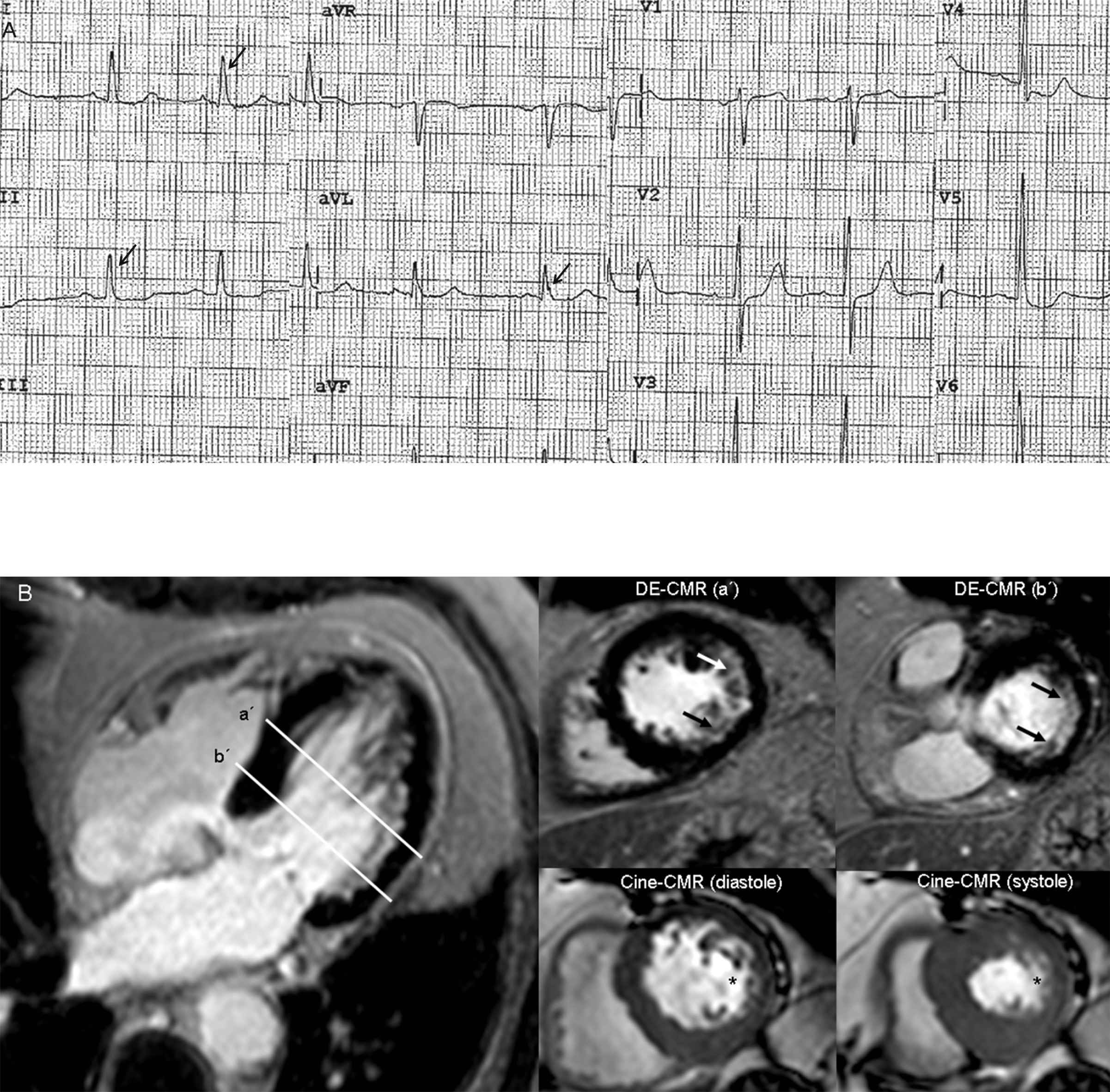
Eighty-one year old male with multiple vessel disease and previous revascularization. The ECG (panel A) shows absence of pathological Q waves and a narrow QRS complex (94 ms). Nevertheless, QRS fragmentation (arrows) is present in DI, DII, and aVL leads. Evidence of a previous subendocardial myocardial infarction on left circumflex territory is detected on delayed-enhancement cardiac magnetic resonance (DE-CMR, panel B). In addition, cine-CMR images show wall motion abnormalities (*) within inferolateral segments, with a left ventricular ejection fraction of 61%.

