Vascular dysfunction: At the heart of cardiovascular disease, cognitive impairment and depressive symptoms☆
Review linked to Career Development Lecture, Artery Meeting, Copenhagen, 2016.
- DOI
- 10.1016/j.artres.2017.05.002How to use a DOI?
- Keywords
- Arterial stiffness; Endothelial dysfunction; Cerebral small vessel disease; Dementia; Depression
- Abstract
Vascular dysfunction may be an important pathway through which ageing and other factors, such as diabetes and obesity, can cause diseases of the heart and brain. Vascular dysfunction includes dysfunction of large arteries (due to arterial stiffness), the microcirculation (microvascular dysfunction) and endothelium (endothelial dysfunction). We have investigated, in a series of epidemiological studies, the role of vascular dysfunction in the pathogenesis of cardiovascular disease, dementia and depression. Data were used of The Hoorn Study, The AGES-Reykjavik Study, The Maastricht Study and The SUVIMAX2 Study. In addition, we did two systematic reviews and an individual participant data meta-analysis.
- Copyright
- © 2017 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
We found that stiffening of the carotid artery is independently associated with incident stroke, but not with coronary heart disease. Furthermore, carotid stiffness improved stroke risk prediction beyond Framingham and cfPWV. In addition, femoral artery stiffening was independently associated with incident cardiovascular disease.
Brain MRI studies showed that cerebral small vessel disease is associated with cognitive decline and incident depressive symptoms. In addition, arterial stiffening was associated with cognitive impairment and depressive symptoms, and this association was mediated by cerebral small vessel disease. We also found that endothelial dysfunction is associated with more depressive symptoms. Finally, we showed the presence of interaction (synergy) with regard to cardiovascular risk, between endothelial dysfunction and type 2 diabetes.
From a clinical point of view, these associations are important as they suggest that efforts at favourably influencing vascular dysfunction can have significant public health implications via prevention of cardiovascular disease, dementia and depression.
Life expectancy has dramatically increased and will continue to do so in the next decades.1 Ageing is associated with a greatly increased risk of vascular-related diseases of the heart and brain, including coronary heart disease, heart failure, stroke and (vascular) dementia and depression. In recent years, emerging evidence indicates that dysfunction of various elements of the vascular system plays an important role in the pathogenesis of these diseases.2,3 Vascular dysfunction includes dysfunction of large arteries (due to arterial stiffening), the microcirculation (microvascular dysfunction) and endothelium (endothelial dysfunction). Indeed, recent statements of the European Society of Hypertension/European Society of Cardiology4 and the American Heart Association/American Stroke Association5 have indicated arterial stiffness and endothelial dysfunction as important, potentially modifiable risk factors for cardiovascular disease and cognitive impairment. However, the exact role of arterial stiffness and microvascular and endothelial dysfunction in the pathogenesis of these diseases is incompletely understood, and their clinical utility remains controversial. Therefore, we have investigated, in a series of epidemiological studies, the role of arterial stiffness and microvascular and endothelial dysfunction in the pathogenesis of cardiovascular disease, cognitive impairment and depressive symptoms. This paper discusses the key findings of our recent work and their potential clinical implications.
