Resistance training-induced decreases in central arterial compliance is associated with increases in serum thromboxane B2 concentrations in young men
- DOI
- 10.1016/j.artres.2018.08.001How to use a DOI?
- Keywords
- Resistance training; Carotid arterial compliance; Vasoconstrictive mediator; Platelet aggregation; Young men
- Abstract
Background: Reduction in central arterial compliance is an independent risk factor for cardiovascular disease, and is caused by high-intensity resistance training. The thromboxane has both potent vasoconstrictive and platelet aggregation effects, and is associated with cardiovascular diseases. However, whether thromboxane is involved in resistance training-induced decrease in central arterial compliance is unclear. The present study aimed to investigate relationships between circulating thromboxane levels and central arterial compliance in both cross-sectional and longitudinal (i.e., resistance training) designs.
Methods and results: First, in a cross-sectional study, we assessed association between circulating thromboxane concentrations and central arterial compliance in 63 young men, who showed significant negative correlation between those parameters. Second, in a longitudinal study, we examined effects of high-intensity resistance training on circulating thromboxane concentrations and central arterial compliance and relationship among changes from baseline in those parameters. Young sedentary men were assigned to control (n = 7) or training (n = 17) groups. Subjects in training group underwent four-week supervised high-intensity resistance training. Resistance training significantly elevated circulating thromboxane concentrations and decreased central arterial compliance; no significant change was observed in control group, and there was significant correlation between changes in those parameters.
Conclusions: circulating thromboxane is possible mechanism explaining resistance training-induced decrease in central arterial compliance in young men.
- Copyright
- © 2018 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
The guidelines of the American College of Sports Medicine and the American Heart Association recommend moderate-to high-intensity resistance training because resistance training can elicit substantial increases in physical fitness and some health-related factors such as muscle strength, bone mineral density, and insulin sensitivity.1,2 In contrast, it has been reported that high-intensity resistance exercise and resistance training [≥75% one-repetition maximum (1RM)] decrease central arterial compliance (CAC),3,4 although low-intensity resistance exercise and resistance training increase arterial compliance5,6 and moderate-intensity resistance training does not change arterial compliance.7 Moreover, a meta-analysis revealed that only high-intensity resistance training was significantly associated with a decrease in arterial compliance.8 It is possible that decreased arterial compliance with both acute and chronic resistance exercise is related to high-intensity and is not found in moderate- and low-intensity exercise. Deteriorated vascular function (i.e., decreased CAC) is an independent risk factor for future cardiovascular diseases.9 Thus, a decrease in CAC induced by resistance training may increase the future risk for cardiovascular diseases. However, the mechanisms underlying resistance training-induced decrease in CAC have not been clarified yet.
The arterial compliance is regulated by the composition of elastin and collagen (structural elements) and the vasoconstrictor tone exerted by smooth muscle cells (functional elements). The elastin/collagen composition of the arterial wall is a more slowly changing component that contributes to arterial compliance.10 As such, it is unlikely that this may be a physiological mechanism underlying decrease in arterial compliance induced by short-term resistance training. In contrast, the functional elements are regulated by some vasoconstrictive mediators.11,12 In particular, among vasoconstrictive mediators, thromboxane (TX) is produced from the platelets and has potent vasoconstrictory and platelet aggregation effects.13–15 The TX receptor exists on vascular smooth muscle cell.16 Thus, increased circulating TX concentrations are possibly associated with decreased arterial compliance. In contrast, high-intensity single bout of resistance exercise causes platelet aggregation,17 which strongly suggests that high-intensity resistance exercise may increase circulating TX concentrations. However, the effect of high-intensity resistance exercise on circulating TX concentrations has not been clarified yet. Moreover, whether increased circulating TX concentrations are associated with resistance training-induced decrease in arterial compliance is unclear.
Accordingly, the aim of the present study was to investigate whether circulating TX concentrations are related to arterial compliance and whether the resistance training-induced decrease in arterial compliance is associated with changes in circulating TX concentrations. We hypothesized that (a) circulating TX concentrations are associated with arterial compliance and (b) resistance training increases circulating TX concentrations, which is associated with a resistance training-induced decrease in arterial compliance. To test our hypothesis, in experiment 1, we examined the relationship between circulating TX concentrations and arterial compliance in a cross-sectional study in young men. In experiment 2, we investigated the effects of a four-week-long resistance training on circulating TX concentrations and arterial compliance in young men.
