Antiretroviral treatment and time since HIV-1 diagnosis are associated with large artery stiffness in sub-Saharan African HIV-1 patients
- DOI
- 10.1016/j.artres.2016.09.002How to use a DOI?
- Keywords
- Non-nucleoside reverse transcriptase inhibitors; Nucleoside reverse transcriptase inhibitors; HIV; Aortic stiffness; Aortic pulse wave velocity; Augmentation index; Tanzania
- Abstract
Background: HIV-1 infection in northern populations is associated with increased large artery stiffness, both in the absence and presence of combination antiretroviral treatment (cART). It is unclear if similar changes occur in sub-Sahara African HIV-infected persons. The study aimed to determine whether HIV-1 infection with and without cART is associated with large artery stiffness in a cohort of HIV-1-infected patients in Tanzania.
Methods: In this cross-sectional study, 146 subjects were recruited: 40 uninfected controls, 51 HIV-1-infected untreated persons, and 55 on cART for at least 12 months. Patients were screened for history of hepatic, renal, haematological or cardiovascular disease, diabetes, dyslipoproteinaemia, and hypertension. Following screening, 4 untreated and 21 cART HIV-1 patients were excluded; leaving 47 HIV-1-infected untreated and 34 cART patients to be studied. cART included first line treatment: lamivudine/zidovudine with nevirapine or efavirenz. Large artery stiffness was assessed using applanation tonometry via pulse wave analysis to determine aortic pulse wave velocity (aPWV) and augmentation index corrected to a heart rate of 75 bpm (AIx@HR75).
Results: Aortic PWV was higher (p = 0.017) in the HIV-1-infected patients on cART (aPWV: 8.2 ± 1.8 m/s) vs. untreated patients (7.3 ± 1.5 m/s) and independent of blood pressure differences. AIx@HR75 was significantly increased in cART HIV-1 (29 ± 8%) vs. untreated HIV-1 patients (23 ± 10%; p = 0.038) and controls (23 ± 10%; p = 0.032). Duration of HIV-1 and cART were independent predictors of arterial stiffening in HIV-1 patients.
Conclusions: The surrogate markers for arterial stiffness suggest an increased cardiovascular risk in sub-Saharan African HIV-1 patients on first line therapy.
- Copyright
- © 2016 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Recent estimates indicate that ~56% of HIV-1 infected people initiated combination antiretroviral treatment (cART) in sub-Saharan Africa in 2012, representing a dramatic 100-fold increase in therapy since 2003.1 However, similar to other sub-Saharan countries, cART coverage is ~60% in Tanzania, far below universal access coverage.2 Nevertheless, while a surge in cART is underway, its favourable effects to reduce AIDS mortality3 may be complicated by emerging cardiovascular abnormalities.4
Soon after the introduction of cART in North America and Europe, unexpected vascular events among HIV-1-infected patients generated concern.5,6 While some retrospective studies do not show evidence that short-term cART is linked to elevated rates of cardiovascular events,7–10 it is becoming increasingly clear that longer exposure to protease inhibitors and some nucleoside analogues of reverse transcriptase inhibitors (i.e., abacavir) does.11,12 Indeed, the Data collection on Adverse events of Anti-HIV Drugs (DAD) study reported a significant association between cumulative protease inhibitor exposure and risk of myocardial infarction.13 Nevertheless, isolating the association between cART and cardiovascular risk in HIV-1-infected patients is inherently problematic, especially because untreated HIV-1 infection per se is associated with coronary artery disease.14–16
HIV-1 infection, both in the absence and presence of cART, is also linked to vascular abnormalities, particularly reduced endothelium-dependent vasodilation17 and increased carotid-artery media thickness.18 In HIV-1 patients in North America and Europe, large elastic artery stiffness, a marker of heightened cardiovascular risk and an independent predictor of adverse vascular events,19 is elevated20 and appears to be unfavourably affected by the presence of cART.21,22 While arterial stiffness in sub-Saharan HIV-1-infected patients may contribute to accelerated atherosclerotic disease and may be exacerbated by cART, data are limited and findings have been inconsistent.23,24
The aim of the present study was to determine whether HIV-1 infection with and without first line cART with non-nucleoside and nucleoside reverse transcriptase inhibitors is associated with large artery stiffness in a cohort of sub-Saharan Africa HIV-1-infected patients in Tanzania.