Stiffening of elastic and muscular segments: distinct pathways in the pathogenesis of cardiovascular events
There are substantial differences in properties between elastic and muscular segments, and it has been suggested6 that stiffening of these segments are differentially associated with cardiovascular events. Stiffening of elastic segments (e.g. the carotid artery and ascending aorta) may be more strongly associated with stroke than coronary heart disease, because stiffening of these segments leads to a high pulsatile pressure and flow load on the brain.7 In addition, stiffening of the carotid artery may lead to stroke through local development of rupture-prone atherosclerotic plaques.8 In contrast, stiffening of muscular segments (e.g. the femoral artery and descending aorta) may be more strongly associated with coronary heart disease events than stroke, because muscular and coronary arteries show similar arterial wall characteristics (i.e. presence of abundant smooth muscle cells and a high collagen/elastin ratio9), and, therefore, stiffening of muscular segments may serve as a proxy for stiffening of the coronary vasculature. We used data of The Hoorn Study10 on local distensibility measurements of the carotid and femoral arteries to investigate elastic and muscular artery stiffness.7 In line with the above hypothesis, the findings indicated that stiffening of the carotid and femoral arteries are associated with a higher cardiovascular event incidence and greater all-cause mortality risk, independently of each other, and independently of carotid-femoral pulse wave velocity (cfPWV). We further elaborated upon these findings and performed a systematic review and an aggregate data and an individual participant data meta-analysis11 on the association between carotid stiffness and incident cardiovascular events. The results showed that carotid stiffening is associated with a higher stroke incidence, but not with coronary heart disease events. The association between carotid stiffness and incident stroke was independent of cardiovascular risk factors and independent of cfPWV. In addition, estimation of carotid stiffness modestly improved stroke risk prediction beyond Framingham stroke risk score factors and cfPWV. From a clinical point of view, these observations are important, as they identify carotid and femoral stiffness as potential separate targets for stroke and coronary heart disease risk lowering therapy. In addition, the findings provide proof of principle that carotid stiffness can have additional value as a risk predictor of stroke beyond the Framingham stroke risk score factors and cfPWV.
Arterial stiffening and endothelial dysfunction: causes of cerebral microvascular damage, cognitive impairment and depressive symptoms