Materials and methods
Subjects
In experiment 1, 63 young men (age, 20–36 years) were enrolled in a cross-sectional study. In experiment 2, 24 young men (age, 20–35 years) were enrolled in a longitudinal study. Applicants for control and resistance training were recruited as each subject in control (n = 7) and training (n = 17) groups, respectively. All subjects were recruited from the local community through flyers, e-mails, and information sharing. None of the subjects had participated in any resistance or endurance training regularly. All subjects were non-smokers and cardiovascular disease-free, as indicated by their medical history. None of the subjects were taking cardiovascular medications. The subjects were instructed to maintain current eating behaviors for the duration of the intervention. The present study was conducted in accordance with the Declaration of Helsinki and was approved by the ethical committee of the University of Tsukuba. All subjects provided informed written consent.
Sample size estimation
The sample size was calculated based on the previous studies18,19 that circulating levels of vasoactive substances are associated with arterial compliance (experiment 1) and high-intensity resistance training decreases arterial compliance (experiment 2). Considering a power of 0.80 and an α level of 0.05, in experiment 1, a total sample size of 38 was found to be necessary by “bivariate normal model” using a general stand-alone power analysis program (G*Power 3; Heinrich Heine, Düsseldorf, Germany). Furthermore, in experiment 2, a total sample size of 22 was found to be necessary by “analysis of variance for fixed effects, special, main effects, and interactions” using a general stand-alone power analysis program. We finally decided to set a total sample size of 63 in experiment 1. Also, we finally decided to set the sizes of the control and training groups at 7 and 17 subjects, respectively, in experiment 2.
Experimental design
In experiment 1, characteristics of subjects, hemodynamics, carotid arterial compliance, and circulating TX concentrations were measured in all subjects. In experiment 2, we measured hemodynamics, muscle strength, carotid arterial compliance, and circulating TX concentrations before and after the intervention for four weeks in all subjects.
Measurements
Before each test, subjects abstained from caffeine and fasted for at least 12 h. All subjects were studied at least 48 h after they last exercised, to avoid acute effects of exercise. All measurements were performed at constant room temperature (23–25 °C), in a quiet room, after the subjects had rested in a supine position for at least 15 min. In experiment 2, the subjects were tested at the same time of the day throughout the study period to avoid potential diurnal variations.
Strength testing
In experiment 2, the maximal muscular strength of all subjects was tested before and after intervention using bicep curls. All subjects performed warm up exercise, constituting 10 repetitions with 5 kg weights, and afterwards, 1RM was obtained based on the established guideline.20 All repetitions of maximal strength test were performed in 3-s eccentric (lowering) and concentric (lifting) phases. Subjects repeated the actions at approximately constant velocities and frequencies with the aid of a metronome. Relative strength was calculated as follows: Relative strength = 1RM/body mass. The day-to-day coefficients of variation were 1.0 ± 1.8% and 1.4 ± 1.4% for 1RM and relative strength, respectively.
Carotid arterial compliance
Common carotid artery echography immediately after applanation of tonometrically obtained arterial pressure from the carotid artery permits noninvasive determination of dynamic carotid arterial compliance. The common carotid artery diameter was measured from the images derived from an ultrasound machine (Logiq E; GE Healthcare, Tokyo, Japan) equipped with a high-resolution linear-array transducer as previously described.4,21 A longitudinal image of the cephalic portion of the common carotid artery was acquired 1–2 cm distal to the carotid bulb. The computer images were analyzed by using image analysis software (Image J, Maryland, USA). The same investigator performed all image analyses. The diameter of the arterial lumen at minimal diastolic relaxation and maximal systolic expansion was measured at three points per frame, and the points were then averaged. Carotid arterial pressure waveforms were obtained with arterial applanation tonometry using an array of 15 micro-piezoresistive transducers (form PWV/ABI; Colin Medical Technology, Komaki, Japan).22 These waveforms were calibrated by equating the carotid mean arterial pressure, diastolic blood pressure (BP) to the brachial mean arterial pressure, and diastolic BP. Each parameter was averaged over 10–15 continuous beats. Brachial BP was measured with the oscillometric method using the automated polygraph apparatus (form PWV/ABI; Colin Medical Technology, Komaki, Japan). Heart rate was computed from ECG. Carotid arterial compliance was calculated by using the equations [(D1 – D0)/D0]/[2(P1 – P0)]π(D0)2], where D1 and D0 are the maximal and minimum diameters, and P1 and P0 are the highest and lowest BPs.4,23 The daily coefficient of variation of carotid arterial compliance was 6.8% ± 2.7% in our laboratory. The β-stiffness index was analyzed using the equations: ln (P1/P0)/[(D1 – D0)/D0], the β-stiffness index is an index of carotid arterial compliance adjusted for distending pressure.4
Circulating TX concentrations
Each blood sample was placed in a chilled serum separator tube and then centrifuged at 3000 × g for 15 min at 4 °C. The serum samples were stored at −80 °C until the assay. Among TXs, thromboxane-A2 (TXA2) has a potent vasoconstrictory effect.16 However, TXA2 is metabolized to thromboxane-B2 (TXB2), with a half-life of 30 s.24 Therefore, we measured the circulating TXB2 concentrations in the present study. Serum concentrations of TXB2 were measured using an enzyme-linked immunoassay kit (Enzo Life Sciences, New York, USA). The intra-assay coefficient of variation of TXB2 was 3.1 ± 1.3%, as previously described (TXB2, ADI-900-002, Enzo Life Sciences).