Methods
Sample population
One hundred-six non-smoking HIV-1-infected adult patients (51 treatment-naïve and 55 cART) recruited from the Kilimanjaro Christian Medical Centre (KCMC) Infectious Diseases Clinic in Moshi, Tanzania participated in the study. Treatment-naïve patients were included if they presented with CD4+ cells ≥500 cells/μL while cART patients were included if CD4+ T cells were <350 cells/μL. We recruited cART-naïve patients with CD4+ cells ≥500 cells/μL to allow more time before treatment while CD4+ T cells in cART patients were <350 cells/μL (the stage at which HIV-1-infected patients begin cART). This allowed uninterrupted comparison between the HIV-1 groups. HIV-1-infected patients were screened for history of hepatic, renal, haematological or cardiovascular disease, diabetes, dyslipoproteinaemia, and hypertension. Following screening, 4 of the 51 untreated HIV-1 patients were excluded based on history of cardiovascular disease and diabetes. Twenty-one of the 55 cART patients were excluded for history of diabetes (n = 5), cardiovascular disease (n = 7), deep vein thrombosis (n = 1), hypertriglyceridemia (n = 7), and hypertension (n = 1). The final sample was composed of 81 HIV-1 patients: 47 untreated HIV-1-infected and 34 HIV-1-infected patients on cART. Antiretroviral treatment consisted of nucleoside and non-nucleoside reverse transcriptase inhibitors with lamivudine (150 mg) and zidovudine (300 mg) twice daily combined with either nevirapine (200 mg) twice daily or efavirenz (600 mg) once daily for at least 12 months. Forty HIV-1 uninfected subjects served as controls. Controls were recruited from the KCMC staff and surrounding community. All participants gave their informed consent to participate in the study, which was approved by the institutional ethics committee at KCMC. All experiments conformed to the Declaration of Helsinki.
Body composition and metabolic measurements
Body mass and height were measured using a medial beam balance (Cardinal Scale Manufacturing Co, Webb City, USA). Abdominal obesity was estimated by waist circumference measured at the smallest part of the abdomen, at the highest part of the iliac crest at the end of normal expiration. Percent body fat was estimated by measuring the skinfold thickness (Lange, Beta Technology Inc., Cambridge, MD, USA) at four sites (biceps, triceps, sub scapular and suprailiac) to the nearest 0.2 mm. Skinfold measurements were repeated twice, averaged, and summed. The Durnin–Womersley equation was used to estimate percent fat from the sum of the four skinfolds, age, and sex.25 Fasting plasma lipid, lipoprotein and glucose concentrations were determined by enzymatic-colourimetric method affiliated with the KCMC Biotechnology Laboratory. CD4+ T cells were measured using flow cytometry (FACS Calibur, Becton Dickinson and Company, San Jose, CA, USA). In the HIV-1 cART patients, we considered the CD4+ T cell count at the time of cART initiation (enrolment) to be their nadir. Blood pressure was measured by KCMC physicians with an automatic sphygmomanometer (Omron, Kyoto, Japan) in a quiet, private room after resting for at least 5 min with the average of three readings recorded.
Large artery stiffness
Large arterial stiffness measurement met Expert Consensus standard procedures established by the European Network for Non-invasive Investigation of Large Arteries.26 Following an overnight fast, subjects reported to the KCMC Biotechnology laboratory and rested supine for 15 min in a temperature-controlled room (22 ± 1 °C). Arterial stiffness was assessed in the non-dominant arm using applanation tonometry (SphygmoCor, AtCor Medical, Itasca, IL USA) via pulse wave analysis to determine aortic pulse wave velocity (aPWV) and augmentation index at heart rate of 75 bpm (AIx@HR75).27 Using a hand-held pressure transducer, aPWV was determined by measuring the time delay in the travel of the forward moving pressure wave between the carotid and femoral arteries using the foot-to-foot method. ECG-gated R-wave arterial pulse waveforms were obtained transcutaneously over the carotid and femoral arteries to determine the velocity of the pressure wave. Travel distance of the pressure wave was measured by the distance from the carotid artery and centre of suprasternal notch subtracted from the distance from the centre of the suprasternal notch to the femoral artery. The average of 10 cardiac cycles at each site was used for analysis.