Arterial stiffening leads to an increased pulsatile pressure and flow load, which may damage the microcirculation.12–14 The brain may be particularly prone to the detrimental effects of this increased load, because its microcirculation is characterized by low impedance, allowing the pulsatile load to penetrate deeply into its microvascular bed.13,14 This increased pulsatile load may directly cause cerebral microvascular dysfunction and damage, despite blood pressure-related protective auto-regulatory mechanisms. Alternatively, the increased pulsatile load may induce a microvascular remodelling response, which initially serves to limit the penetration of the pulsatile load in the microcirculatory system by raising vascular resistance. Yet, this protective response may ultimately become unfavourable leading to impaired vasoreactivity and microvascular ischaemia.13,14 It is, moreover, likely that these mechanisms operate simultaneously (Fig. 1). In addition, arterial stiffening may cause excessive blood pressure variability,15 which may further sensitize high-flow organs to the harmful effects of impaired microvascular vasoreactivity.14 Furthermore, a recent study16 has suggested that arterial stiffening may lead to cerebral (microvascular) damage via acceleration of cerebral β-amyloid accumulation, although the exact mechanism underlying this association is incompletely understood. Finally, endothelial dysfunction may lead to cerebral microvascular damage via multiple mechanisms, including impairment of the process of neurogenesis,17 impaired cerebral blood flow regulation18 and disturbance of blood–brain barrier function.19
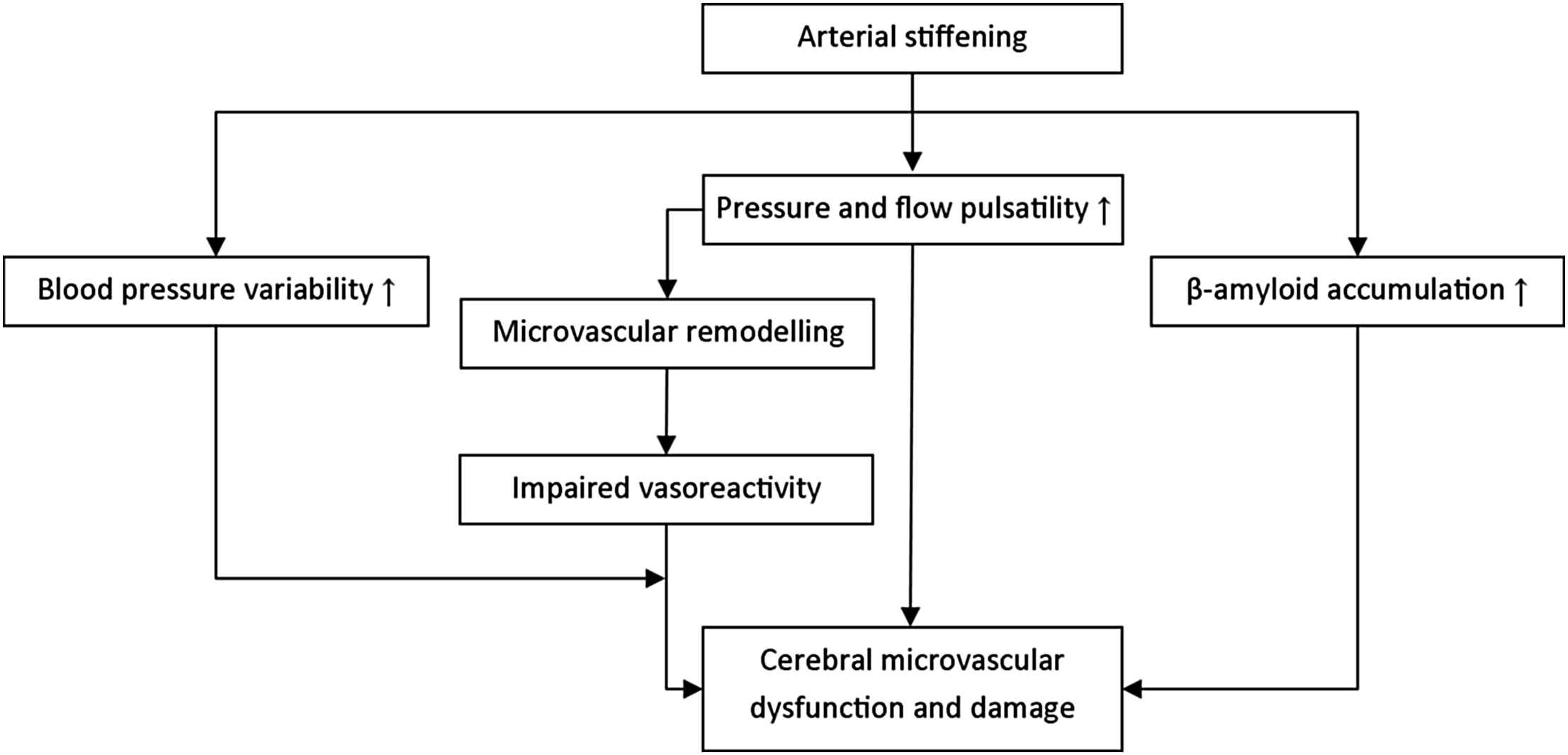
Potential pathways through which arterial stiffening can lead to cerebral microvascular dysfunction and damage.
Cerebral microvascular damage leads to neuronal cell death, diminished neuronal connectivity and, ultimately, dysfunction of the brain.19 Brain dysfunction can manifest itself as cognitive impairment, including dementia. In addition, it has been suggested20 that cerebral microvascular damage leads to depression via disruption of deep and frontal brain structures or their connecting pathways involved in mood regulation, in particular in older individuals (vascular depression hypothesis). Depression has a bimodal age distribution with peaks in the early third and in ninth decades suggesting the presence of different causes of depression in young and older individuals.21 In line with the vascular depression hypothesis, a recent randomized clinical trial22 showed that nimodipine, a drug with vasoprotective properties, reduced time to remission of late-life depression.
