The Epidemiology of HIV in Oman, 1984–2018: A Nationwide Study from the Middle East
- DOI
- 10.2991/jegh.k.191208.001How to use a DOI?
- Keywords
- HIV/AIDS; epidemiology; Oman; Middle East; late HIV diagnosis
- Abstract
We used population-based data on all diagnosed people living with Human Immunodeficiency (HIV) reported to the National AIDS Programme in 1984–2018 to describe the HIV epidemiology in Oman. A total of 3060 Omanis were diagnosed with HIV from 1984 to 2018. The proportions of new infections attributed to sexual contact accounted for 56.3% (376/668) in 1984–1996 compared with 80.7% (630/780) in 2013–2018. Of 1417 patients with a documented CD4 count at the entry of care, 45.3% had a baseline CD4 count of <200 cells/mm3. Compared with heterosexuals, homosexuals had higher rates of advanced HIV disease [42.7% (388/908) vs 50.4% (136/270), respectively]. Rates of advanced disease and death within a year of HIV diagnosis rose consistently with age at diagnosis. Approximately half (48.8%) of the patients diagnosed in 1984–2018 had died by December 2018. The majority (85.6%; 572/668) of people who were diagnosed in 1984–1997 had died compared with 12.7% (99/780) of those diagnosed in 2013–2018. However, people died more recently had a higher proportion of death within a year of HIV diagnosis [74.7% (74/99) in 2013–2018 compared with 13.8% (79/572) in 1984–1996]. This study shows that the HIV epidemic in Oman is a low-prevalence one. Of concern, a large proportion of new HIV diagnoses continued to present late, which has resulted in a substantial increase in short-term mortality over the past 20 years. Nevertheless, we observed a remarkable decline in overall mortality over time, which may be explained by the improvement in the quality of HIV care in Oman.
- Copyright
- © 2020 The Authors. Published by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Despite the remarkable progress in Human Immunodeficiency (HIV) treatment [1,2], HIV infection remains a major global health threat. The Joint United Nations Programme on HIV and AIDS (UNAIDS) global AIDS report shows that there were 37.9 million People Living with HIV/AIDS (PLWHA) in 2018; 1.7 million became newly infected, and 770,000 people died from AIDS-related illnesses in 2018 [3]. Since 2010, the global AIDS-related mortality has declined by 33%, but it has increased by 9% in the Middle East and North Africa (MENA) region. Besides, the new global HIV infections declined from 2.1 million in 2010 to 1.7 million in 2018; however, the annual number of new HIV infections in the MENA has increased by 10% over the same period [3]. Detailed knowledge of the HIV epidemic through data collection and analysis is crucial to guide policies and programs in the MENA region. Unfortunately, the most recent UNAIDS global AIDS report lacked data on more than half of the 21 countries in the MENA region [3].
Oman is situated in the Arabian Peninsula, with a total population of 4,658,295; of these, 2,666,934 (57.3%) are Omanis [4]. The first case of HIV/AIDS in Oman was diagnosed in 1984, and the National AIDS Programme (NAP) was formed in 1996. There are currently 14 public treatment centers offering free HIV care including Antiretroviral Therapy (ART), HIV genotyping, HIV Viral Load (VL), and CD4 counts. The country adopted treatment for all, irrespective of CD4 cell count, in December 2015 [5]. This study aims to provide a descriptive analysis of the HIV epidemiology in Oman in 1984–2018. We will examine the change in the HIV epidemic in the country, with particular emphasis on trends of new HIV diagnoses, all-cause mortality, and survival. We hope to produce a summary that will inform policymakers and stakeholders’ targeted interventions.
2. MATERIALS AND METHODS
In this analysis, we included all Omani HIV cases reported to the NAP from 1984 through December 2018. We used a centralized national HIV surveillance dataset. The ministry of health has been collecting individual-based HIV data since 1984. Data include demographics, HIV risk factor, reason for HIV testing, baseline CD4 count, baseline HIV VL, Hepatitis B Virus (HBV) status, Hepatitis C Virus (HCV) status, and ART details. Positive HBV status was defined as positive Hepatitis B Surface Antigen and positive HCV status was defined as positive HCV antibodies. Both baseline CD4 count and HIV VL were defined as closest measurement to HIV diagnosis. We used the UNAIDS Spectrum modeling software [6] to calculate the estimated number of PLWHA in Oman by December 2018.