Body composition
Anthropometric measurements were taken with subjects’ barefoot and wearing only light clothing. Height was measured to the nearest 0.1 cm using a stadiometer (AD-6227R, A&D Co., Ltd., Tokyo, Japan). Body mass, body fat percentage, and lean body mass were measured to the nearest 0.1 kg on a calibrated digital scale (InBody 770, InBody Japan, Tokyo, Japan) and adjusted for the estimated clothing mass by subtracting 0.5 kg. Daily coefficients of variation for the two trials were 0.1% ± 0.1%, 0.2% ± 0.1%, 0.5% ± 0.2%, 3.4% ± 2.7%, and 0.4% ± 0.4% for height, body mass, body mass index, body fat percentage, and lean body mass, respectively.
Resistance training intervention
In experiment 2, the subjects in training group underwent supervised resistance exercises thrice a week, during the four-week-study. During the training session, the subjects completed five sets of 10 repetitions of bicep curls at 75% of 1RM, with a 2-min inter-set rest period.19,25 We selected bicep curls, which induce an increase in arterial stiffness (i.e., decrease in arterial compliance) as well as whole-body resistance training.4,19,25 All repetitions of the resistance training were performed in a 3-s eccentric (lowering) and concentric (lifting) phases.19 The subjects repeated the actions at approximately constant velocities and frequencies with the aid of a metronome. The loads were increased for the following exercise sessions when subjects were able to complete 10 repetitions in the third set. Each training session lasted for approximately 20 min. The resistance exercise was performed until concentric failure, afterward remaining sets were completed with the support of assistants. Except for routine activities during training, all other exercises (resistance training, anaerobic exercise, and aerobic exercise) were prohibited.
Statistical analysis
The Shapiro–Wilk test was used to evaluate the normality of distributions. Data were expressed as mean ± standard deviation unless indicated otherwise. In experiment 1, because serum TXB2 concentrations were not a normal distribution, the relationship between carotid arterial compliance and serum TXB2 concentrations was assessed by Spearman rank correlation coefficient (rs) analysis. It is worth noting that advancing age can elicit increased urinary TXB2 concentrations and decreased carotid arterial compliance23,26; therefore, the correlation was adjusted for age. In experiment 2, unpaired sample t-tests were used to examine differences between the groups on characteristics of subjects. A two-way analysis of variance with repeated measures was used to evaluate the interaction (group*time) on hemodynamics, muscle strength, carotid arterial compliance, and serum TXB2 concentrations. When a significant interaction was detected, specific mean comparisons were performed to identify significant differences within each intervention. When a significant F values were obtained, a post-hoc test using the Bonferroni method was performed to identify the significant differences among the mean values. Moreover, we conducted the comparison of changes in arterial compliance and serum TXB2 concentrations between groups using a non-parametric Mann–Whitney U test. Because changes in serum TXB2 concentrations and arterial compliance were not a normal distribution, the relationship between the changes from baseline in serum TXB2 concentrations and carotid arterial compliance in all subjects was assessed using Spearman rank correlation coefficient (rs) analysis. In addition, for the same reason described above, the correlation was adjusted for age. In all tests, a two-tailed P value < 0.05 was accepted as statistically significant. All statistical analyses were performed using SPSS Statistics version 24.0 for Windows (IBM SPSS Japan Inc., Japan).
Results
Experiment 1
We investigated whether serum TXB2 concentrations are associated with carotid arterial compliance in young men. Table 1 shows the subjects’ characteristics, hemodynamics, carotid arterial compliance, and serum TXB2 concentrations. We found a significant negative correlation between carotid arterial compliance and serum TXB2 concentrations (Fig. 1, rs = −0.26, P < 0.05). After adjustment for age, serum TXB2 concentrations were still significantly associated with carotid arterial compliance (partial rs = −0.25, P < 0.05).