Forty-four year old male with a history of previous myocardial infarction. Absence of pathological Q waves or QRS prolongation, whereas QRS fragmentation is observed in inferior leads (arrows, panel A). An extensive, trasnmural, anteroseptal wall myocardial infarction is observed at delayed-enhancement cardiac magnetic resonance (DE-CMR, panel B), involving apical (a), mid (b), and basal (c) segments; with a left ventricular ejection fraction of 23%. Furthermore, a small subendocardial inferior-wall myocardial infarction is detected at mid and basal segments (*).
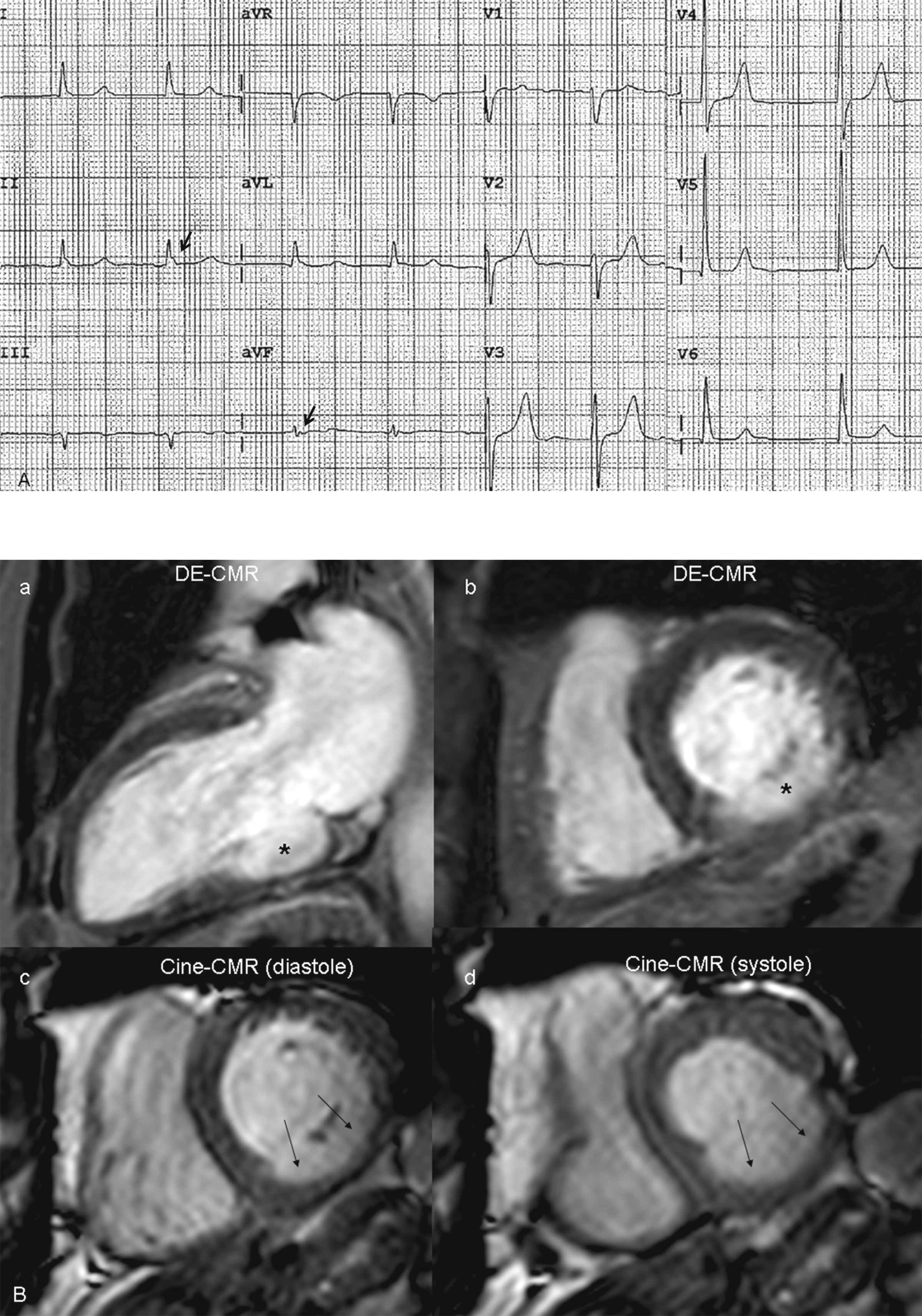
Sixty-nine year old male with a history of previous myocardial infarction. The ECG (panel A) shows absence of pathological Q waves or QRS prolongation; although QRS fragmentation is observed in inferior leads (arrows). A transmural inferior-wall myocardial infarction is observed in basal segments (asterisk in panel B, a and b), with underlying dyskinesia (arrows), and a left ventricular ejection fraction of 43%.