We have provided further evidence of the existence of an association between vascular dysfunction on the one hand and cerebral microvascular damage and brain dysfunction on the other. We conducted a systematic review and aggregate data meta-analysis23 that showed that a consistent association exists between greater arterial stiffness on the one hand and cerebral small vessel disease and cognitive impairment on the other. In addition, we used data of The AGES-Reykjavik Study to further explore these associations. The results showed that: 1) various magnetic resonance imaging (MRI) markers of cerebral small vessel disease are strongly associated with a higher depressive symptom incidence24; 2) cerebral small vessel disease located in the deep (sub-cortical) brain region (i.e. internal and external capsules, thalamus, hippocampus and amygdala combined) is, as compared to disease in other brain regions, more strongly associated with a higher depressive symptom incidence24; 3) arterial stiffening is associated with more (severe) depressive symptoms, and this association is in part mediated by cerebral small vessel disease25; and 4) endothelial dysfunction is associated with more (severe) depressive symptoms.26 Taken together, these studies suggest that both arterial stiffening and endothelial dysfunction may lead to microvascular damage-related brain dysfunction, which may manifest itself as cognitive impairment and/or depressive symptoms. From a clinical point of view, these associations are important as they suggest that efforts at favourably influencing arterial stiffness and endothelial dysfunction can have significant public health implications via prevention of dementia and depression.
Arterial stiffening does not lead to generalized microvascular dysfunction
It has been hypothesized27 that arterial stiffening can also lead to microvascular dysfunction and damage in other organs than the brain (generalized microvascular dysfunction), and that this may explain the association between arterial stiffness and different diseases, including peripheral neuropathy,28 type 2 diabetes29 and osteoporosis.30 However, evidence of an association between arterial stiffness and markers of generalized microvascular dysfunction is lacking. The skin microcirculation is a representative vascular bed to examine generalized microvascular phenomena.31 We therefore investigated the association between arterial stiffness and skin microvascular function.32 For this analysis, data was used of The Maastricht Study and The SUVIMAX2 Study. As it has been hypothesized that individuals with type 2 diabetes are particularly prone to the detrimental effects of increased pressure and flow pulsatility on the microcirculation, because type 2 diabetes is associated with increased microvascular perfusion,14 we additionally investigated whether any association between stiffness and microvascular function is stronger in individuals with type 2 diabetes as compared to those without type 2 diabetes. However, in contrast to the above hypothesis, the results of this study showed that arterial stiffness is not associated with skin microvascular function, irrespective of the presence of type 2 diabetes. This suggests that arterial stiffening alone may not lead to generalized microvascular dysfunction. Possibly, the microcirculation of most organs is able to protect itself against the detrimental effects of arterial stiffening and pressure and flow pulsatility. This may be due to the fact that most organs, with the exception of the brain and kidney, have relatively high microvascular impedance.14 Therefore, most of the increased pulsatile energy is dissipated by arteries and large arterioles proximal to the capillaries.
Endothelial dysfunction and type 2 diabetes synergistically increase cardiovascular risk
In the pathogenesis of cardiovascular events, true interaction (synergy) between risk factors appears rare, i.e. most studies find that risk factors act, and thus increase cardiovascular risk, independently of each other. From a clinical point of view, detection of interaction is, however, important as this identifies key therapeutic targets: interventions aimed at such risk factors are potentially more efficacious than treatment of risk factors which do not interact.33 We used prospective data of The Hoorn Study34 to evaluate the interaction between endothelial dysfunction on the one hand and type 2 diabetes, impaired glucose metabolism and insulin resistance on the other with regard to the risk of cardiovascular events. We investigated this interaction as it has been suggested that individuals with type 2 diabetes are extremely sensitive to the adverse cardiovascular effects of endothelial dysfunction.35 If true, this implies that endothelial dysfunction and type 2 diabetes synergistically increase cardiovascular risk. This may be due to a reciprocal association between endothelial dysfunction and type 2 diabetes, in which endothelial dysfunction acts as both cause36 and consequence37 of type 2 diabetes. In addition, type 2 diabetes may amplify the detrimental effects of endothelial dysfunction on atherothrombosis.38 In accordance with this hypothesis, two recent studies35,39 showed interaction, on a multiplicative scale, with regard to cardiovascular event risk, between type 2 diabetes and endothelial dysfunction. Both studies defined endothelial dysfunction by increased levels of endothelium-derived circulating biomarkers. In The Hoorn Study, we have shown that such interaction is also present between type 2 diabetes and impaired endothelium-dependent flow-mediated dilatation,34 a key functional estimate of endothelium-dependent, nitric oxide-mediated dilatation. In addition, interaction on an additive scale (potentially causal interaction33) was demonstrated, and such interaction was already present in individuals with impaired glucose metabolism or insulin resistance. This study therefore provides strong evidence in favour of causal interaction between endothelial dysfunction and type 2 diabetes in the pathogenesis of cardiovascular events. This interaction is important as it suggests that endothelial dysfunction may act at least partially as the underlying phenomenon which explains the two to three times higher cardiovascular risk seen in type 2 diabetes.38 This also identifies endothelial dysfunction as a key therapeutic target for lowering of cardiovascular risk in type 2 diabetes. In addition, the fact that an interaction was already present in individuals with impaired glucose metabolism and insulin resistance suggests that endothelial dysfunction is an early therapeutic target even before onset of type 2 diabetes.