Twenty radial pulse pressure waveforms were acquired and calibrated against brachial artery systolic and diastolic blood pressure to produce a composite aortic pressure wave. Aortic AIx@HR75 was determined by a computerized transfer function28 that determines the magnitude of difference between the first and second aortic systolic pressure peaks divided by the pulse pressure, expressed as a percent. Greater augmentation of aortic pressure by the second systolic pressure peak produced by peripheral artery wave reflection is a well-established independent predictor of adverse cardiovascular events.29
Data were discarded and repeated if the standard deviation of aPWV and AIx@HR75 were ≥10%. aPWV was repeated if the timing of the R–R interval from the carotid and femoral sites differed by more than 6%. Data was only used if operator index - a measure of waveform reproducibility and signal strength was ≥90%.
Statistical analysis
Differences in subject characteristics, aPWV, and AIx@HR75 were determined by between-groups ANOVA with the Tukey honest significant difference post-hoc method. A Chi-square was used to analyse sex distributions among the groups. Because there were no sex differences in the primary outcome variables in the HIV-1 patients, data were pooled and presented together. The Mann–Whitney U test was used to determine differences in duration of HIV-1 infection between untreated and cART patients. Pearson’s bivariate correlations were used to determine variables that were related to aPWV and AIx@HR75 among all HIV-1 patients. Logarithmic transformation was used for non-normal variables. Variables that were significantly related to arterial stiffness (p < 0.05) among HIV-1 patients earned entry into a stepwise multiple linear regression analysis to identify the independent determinants of stiffness. In the multiple regression model, variables with a related probability of greater than 0.10 were removed. Systolic pressure, age, time since HIV-1 diagnosis, glucose, and waist circumference were included in the model to predict aPWV. Glucose, presence of cART, CD4+at the time of enrolment, time since HIV-1 diagnosis, diastolic pressure, HDL-cholesterol and percent fat were included in the stepwise regression to predict aortic AIx@HR75. Because blood pressure is recognized to be a strong and independent confounder of increased arterial stiffness30 and is suggested to be taken into consideration when interpreting aPWV,31 stiffness results were adjusted for mean arterial pressure and presented as such. Regression diagnostics were performed to ensure that the analyses satisfied assumptions including: 1) linearity between the predictors and dependent variables; 2) normality of respective residuals; 3) homoscedasticity; 4) influence and leverage; and 5) multicollinearity. Regression data is presented as Beta (β) – the value given to a predictor variable in the regression equation, the combined correlation coefficient between the dependent variable and the predictor variables (R), explained variance (R2), and the unstandardized regression coefficients (B) with 95% Confidence Intervals. Statistical significance was set at p < 0.05. Analyses were carried out using IBM SPSS Statistics for Windows (version 20; IBM Corp., Armonk, N.Y., USA).
Results
Table 1 presents characteristics for the controls and HIV-1-infected patients. Age and all anthropometric characteristics including BMI, body fat and waist circumference were similar among the groups. In addition, there were no differences in diastolic pressure or plasma LDL-cholesterol, triglycerides and glucose between controls and HIV-1-infected patients. Systolic pressure and mean arterial pressure were significantly higher in untreated HIV-1-infected patients compared with HIV-1-infected patients on cART (p = 0.036 and 0.03, respectively). Likewise, HDL-cholesterol was significantly higher in HIV-1-infected patients on cART compared with untreated HIV-1-infected patients (p = 0.006). Compared to controls, women were overrepresented in both treated and untreated the HIV-1-infected patients (χ2 = 15.0; p < 0.001 for treated; χ2 = 13.4; p < 0.001 for untreated).