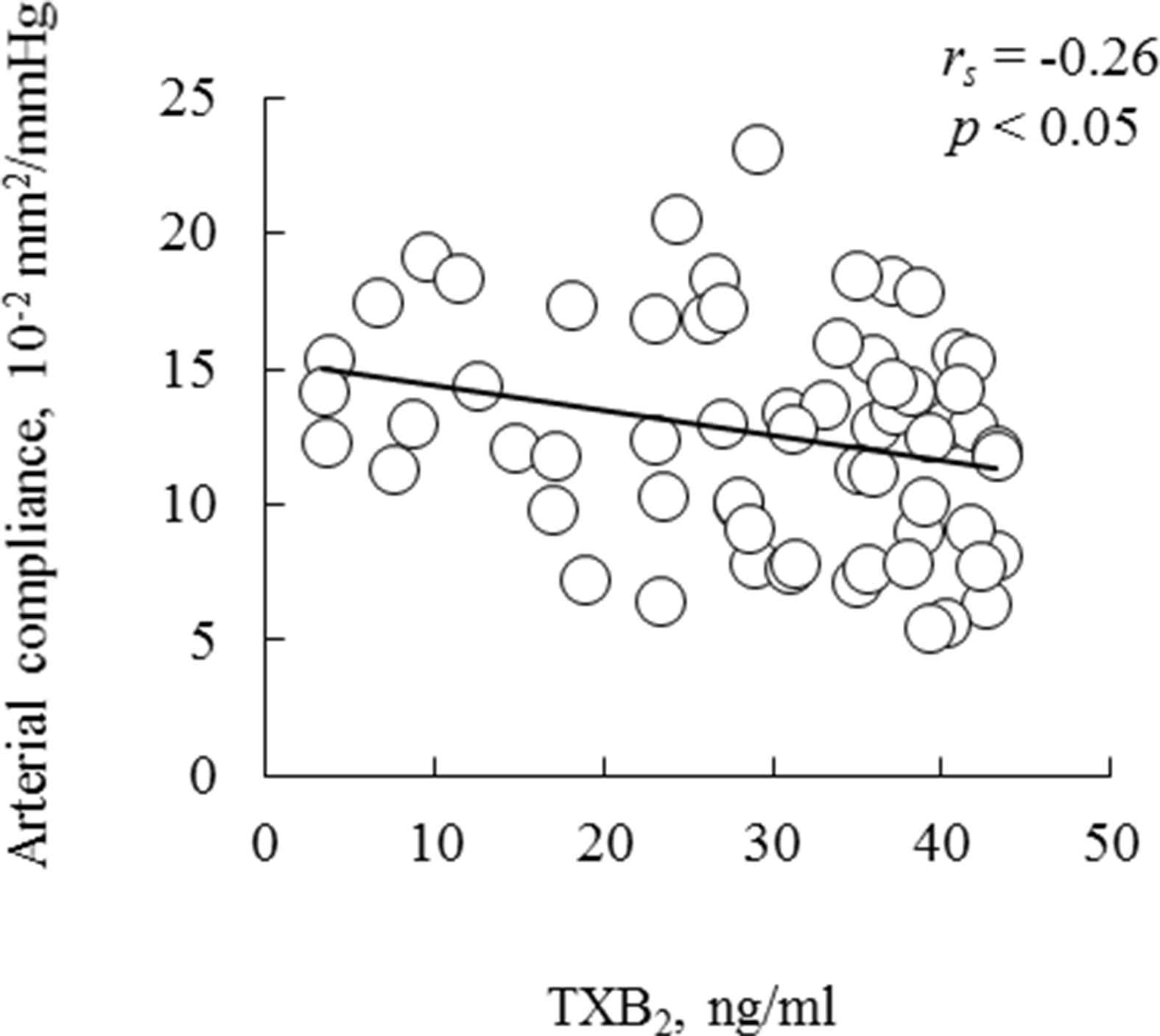
Correlation between serum TXB2 concentrations and carotid arterial compliance in young men. The line in the scatter plots shows a significant correlation by Spearman rank correlation coefficient analysis. Abbreviations: TXB2- thromboxane B2.
| Variables | |
|---|---|
| Number of subjects | 63 |
| Age, years | 25 ± 4 |
| Height, cm | 172 ± 6 |
| Body mass, kg | 69.8 ± 12.2 |
| Body mass index, kg m−2 | 23.6 ± 3.9 |
| Body fat, % | 19.5 ± 7.2 |
| Lean body mass, kg | 52.6 ± 6.0 |
| Systolic BP, mmHg | 116 ± 10 |
| Mean BP, mmHg | 86 ± 8 |
| Diastolic BP, mmHg | 68 ± 7 |
| Heart rate, bpm | 58 ± 9 |
| Arterial compliance, 10−2 mm2/mmHg | 13 ± 4 |
| Thromboxane B2, ng/ml | 29.3 ± 11.6 |
Note: Values are means ± SD. BP indicates blood pressure.
Characteristics and hemodynamics of selected subject in experiment 1.