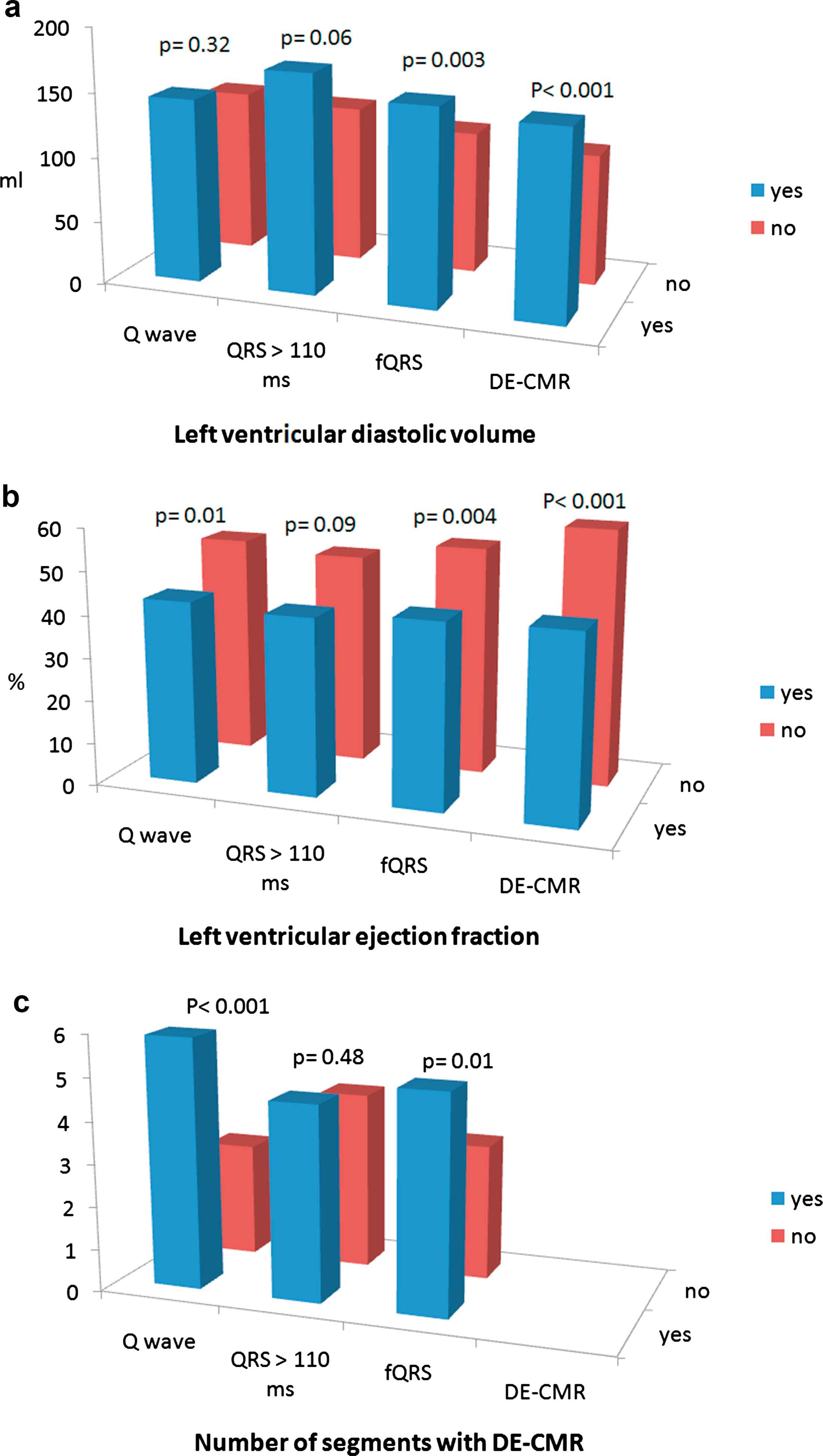
Bar graph showing the myocardial phenotype [(left ventricular end diastolic volume, left ventricular ejection fraction, and number of segments with evidence of fibrosis at delayed-enhancement cardiac magnetic resonance (DE-CMR))] according to the presence or absence of electrocardiographic and CMR variables expected to achieve an accurate discrimination between favorable or adverse phenotype.