| Variable | Controls (n = 40) | HIV-1 untreated (n = 47) | HIV-1 cART (n = 34) |
|---|---|---|---|
| Age, year | 45 ± 5 | 45 ± 6 | 45 ± 4 |
| Sex, men/women | 25/15 | 11/36a | 6/28a |
| Body mass, kg | 69.1 ± 12.4 | 66.8 ± 11.2 | 63.3 ± 12.3 |
| BMI, kg/m2 | 25.5 ± 4.2 | 25.1 ± 3.9 | 24.8 ± 4.1 |
| Body fat, % | 33 ± 8 | 37 ± 6 | 36 ± 7 |
| Waist circumference, cm | 86 ± 10 | 85 ± 9 | 86 ± 12 |
| Systolic BP, mm Hg | 126 ± 12 | 128 ± 10 | 122 ± 11b |
| Diastolic BP, mm Hg | 75 ± 6 | 77 ± 5 | 74 ± 8 |
| MAP, mm Hg | 92 ± 7 | 94 ± 6 | 90±8b |
| LDL-cholesterol, mmol/l | 2.9 ± 0.1 | 2.6 ± 0.1 | 2.8 ± 0.1 |
| HDL-cholesterol, mmol/l | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.4 ± 0.1b |
| Triglycerides, mmol/l | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 |
| Glucose, mmol/l | 4.7 ± 0.1 | 4.6 ± 0.1 | 4.7 ± 0.1 |
| CD4 at enrolment, cells/μL | – | 702 ± 157 | 278 ± 53b |
| CD4 (most recent), cells/μL | – | 466 ± 182 | 590 ± 207b |
| Median HIV-1 diagnosis, months | – | 21.7 | 38.7 |
| Median cART, months | – | – | 32.4 |
Values are means ± SD unless otherwise noted. M, men; W, women; BMI, body mass index; BP, blood pressure; MAP, mean arterial pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; cART, combination antiretroviral treatment.
p = 0.001 by χ2 for sex distribution in both groups of HIV-1 patients.
p < 0.05 vs. HIV-1 untreated.
Subject characteristics.
There were no group differences (p = 0.89) in most recent CD4+ T cell counts. The median duration of time since HIV-1 diagnosis was 27.2 months (Interquartile range (IQR): 12.0–108.4 months). However, the time since HIV-1 diagnosis was significantly greater in HIV-1-infected patients on cART compared to untreated patients (mean rank: 55.0 vs.30.9 respectively, p = 0.001). The median duration of cART was 32.4 months (IQR: 21.3–48.5 months) (Table 1).
Large artery stiffness in HIV-1 patients
Haemodynamic data of pulse wave analysis are shown in Table 2. Peripheral mean systolic and pulse pressure were higher and peripheral diastolic pressure lower compared with corresponding mean central values for each group (p < 0.001). There were no differences among groups in central and peripheral blood pressure variables at the time of pulse wave analysis.
| Variable | Controls (n = 40) | HIV-1 untreated (n = 47) | HIV-1 cART (n = 34) | ANOVA |
|---|---|---|---|---|
| Central systolic BP | 115 ± 12 | 117 ± 11 | 113 ± 10 | 0.246 |
| Peripheral systolic BP | 125 ± 12a | 127 ± 11a | 123 ± 11a | 0.239 |
| Central diastolic BP | 76 ± 6 | 78 ± 6 | 78 ± 8 | 0.366 |
| Peripheral diastolic BP | 75±6a | 77±6a | 76±8a | 0.502 |
| Central MAP | 89 ± 7 | 91 ± 7 | 89 ± 8 | 0.387 |
| Peripheral MAP | 91 ± 7 | 93 ± 7 | 91 ± 9 | 0.382 |
| Central PP | 39 ± 9 | 39 ± 9 | 36 ± 7 | 0.151 |
| Peripheral PP | 50 ± 10a | 51±9a | 46±7a | 0.054 |
| PP amplification (ratio) | 1.31 ± 0.17 | 1.32 ± 0.14 | 1.31 ± 0.16 | 0.949 |
| Heart rate (beats/min) | 66 ± 11 | 76±9b | 76 ± 11b | 0.001 |
Values (mm Hg) are means ± SD. cART, combination antiretroviral treatment; BP, blood pressure; MAP, mean arterial pressure; PP, pulse pressure.
p<0.001 for corresponding within-group central variable.
p < 0.01 versus controls.
Central and peripheral haemodynamics during pulse wave analysis.
Aortic PWV was elevated (p = 0.039) among all HIV-1-infected patients (HIV-1 and HIV-1 cART; 7.7 ± 1.7 m/s) compared with controls (7.0 ± 1.2 m/s). Aortic PWV differed significantly among the three groups (p < 0.001) and was markedly elevated by ∼1 m/s (p = 0.017) in the HIV-1-infected patients on cART (8.2 ± 1.8 m/s) compared with untreated HIV-1-infected patients (7.3 ± 1.5 m/s; Fig. 1 part A). There was no difference in aPWV between untreated HIV-1-infected patients and control subjects (7.3 ± 1.5 m/s vs. 7.0 ± 1.2 m/s; p = 0.401).