Experiment 2
We investigated the effects of resistance training on serum TXB2 concentrations and arterial compliance in young men. In the training group, subjects completed all training sessions (i.e., a total of 12 training sessions in four weeks). At the baseline, no significant differences were found in any of the parameters between the control and training groups (Tables 2 and 3, Fig. 2). In addition, no significant interactions were observed in BPs (systolic BP, mean BP, and diastolic BP), heart rate, and carotid arterial distension between the control and training groups (Table 3); among these parameters, only carotid arterial distension was decreased after resistance training (P < 0.05). We found significant interactions in β-stiffness index, 1RM, and relative strength between the two groups (β-stiffness index: F = 8.32, P < 0.01; 1RM: F = 38.19, P < 0.001; relative strength: F = 35.83, P < 0.001); β-stiffness index, 1RM, and relative strength significantly increased after the four-week training (three parameters, P < 0.001).
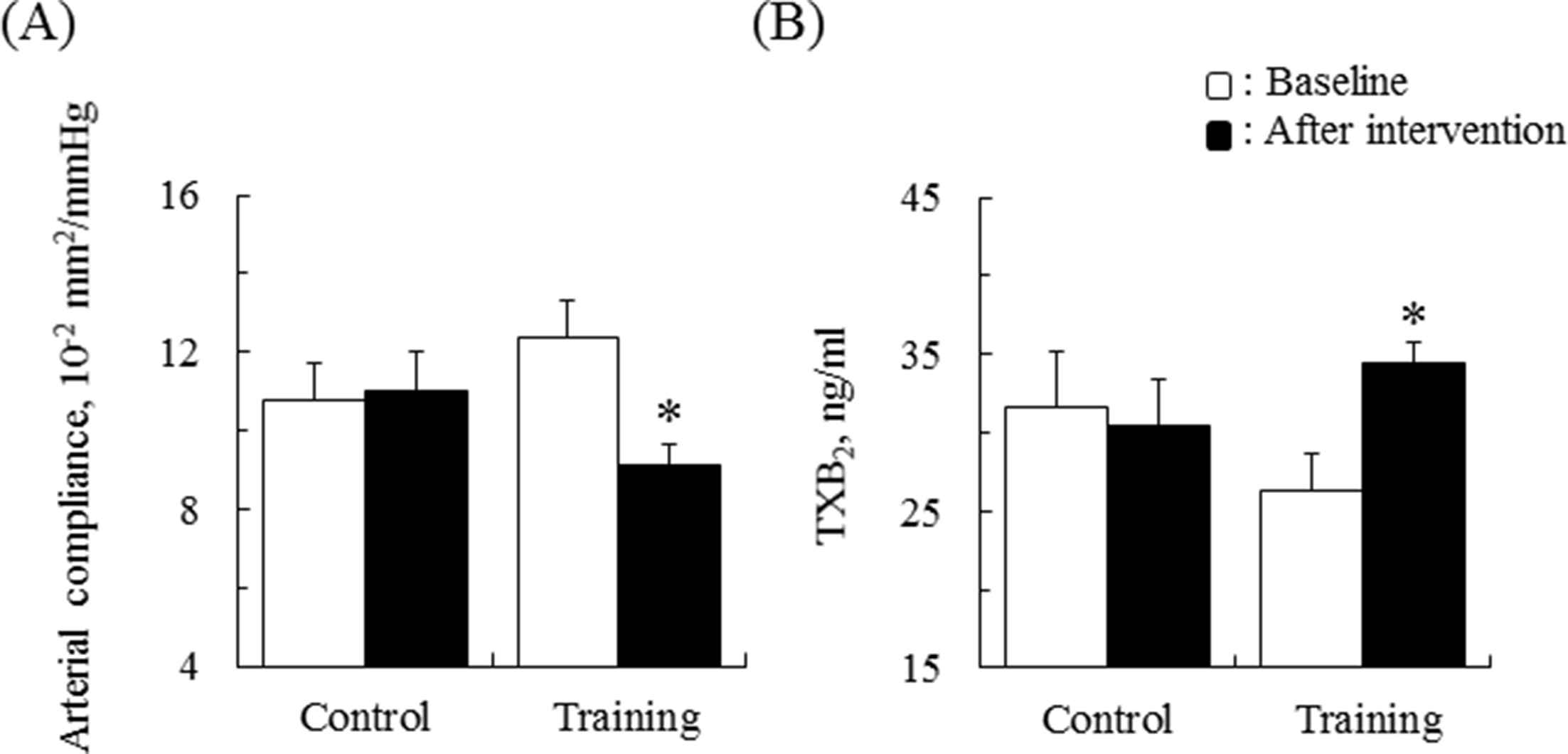
Changes in (A) carotid arterial compliance and (B) serum TXB2 concentrations for both the control and training groups by two-way ANOVA. Two-way ANOVA reveals a significant interaction in both (A) and (B). P < 0.005 by post-hoc analysis shows the significant difference compared with baseline in the training group. Data are presented as mean ± SE. Abbreviations: TXB2- thromboxane B2; ANOVA-analysis of variance.
| Variables | Control | Training |
|---|---|---|
| Number of subjects | 7 | 17 |
| Age, years | 25 ± 2 | 25 ± 4 |
| Height, cm | 173 ± 5 | 173 ± 6 |
| Body mass, kg | 76.5 ± 13.2 | 70.2 ± 12.5 |
| Body mass index, kg m−2 | 25.7 ± 4.8 | 23.5 ± 3.9 |
| Body fat, % | 21.5 ± 9.1 | 19.6 ± 7.4 |
| Lean body mass, kg | 59.0 ± 4.4 | 55.5 ± 6.5 |
Note: Values are means ± SD.