Pathophysiological model
Based on our findings, we propose the following pathophysiological model for the interrelated role of arterial stiffening and microvascular and endothelial dysfunction in the pathogenesis cardiovascular disease, cognitive impairment and depressive symptoms (Fig. 2). Endothelial dysfunction is associated with a marked increased cardiovascular risk, particularly so in individuals with type 2 diabetes, impaired glucose metabolism or insulin resistance. In addition, arterial stiffening leads to an increased risk of cardiovascular events. Importantly, stiffening of elastic segments (e.g. the carotid artery and ascending aorta) and muscular segments (e.g. the femoral artery and descending aorta) may lead to cardiovascular events independently of each other via distinct pathways. Stiffening of elastic arteries is most strongly associated with incident stroke, whereas stiffening of muscular arteries may be most strongly associated with incident coronary heart disease. Furthermore, arterial stiffening may lead to cerebral microvascular damage, which, in turn, may lead to brain dysfunction. Brain dysfunction can manifest itself as cognitive impairment and/or depressive symptoms. Finally, endothelial dysfunction may lead to brain dysfunction. This may be through multiple (partially interdependent) pathways, including impairment of the process of neurogenesis, impaired cerebral blood flow regulation, blood–brain barrier dysfunction and microvascular damage.
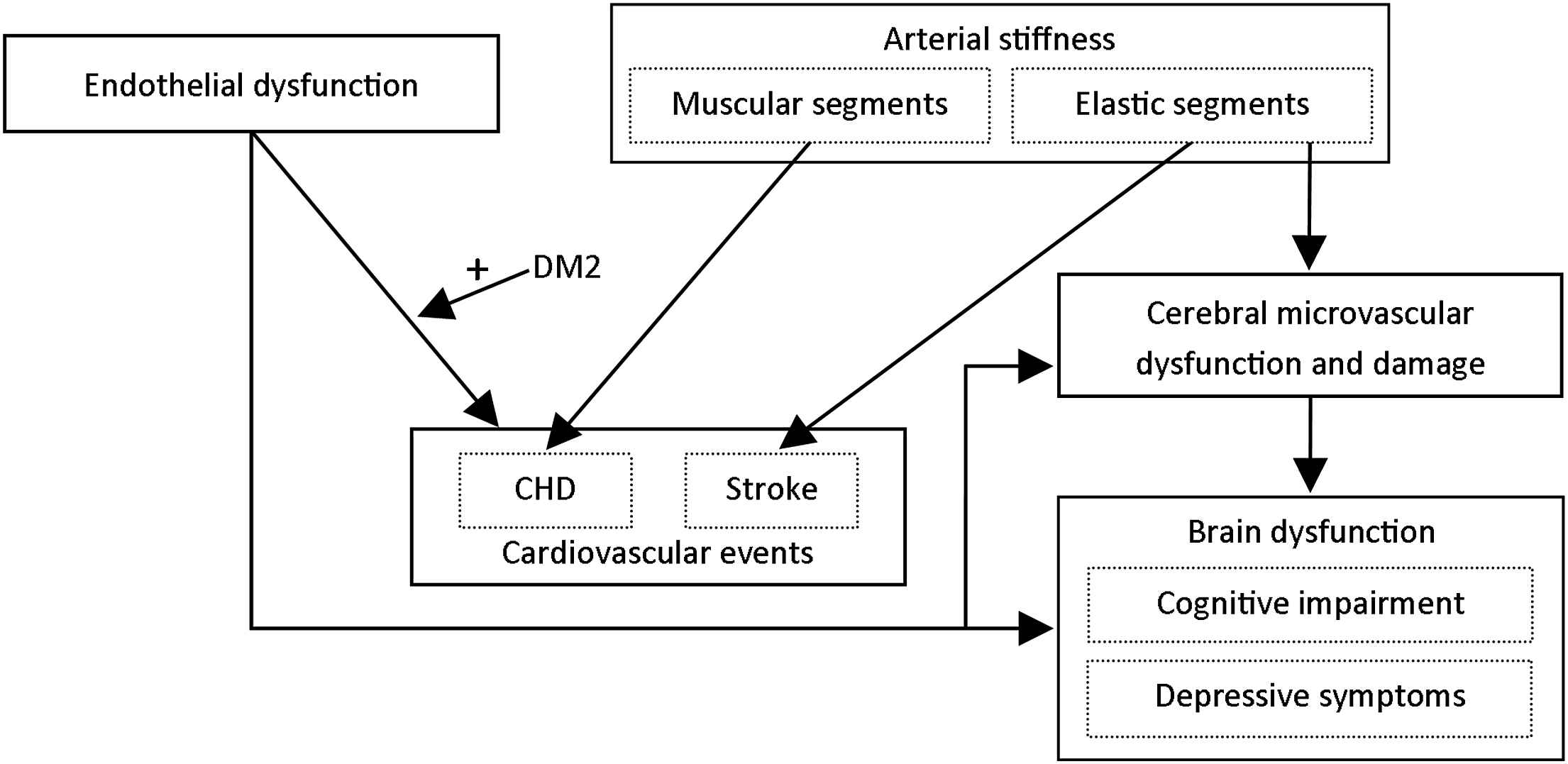
Proposed pathophysiological model for the role of arterial stiffening, microvascular dysfunction and endothelial dysfunction in the pathogenesis of cardiovascular events, cognitive impairment and depressive symptoms. DM2 = type 2 diabetes; CHD = coronary heart disease.
Future perspectives