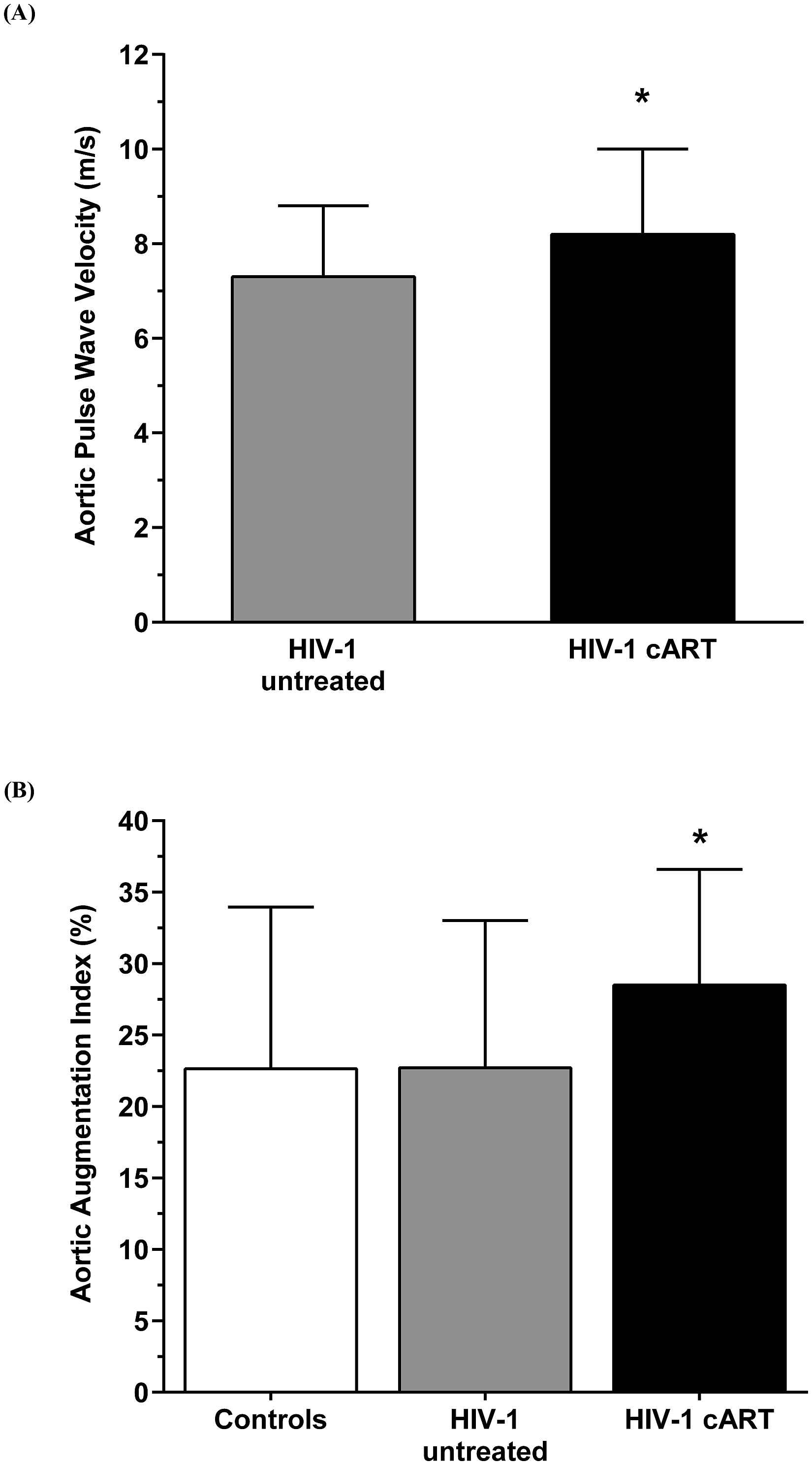
(A) Aortic pulse wave velocity (m/s) between the HIV-1 untreated and HIV-1 patients on cART for a minimum of 12 months. Values are mean ± SD *p = 0.017 adjusted for mean arterial pressure. (B) Aortic augmentation index (%) at heart rate of 75 bpm among the three groups. HIV-1 patients on cART showed elevated augmentation of aortic pressure, an indicator of earlier peripheral artery wave reflection. *p = 0.038 versus controls and p = 0.032 versus HIV-1 untreated.