Selected subject characteristics in experiment 2.
| Variables/Group | Time point | Interaction | |
|---|---|---|---|
| Baseline | After intervention | ||
| Systolic BP, mmHg | |||
| Control | 115 ± 10 | 115 ± 7 | F < 0.001 |
| Training | 118 ± 10 | 118 ± 8 | P = 1.00 |
| Mean BP, mmHg | |||
| Control | 83 ± 8 | 86 ± 6 | F = 0.73 |
| Training | 88 ± 8 | 88 ± 8 | P = 0.40 |
| Diastolic BP, mmHg | |||
| Control | 65 ± 6 | 66 ± 6 | F = 0.002 |
| Training | 69 ± 8 | 70 ± 9 | P = 0.97 |
| Heart rate, bpm | |||
| Control | 57 ± 6 | 56 ± 10 | F = 0.64 |
| Training | 60 ± 9 | 61 ± 9 | P = 0.43 |
| Arterial distension, mm | |||
| Control | 0.61 ± 0.18 | 0.63 ± 0.31 | F = 3.01 |
| Training | 0.51 ± 0.13 | 0.43 ± 0.13 | P = 0.10 |
| β-stiffness index, U | |||
| Control | 5.8 ± 1.1 | 5.6 ± 0.6 | F = 8.32 |
| Training | 4.9 ± 1.1 | 7.1 ± 1.6* | P < 0.01 |
| 1RM biceps curls, kg | |||
| Control | 23 ± 3 | 23 ± 3 | F = 38.19 |
| Training | 21 ± 3 | 25 ± 4* | P < 0.001 |
| Relative strength, 1RM/body mass | |||
| Control | 0.30 ± 0.03 | 0.30 ± 0.03 | F = 35.83 |
| Training | 0.31 ± 0.04 | 0.36 ± 0.06*# | P < 0.001 |
Note: Values are means ± SD. BP indicates blood pressure; 1RM indicates one repetition maximum.
P < 0.005 vs baseline;
P < 0.05 vs control group.
Changes in hemodynamics and muscle strength.
After the intervention, we found a significant interaction on changes in carotid arterial compliance between the groups (F = 8.70, P < 0.01); carotid arterial compliance was significantly decreased after intervention in the training group (Fig. 2A, control: from 11 ± 2 to 11 ± 3 10−2 mm2/mmHg, N.S. training: from 12 ± 4 to 9 ± 2 10−2 mm2/mmHg, P < 0.005). Significant interaction on changes in serum TXB2 concentrations was found between the control and training groups (F = 4.76, P < 0.05); serum TXB2 concentrations were significantly increased only in the training group (Fig. 2B, control: from 31.7 ± 9.2 to 30.4 ± 7.8 ng/ml, N.S.; training: from 26.3 ± 9.9 to 34.4 ± 5.4 ng/ml, P < 0.005). Furthermore, the changes in carotid arterial compliance (decrease) and serum TXB2 concentrations (increase) were significantly greater in the training group than in the control group (both, P < 0.05).
As shown in Fig. 3, we found a significant negative correlation between changes in serum TXB2 concentrations and those in carotid arterial compliance before and after the intervention (rs = −0.56, P < 0.005). In addition, the relationship between changes in serum TXB2 concentrations and those in carotid arterial compliance remained significant after controlling for age (partial r = −0.42, P < 0.05).