The present studies provide further evidence for a role of vascular dysfunction in the pathogenesis of cardiovascular disease, cognitive impairment and depressive symptoms. This identifies vascular dysfunction as an important target in the prevention and treatment of these diseases or symptoms. Some of the associations were, however, evaluated in cross-sectional studies only25,26,32; longitudinal studies are, therefore, needed to assess the temporality of these associations. Ideally, such studies should use an extensive phenotyping approach with detailed measurements on the function and structure of the (micro)vasculature as well as measurements on cardiac and cerebral function, including assessment of (subtypes of) dementia and clinical depression. Such an approach will help to further understand the complex associations between vascular dysfunction and cardiovascular disease. In addition, new epidemiological techniques have become available for (etiologic) observational research, including use of instrumental variables (e.g. Mendelian randomization techniques), adjustment for time-dependent confounding, and estimation of the potential impact of unmeasured confounders. These techniques may help to further minimize the influence of bias in observational research. Finally, clinical trials are warranted to identify successful (non)pharmacological treatment strategies to counter the adverse effects of vascular dysfunction. Such trials are currently conducted; their results are highly anticipated.
Conflict of interest
None.
References
Cite this article
TY - JOUR AU - T.T. van Sloten PY - 2017 DA - 2017/05/23 TI - Vascular dysfunction: At the heart of cardiovascular disease, cognitive impairment and depressive symptoms☆ JO - Artery Research SP - 18 EP - 23 VL - 19 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2017.05.002 DO - 10.1016/j.artres.2017.05.002 ID - vanSloten2017 ER -