Unlike aPWV, there was no difference in aortic AIx@HR75 bpm between the total cohort of HIV-1-infected patients (25 ± 10%) compared with controls (23 ± 11%). However, HIV-1 patients on cART showed elevated augmentation of aortic pressure. Similar to the higher aPWV, aortic AIx@HR75 was significantly elevated in HIV-1-infected patients on cART (29 ± 8%) compared with untreated HIV-1-infected patients (23 ± 10%, p = 0.038) and control patients (23 ± 10%, p = 0.032; Fig. 1 part B). There were no differences in aortic AIx@HR75 between untreated HIV-1-infected patients and controls.
Independent predictors of arterial stiffness
In the total cohort of HIV-1-infected patients, aPWV was significantly related to systolic pressure (r = 0.35; p = 0.001), age (r = 0.34; p = 0.004), time since HIV-1 diagnosis (r = 0.27; p = 0.015), glucose (r = 0.26; p = 0.019), and waist circumference (r = 0.25; p = 0.022). Aortic AIx@HR75 was significantly related to glucose (r = 0.34; p = 0.002), cART (r = 0.29; p = 0.008), CD4+ T cells at enrolment (r = −0.27; p = 0.017), time since HIV-1 diagnosis (r = 0.25; p = 0.022), diastolic pressure (r = 0.23; p = 0.043), HDL-cholesterol (r = 0.22; p = 0.044) and percent fat (r = 0.23; p = 0.046). After performing stepwise multiple linear regression analyses, the prediction model, shown in Table 3 (top panel) was statistically significant (F = 7.441, p < 0.0001) and accounted for 23% of the variance of aPWV (R2 = 0.229, Adjusted R2 = 0.199). Age (β = 0.236; B = 0.066), time since HIV-1 diagnosis (β = 0.267; B = 0.021) and systolic pressure (β = 0.259; B = 0.053) independently predicted aPWV, with HIV-1 diagnosis a stronger predictor. A one-year increase in time since HIV-1 diagnosis was independently related to a 0.24 m/s increase in aPWV after controlling for the effects of age and systolic blood pressure. The independent contributions of the predictors remained significant despite including additional covariates (sex, CD4+ T cells at enrolment and most recent, viral load, LDL and HDL-cholesterol, triglycerides, anthropometrics, and mean arterial pressure) in the regression model.
| Predictor Variable | Multiple R | R square | Unstandardized regression coefficient B | 95% confidence Interval | P | |
|---|---|---|---|---|---|---|
| Aortic PWV (m/s) | Age | 0.338 | 0.114 | 0.066 | 0.000 to 0.131 | 0.002 |
| Time since HIV-1 diagnosis | 0.410 | 0.168* | 0.021 | 0.008 to 0.035 | 0.001 | |
| Systolic blood pressure | 0.479 | 0.229* | 0.053 | 0.021 to 0.084 | <0.001 | |
| Aortic AIx (%) | Glucose | 0.332 | 0.110 | 0.041 | 0.013 to 0.068 | 0.004 |
| Presence of cART | 0.430 | 0.185* | 0.066 | 0.026 to 0.106 | 0.002 | |
| Diastolic blood pressure | 0.509 | 0.259* | 0.004 | 0.001 to 0.007 | <0.001 |
The multivariate regression included all variables that were significantly related to arterial stiffness as determined from the Pearson’s bivariate analysis. The independent predictor variables entering the final model are reported. PWV, pulse wave velocity; AIx, augmentation index; cART, combination antiretroviral treatment;
Significant change in R square (p < 0.01).
Independent predictors of aortic PWV (top panel) and aortic AIx@75 (bottom panel) among HIV-1 patients.
With respect to AIx@HR75, glucose, presence of cART, CD4+ T cells at enrolment, time since HIV-1 diagnosis, diastolic pressure, HDL-cholesterol, and percent fat were included in the stepwise regression model. The prediction model, shown in the bottom panel of Table 3, was statistically significant (F = 9.017, p < 0.0001) and accounted for 26% of the variance of aortic AIx@HR75 (R2 = 0.26, Adjusted R2 = 0.231). Glucose (β = 0.293; B = 0.041), presence of cART (β = 0.332; B = 0.066), and diastolic pressure (β = 0.276; B = 0.004) independently predicted AIx@HR75, with cART being more significant than other predictors. When taking into account the influence of glucose and diastolic blood pressure, the presence of cART was independently associated to a rise in AIx@HR75 by 7%. The independent contributions of glucose, cART, and diastolic pressure remained significant despite including additional covariates (age, sex, most recent CD4+ T cells, viral load, LDL-cholesterol, triglycerides, anthropometrics, and mean arterial pressure) in the regression model.