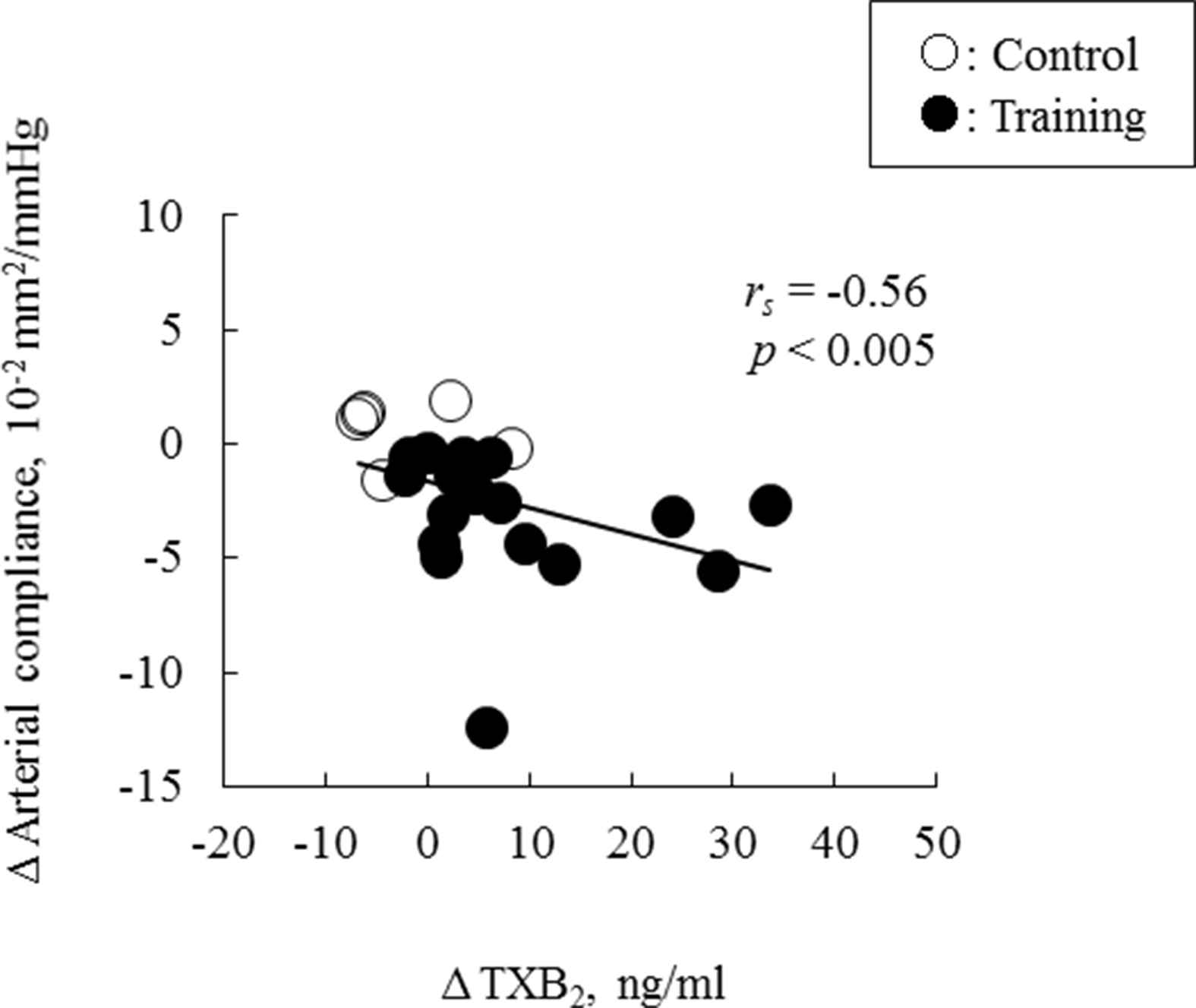
The relationship between changes in the carotid arterial compliance and in serum TXB2 concentrations, before and after the intervention. The line in the scatter plots shows a significant correlation by Spearman rank correlation coefficient analysis. Abbreviations are the same as in Fig. 1.
Discussion
The salient findings of the present study were as follows: First, in a cross-sectional study, a significant negative correlation was found between circulating TXB2 concentrations and carotid arterial compliance in young men. Second, in a longitudinal study, the four-week resistance training significantly increased circulating TXB2 concentrations, which was significantly associated with the resistance training-induced decrease in carotid arterial compliance in young men. Therefore, we suggest that the increases in circulating TXB2 concentrations were partly involved in the decrease in CAC after the resistance training.
TXA2 is produced from the platelets and has a potent vasoconstrictory effect.14 A previous study demonstrated that circulating concentrations of TXB2 in subjects with high central BP are three-fold higher than that in subjects with low central BP.27 In addition, increasing urinary concentrations of TXB2 have been reported to be associated with an increasing risk of cardiovascular events, particularly myocardial infarction and cardiovascular death.28 Thus, increased TXB2 concentrations may be associated with deteriorated vascular functions, and cardiovascular disease. In our cross-sectional study, circulating TXB2 concentrations were significantly negatively correlated with CAC in young men. Furthermore, after resistance training intervention, circulating TXB2 concentrations were significantly elevated, arterial compliance was significantly decreased, and the changes in circulating TXB2 concentrations were significantly negatively associated with a change in CAC in young men. In summary, our results suggest that the increased circulating TXB2 concentrations participate, at least in part, in the mechanisms underlying resistance training-induced decrease in CAC, in young men.