| QRS fragmentation | |||
|---|---|---|---|
| Presence (n = 49) | Absence (n = 36) | p Value | |
| QRS duration (ms) | 111.6 ± 25.4 | 102.2 ± 24.5 | 0.09 |
| LV diastolic diameter (mm) | 55.5 ± 10.5 | 49.0 ± 6.7 | 0.002 |
| LV systolic diameter (mm) | 42.5 ± 13.5 | 33.0 ± 8.4 | <0.001 |
| LV diastolic volume (ml) | 153.6 ± 81.6 | 111.5 ± 41.4 | 0.003 |
| LV systolic volume (ml) | 95.3 ± 80.0 | 56.7 ± 40.3 | 0.005 |
| LV ejection fraction (%) | 43.2 ± 15.9 | 53.6 ± 16.3 | 0.005 |
| LV ejection fraction <40% | 22 (45%) | 9 (25%) | 0.07 |
| Left atrium area (cm2) | 24.6 ± 5.9 | 22.6 ± 6.1 | 0.14 |
| RV diastolic volume (ml) | 91.1 ± 21.5 | 84.8 ± 23.1 | 0.21 |
| RV systolic volume (ml) | 36.8 ± 16.1 | 33.1 ± 11.8 | 0.26 |
| RV ejection fraction (%) | 61.0 ± 9.8 | 61.7 ± 6.0 | 0.74 |
| LV mass (g) | 110.8 ± 31.8 | 92.8 ± 28.1 | 0.01 |
| Number of segments with DE | 5.1 ± 3.4 | 3.2 ± 3.1 | 0.01 |
| Scar index | 1.85 ± 0.7 | 1.49 ± 0.6 | 0.02 |
LV refers to left ventricular, RV to right ventricular, and DE to delayed-enhancement.
Relationship between the presence of QRS fragmentation and both morphological and functional parameters.
Comparison between categorized predictive variables
We further evaluated the myocardial phenotype according to the presence or absence of electrocardiographic and DE-CMR variables expected to achieve an accurate discrimination between favorable or adverse phenotype: Q wave (n = 40), QRS duration >110 ms (n = 22), presence or fQRS (n = 49), and presence of DE (n = 64).
As portrayed in Fig. 4, presence of QRS duration >110 ms was only a modest predictor of an adverse myocardial phenotype, showing only trends towards larger LV volumes and lower LVEF, and failing to identify significant differences regarding the extent of fibrosis. The LV end diastolic volume did not differ between patients with or without pathological Q waves (143.8 ± 51.5 ml vs. 128.5 ± 83.7, p = 0.32), whereas the LVEF and the number of segments with fibrosis were significantly different according to the presence of a pathological Q wave (Fig. 4).
The presence of fQRS was able achieve an accurate discrimination between all these variables. In parallel, the presence of any DE was also strongly related to a worse myocardial phenotype (Fig. 2). In particular, patients with absence of DE showed a normal LV diastolic volume (102.3 ± 30.5 ml) and LVEF (59.8 ± 13.6%). Finally, comparison of ROC curves for the detection of myocardial fibrosis did not yield significant differences between curves, with the following areas under the curve; pathological Q wave 0.69 (0.58–0.78), QRS >110 ms 0.51 (0.40–0.62), and fQRS 0.66 (0.55–0.76).
Discussion
Our main finding was that while the presence of pathological Q waves on resting ECG was associated to systolic function and to the extent of fibrosis on DE-CMR; fQRS was the only QRS-derived variable systematically and more closely related to LV size, LV systolic function, and to the presence and extent of fibrosis.
Pathological Q waves and QRS duration
In the past decades, a significant decline in the incidence of Q-wave myocardial infarction has been witnessed. In addition, it has been reported that pathological Q waves may regress or disappear in up to 25%–63% of patients; yielding a low sensitivity.14 Recent studies have identified prolonged QRS duration on resting ECG as a predictor of SCD. It has been hypothetized that QRS duration may reflect the presence of increased fibrosis of the myocardium even in the absence of severe LV dysfunction.
In our investigation, the presence of pathological Q waves was related to the LVEF and to the extent of fibrosis at DE. Nevertheless, it was not related to LV size and, in line with the aforementioned low sensitivity shown in previous studies, a significant number of patients with fibrosis failed to show pathological Q waves.