Stepwise multivariate regression was also performed in the uninfected control group to identify independent predictors of aPWV and AIx@HR75. After controlling for all potential covariates in the model, diastolic pressure (p = 0.002) and sex (p = 0.004) were the only significant predictors of aPWV and AIx@HR75 respectively in the control group.
Discussion
The primary new finding of the present study is that, as demonstrated by significantly higher aPWV and AIx@HR75, cART for at least 12 months was associated with worse large elastic artery stiffness in HIV-1 patients. Time since HIV-1 diagnosis and the presence of cART were strong independent predictors of elevated aPWV and greater augmentation of aortic pressure, respectively. These findings are the first to be reported regarding stiffening of large arteries in HIV-1-infected patients in relation to cART in Tanzania.
In contrast to prior reports from North America and Europe demonstrating increased arterial stiffness with untreated HIV-1 infection, our findings suggest that arterial stiffness is not elevated in sub-Saharan Africa treatment-naïve patients. These data are in contrast to an Italian study conducted by Schillaci et al., who were one of the first to demonstrate increased stiffening in untreated HIV-1-infected patients.20 Similarly, Ngatchou and colleagues reported increased stiffness in untreated native Cameroonian HIV-1-infected patients.32 However, because nearly half of their patients also had metabolic syndrome, it is difficult to determine if the changes in arterial stiffness were mediated by HIV-1 or by accompanying risk factors, or both. A novel aspect of the present study that significantly extends beyond prior research was that the HIV-1-infected patients were free of major comorbidities and were well matched to the uninfected control group. Our findings are in agreement with those of the Rwanda Women’s Inter-association Study24 showing that arterial stiffness was not increased in HIV-1-infected patients free of risk factors.
The major finding of the present study suggests that there is an important interaction between the burden of HIV-1 infection, as measured by the time since HIV-1 diagnosis, and cART that contributes to stiffening in sub-Saharan HIV-1-infected patients. While aortic PWV was ∼1 m/s higher in the HIV-1-infected cART group compared with treatment-naïve patients and uninfected controls, the time since HIV-1 diagnosis was significantly longer in treated patients and was an independent predictor of aPWV. Therefore, it is prudent to recognize the likelihood that chronic HIV-1-associated inflammation and immune dysfunction may mediate arterial stiffening in cART patients. For example, stiffening of elastic arteries in patients on cART may be attributed to HIV-associated oxidative and inflammatory stress, which has been shown to fragment the elastic laminae and promote collagen and fibrosis deposition,33 reducing arterial elasticity.34 Of particular concern is that cART may amplify the oxidative and inflammatory stress in HIV-1-infected persons,35 promoting greater arterial stiffening.36,37
While the relationship between immunodeficiency and arterial stiffness has not been conclusively established, some studies have reported an independent association between greater arterial stiffness and low nadir CD4+ T cells.38,39 For example, multivariate adjusted data from Ho and colleagues indicate that nadir CD4+ T-cell counts below 350 cells/μl were independently associated to increased aortic augmentation and PWV in HIV-infected treated individuals from a US-based cohort.38 These findings suggest that immunodeficiency may also influence arterial stiffness. However, in the present study the degree of immunodeficiency – as defined by nadir CD4 counts prior to initiation of cART was ∼294 cells/μL, quite higher than the nadir (84 cells/μL) reported in the study by Ho et al. As a result, CD4+ T cell counts at the time of enrolment were not associated with arterial stiffness in the present study.
Increased arterial stiffness is clinically relevant as findings from a recent meta-analysis indicate that for every 1 m/sec increase in aPWV, the risk for cardiovascular events rises by 15%.40 Moreover, earlier central artery wave reflection is an independent predictor of major adverse cardiovascular events; the multivariate adjusted hazard ratio for unstable angina, stroke, coronary revascularization, acute myocardial infarction and sudden death is increased by 33% for each 10% increase in AIx@HR75.29 Increased arterial stiffness may represent an important link contributing to atherosclerotic vascular disease associated with HIV-1 infection and cART.