The possible mechanisms underlying the resistance training-induced increases in circulating TXB2 concentrations could be explained by the sympathetic nervous system. Inhibition of alpha-1 and alpha-2 adrenergic receptors has been reported to decrease circulating TXB2 concentrations.29 In addition, resistance training increases circulating norepinephrine concentrations,30 which increase alpha- and beta-adrenergic effects.31 Hence, the resistance training-induced increase in circulating norepinephrine concentrations possibly contributes to increased circulating TXB2 concentrations. However, further studies are necessary to elucidate the mechanisms underlying the increase in circulating TXB2 concentrations caused by resistance training.
In the present study, our data showed that increased circulating TXB2 concentrations induced by resistance training are significantly related to decreased CAC in young men. Several mechanisms can be proposed as follows: First, increased vasoconstrictor tone exerted by smooth muscle cells aggravates arterial compliance.32,33 TX is produced from the platelets and has potent vasoconstrictory and platelet aggregation effects.13–15 The TX receptor exists on vascular smooth muscle cell.16 Following the occupation of TX receptor on vascular smooth muscle cell, the contraction is elicited through coupling to either Gq/11 or, to a greater extent, G12/13 evokes the biosynthesis of inositol 1,4,5-trisphophate and activation of specific RhoA guanine nucleotide exchange factors that in turn activate Rho kinase.34 Such a contraction is significant because it is slow and lasting.35 Hence, increased circulating TXB2 concentrations induced by the resistance training possibly caused a direct decrease in CAC in the present study. Second, one animal study showed that TXA2 antagonist decreases the oxidative stress.36 We have previously reported that a single bout of high-intensity eccentric exercise increases the oxidative stress in young men.37 Moreover, activation of oxidative stress provokes vasoconstriction in the central artery.38 Thus, oxidative stress possibly contributes to the relationship between changes in circulating TXB2 concentrations and CAC, which are induced by the resistance training. Future studies are necessary to test these hypotheses.
There are several noteworthy limitations of the present study that should be emphasized. First, the present study was not randomized control trial. Applicants for control and resistance training were recruited as each subject in control and training groups, respectively. Moreover, the sample size of the present study was small. However, the sample power in the present study was 0.81 for arterial compliance. This means that 81% of studies would be expected to yield a significant effect, rejecting the null hypothesis that the odds are 1.0, and suggesting that the present study has substantial statistical power. Further studies are necessary to investigate a randomized control trial of substantial statistical power. Second, the present study was not included women. Since female hormone (i.e., estrogen) influences arterial compliance,39 we recruited only men, which limits the generalizability of this study. Further studies are needed to examine this issue. Third, we adapted biceps curls as high-intensity resistance training used in previous study. However, bicep curls exercise is difficult to gain generalizability. Further interventional studies are needed using whole-body resistance training. Fourth, the present study conducted arterial compliance analysis using calipers at three different points. A number of previous studies from our laboratory and others have reported that in exercise training intervention, arterial compliance has been conducted using calipers at three different points.4,23,40,41 Also, the daily coefficient of variation of arterial compliance was 6.8% in our laboratory, as well as 7.5% of coefficient of variation using wall tracking device for carotid artery distension.42 Thus, we believe that arterial compliance analysis using calipers at three different points has similar good repeatability to using wall tracking device.
In conclusion, the particularly novel findings in the present study are as follows: circulating TXB2 concentrations were significantly negatively correlated with CAC in a cross-sectional study in young men. The resistance training significantly increased circulating TXB2 concentrations, which was significantly associated with a resistance training-induced decrease in CAC in a longitudinal study in young men. These findings suggest that the increases in circulating TXB2 concentrations partly contribute to the deterioration in CAC, resulting from resistance training in young men.
Grant
The present study was supported by in part by a grant from
Conflicts of interest
The authors declare no conflicts of interest, financial or otherwise.
Acknowledgments
We would like to thank Dr. Satoshi Sakai and Dr. Takashi Miyauchi for scientific support.
References
Cite this article
TY - JOUR AU - Kaname Tagawa AU - Song-Gyu Ra AU - Hiroshi Kumagai AU - Yuriko Sawano AU - Kosaku Yamamoto AU - Toru Yoshikawa AU - Youngju Choi AU - Yasuko Yoshida AU - Kazuhiro Takekoshi AU - Seiji Maeda PY - 2018 DA - 2018/09/18 TI - Resistance training-induced decreases in central arterial compliance is associated with increases in serum thromboxane B2 concentrations in young men JO - Artery Research SP - 63 EP - 70 VL - 23 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2018.08.001 DO - 10.1016/j.artres.2018.08.001 ID - Tagawa2018 ER -