Besides, QRS duration was highly related to LV size and systolic function, whereas no relationship was found between QRS duration and the extent of fibrosis on DE. Indeed, among patients with evidence of fibrosis, QRS duration did not differ between patients with transmural or subendocardial fibrosis. However, it might be assumed from our results that patients with CHD but a narrow QRS duration are more prone to have a favorable myocardial phenotype, with less left ventricular remodeling and a smaller fraction of patients with impaired systolic function.
QRS fragmentation
The presence of fQRS has been identified as a predictor of major adverse cardiac events and of SCD in patients with CHD, and has been shown to have a better predictive value than pathological Q waves for the detection of myocardial fibrosis.15,16 The presence of myocardial fibrosis is believed to cause an heterogeneous ventricular activation, leading to fQRS.17 Recently, fQRS in patients with CHD and preserved LV systolic function was related to the presence of subclinical global and regional LV dysfunction, as assessed by strain echocardiography.16 However, the evidence relating fQRS and myocardial fibrosis as assessed by DE is both scarce and controversial.18
In the present study, the presence of fQRS was highly related to LV remodeling, systolic dysfunction, and with the presence and extent of myocardial fibrosis.
When compared to other ECG-related ventricular depolarization parameters expected to attain an accurate discrimination between favorable or adverse myocardial phenotypes (pathological Q waves and prolonged QRS duration), the presence of fQRS was consistently identified as a more accurate tool throughout most CMR variables.
On the other hand, the presence of any evidence of myocardial fibrosis as assessed by DE was also strongly related to a worse myocardial phenotype. Above all, patients with CHD but absence of DE showed normal LV volumes and systolic function. This should be emphasized in the context that despite we demonstrated that QRS characteristics are related to the underlying myocardial phenotype, 41% of patients with normal QRS complexes had evidence of fibrosis and 16% had an LVEF lower than 40%.
Interestingly, the presence of fibrosis at DE and fQRS were both associated to an LV remodeling and LV systolic dysfunction, although left atrial size and right ventricular size and function could not be discriminated with neither methods, underscoring the potential role of fQRS as an inexpensive and widely available means to estimate LV morphology and systolic function in patients with CHD.
Our study, though hypothesis generating, highlights the valuable role of different QRS complex characteristics in order to portray the underlying myocardial phenotype in patients with CHD. While the considerably more complex Selvester score has been established as a tool to quantify myocardial scar, the widespread clinical use of this score is limited mainly by its complexity. On the contrary, detection of fQRS is a valuable, reproducible, and straightforward assessment for routine clinical use.19,20
Finally, since a large portion of these patients had normal QRS complexes, our findings seem to support the use of further stratification of patients with CHD with non-invasive imaging tools such as DE-CMR.
A number of limitations should be recognized. The relatively small sample size might potentially lead to selection bias, although we included a high-risk population with 75% patients with evidence of fibrosis. Indeed, such high prevalence of fibrosis might influence the interpretation of the results regarding the ability of QRS characteristics to identify fibrosis. Furthermore, referral bias might potentially affect the results. The extent of irreversible myocardial damage at DE was assessed qualitatively, which is the most common approach. Despite we did not perform a direct quantification (in mm3 or grams), the degree of fibrosis was assessed using the number of segments with DE as well as the previously reported scar index, that takes into account the radial extent of fibrosis.10
Conclusions
In the present prospective study, while the presence of pathological Q waves on resting ECG was associated to systolic function and to the extent of fibrosis on DE-CMR; fQRS was the only QRS-derived variable systematically and more closely related to LV size, LV systolic function, and to the presence and extent of fibrosis.
Conflict of interest statement
None of the authors have conflicts of interest to declare.
Acknowledgment
We would like to thank Dr. Héctor Vetulli for his generous contribution and critical review of the manuscript.
Abbreviations
- DE-CMR
delayed-enhancement cardiac magnetic resonance;
- CHD
coronary heart disease;
- LV
left ventricular;
- fQRS
QRS fragmentation;
- EDV
end diastolic volume;
- ESV
end systolic volume;
- LVEF
left ventricular ejection fraction;
- SCD
sudden cardiac death.
References
Cite this article
TY - JOUR AU - Gaston A. Rodriguez-Granillo AU - Carlos Ingino AU - Carolina Parada-Villavicencio AU - Pedro Lylyk PY - 2014 DA - 2014/05/19 TI - Relationship between QRS characteristics and delayed-enhancement cardiac magnetic resonance in patients with ischemic cardiomyopathy JO - Artery Research SP - 88 EP - 97 VL - 8 IS - 3 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2014.04.002 DO - 10.1016/j.artres.2014.04.002 ID - Rodriguez-Granillo2014 ER -