It is important to point out that many studies have indicated that the adverse effects of cART on cardiovascular risk appear to be due, in part, to metabolic disturbances including dyslipidaemia, insulin resistance, abdominal obesity, hyperglycaemia, and elevated blood pressure.41 For example, in 108 Cameroon HIV-1 patients treated with reverse transcriptase inhibitors, Ngatchou et al. reported that aortic augmentation was significantly elevated compared with treatment-naïve patients. However, the treated patients had markedly higher blood pressure and presented with lower HDL-cholesterol than untreated patients, both of which were shown to be independent predictors of arterial stiffness.23 It is noteworthy that we observed increased stiffness in cART HIV-1-infected patients in the absence of comorbidities that often accompany therapy. This finding suggests that HIV-1 infection and cART may modulate arterial stiffening beyond that attributed to cardiometabolic abnormalities. However, elevated blood pressure and glucose were independent predictors of arterial stiffness in the HIV-1 cohort.
The mechanisms contributing to increased large artery stiffness with untreated HIV-1 infection and in patients on cART, particularly nucleoside and non-nucleoside reverse transcriptase inhibitors are poorly understood. While it has been shown that protease inhibitors42 and abacavir diminish endothelial function43 and are linked to arterial stiffness in HIV-1 patients,21,22 a unique aspect of the present study was that none of the cART patients in our Tanzanian cohort were prescribed these medications. Rather, we show a strong association of arterial stiffening with combination therapy using lamivudine/zidovudine with nevirapine or efavirenz. This finding adds to the accumulating evidence of long-term side effects with the first generation Retroviral Therapy (RT) backbone lamivudine/zidovudine (osteoporosis, lipodystrophy etc.). Our findings provide another argument to re-evaluate ART guidelines in African countries, such as Tanzania and change the first line backbone to second-generation RT inhibitors.
There are important experimental considerations of the present study. First, while we have attempted to identify the independent predictors of aortic PWV and AIx@HR75, we cannot rule out that environmental, genetic and/or lifestyle behaviours may have influenced the results of our group comparisons. In an effort to minimize these concerns, we employed strict inclusion criteria to eliminate the effects of clinically overt cardiometabolic disease and risk factor clustering that has been shown to stiffen arteries. However, this presents a limitation because a greater number (21) of cART patients were excluded (versus five of the untreated patients) based on the inclusion criteria. Excluding these patients, who were likely to have more severe arterial stiffness due in part to vascular disease and risk factors, may attenuate the magnitude of our findings. We also realize that the present study would be strengthened by prospectively measuring changes in large artery stiffness across time rather than via a single observation to isolate the independent and coordinated influences of HIV-1 infection and chronic cART use on changes in stiffness. We are currently completing a prospective longitudinal study that focuses on these unanswered questions in HIV-1 sub-Saharan patients.
Conclusions
These findings provide the first experimental evidence suggesting that cART may modulate vascular stiffening in sub-Saharan HIV-1 patients in Tanzania. Notwithstanding the much-needed expansion of treatment in Tanzania and other sub-Saharan countries, the great benefits of cART should be weighed against the cardiovascular complications associated with arterial stiffening. Routine assessment of large artery stiffness prior to cART and at regular intervals (e.g., bi-yearly) during therapy may help to identify particular groups of patients (i.e., those with pro-stiffening cardiometabolic abnormalities) that require more intensive lifestyle and pharmacological intervention to lower their long-term cardiovascular risk.
Funding
The NUFFIC Scholarship programme, The Netherlands, and an International Fellows Program award from the University of Wisconsin System supported this study. All authors designed the study, contributed and revised manuscript drafts, and approved the final manuscript. MVA, MVF, TF and JB supervised data collection procedures, patient recruitment, and human subject protection. TF and GV performed data collection procedures and developed the dataset. All authors completed the review of literature, data analysis, and prepared text.
Conflict of interest statement
The authors declare no conflicts of interest.
References
Cite this article
TY - JOUR AU - Titus F. Msoka AU - Gary P. Van Guilder AU - Yvo M. Smulders AU - Marceline van Furth AU - John A. Bartlett AU - Michiel A. van Agtmael PY - 2016 DA - 2016/10/03 TI - Antiretroviral treatment and time since HIV-1 diagnosis are associated with large artery stiffness in sub-Saharan African HIV-1 patients JO - Artery Research SP - 34 EP - 41 VL - 16 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2016.09.002 DO - 10.1016/j.artres.2016.09.002 ID - Msoka2016 ER -