We stratified data by sex, age (0–4, 5–14, 15–24, 25–49, and ≥50 years), year of HIV diagnosis, HIV risk factor, reason for HIV testing (HIV-related symptoms, blood bank screening, patient’s request, HIV contact, antenatal screening, and others, such as prisoner, tuberculosis, sexually transmitted infection diagnosis or preemployment health check), and region of residence (Muscat and outside Muscat). HIV risk factor was categorized into heterosexual, homo/bisexual, and others. HIV risk factor was labeled heterosexual and homo/bisexual when the infection was attributed to heterosexual contact and homo/bisexual contact, respectively. “Others” factor for HIV transmission included vertical transmission, Intravenous Drug Use (IDU), and blood transfusion. Year of HIV diagnosis was stratified (based on key national and international milestones in HIV care such as the availability and roll out of ART in the country) into four periods: 1984–1996, 1997–2006, 2007–2012, and 2013–2018. Survival from HIV diagnosis until death by December 31, 2018, was categorized into <1, 1–4, and >4 years after HIV diagnosis. Based on the CD4 count at baseline, we classified stage of HIV diagnosis into advanced HIV disease (CD4 count <200 cells/mm3), late HIV diagnosis (CD4 count 200–349 cells/mm3), and early diagnosis (CD4 count ≥350 cells/mm3). We analyzed data using MS Excel and SPSS. We used Chi-square test as a test of significance for proportions and p-values were calculated and considered significant if <0.05. As we used routinely collected data, we did not seek ethical approval or written consent.
3. RESULTS
3.1. HIV Diagnosis
A total of 3060 Omanis were diagnosed with HIV in the period from 1984 to 2018, of whom 872 (28.5%) were diagnosed in the period from 1997 through 2006 (Table 1). As of December 31, 2018, the majority (85.6%; 572/668) of people who were diagnosed in 1984–1997 had died compared with 12.7% (99/780) of those diagnosed in 2013–2018. Males accounted for more than two-thirds of new diagnoses. The age at diagnosis distribution has changed over time, the percentage of those aged 0–14 years decreased from 14.2% (95/668) in 1984–1996 to 2.9% (23/780) in 2013–2018, while the proportion of patients aged 15–24 years rose from 16.8% (112/668) to 20.4% (159/780) in the same periods. HIV risk factors also differed over time. The proportions of new infections attributed to sexual contact accounted for 56.3% (376/668) of infections in 1984–1996 compared with 80.7% (630/780) in 2013–2018. The majority (71.2%; 555/780) of people diagnosed in 2013–2018 resided outside Muscat compared with 50.1% (335/668) in 1984–1996. Figure 1 shows the HIV cases stratified by year of diagnosis and sex.
| Characteristics (N = 3060) | Year of HIV diagnosis | p-value | |||
|---|---|---|---|---|---|
| 1984–1996 (n = 668), n (%)* | 1997–2006 (n = 872), n (%) | 2007–2012 (n = 740), n (%) | 2013–2018 (n = 780), n (%) | ||
| Alive (n = 1566) | 96 (14.4) | 303 (34.7) | 486 (65.7) | 681 (87.3) | <0.01 |
| Dead (n = 1494) | 572 (85.6) | 569 (65.3) | 254 (34.3) | 99 (12.7) | |
| Sex | |||||
| Male (n = 2212) | 452 (67.7) | 674 (77.3) | 513 (69.3) | 573 (73.5) | <0.01 |
| Female (n = 848) | 216 (32.3) | 198 (22.7) | 227 (30.7) | 207 (26.5) | |
| Residence | |||||
| Muscat (n = 1044) | 333 (49.9) | 321 (36.8) | 165 (22.3) | 225 (28.8) | <0.01 |
| Outside Muscat (n = 2016) | 335 (50.1) | 551 (63.2) | 575 (77.7) | 555 (71.2) | |
| Age at diagnosis (years) | |||||
| 0–4 (n = 77) | 23 (3.4) | 18 (2.1) | 24 (3.2) | 12 (1.5) | <0.01 |
| 5–14 (n = 120) | 72 (10.8) | 23 (2.6) | 14 (1.9) | 11 (1.4) | |
| 15–24 (n = 561) | 112 (16.8) | 152 (17.4) | 138 (18.6) | 159 (20.4) | |
| 25–49 (n = 1958) | 403 (60.3) | 576 (66.1) | 472 (63.8) | 507 (65) | |
| ≥50 (n = 344) | 58 (8.7) | 103 (11.8) | 92 (12.4) | 91 (11.7) | |
| Risk factor | |||||
| Heterosexual (n = 1670) | 301 (45.1) | 467 (53.6) | 431 (58.2) | 471 (60.4) | <0.01 |
| Homosexual/bisexual (n = 503) | 75 (11.2) | 154 (17.7) | 115 (15.5) | 159 (20.3) | |
| Others (n = 410) | 214 (32) | 102 (11.7) | 53 (7.2) | 41 (5.3) | |
| Not documented (n = 477) | 78 (11.7) | 149 (17.1) | 141 (19.1) | 109 (14) | |
| Reason for testing | |||||
| Antenatal screening (n = 112) | 1 (0.1) | 2 (0.2) | 34 (4.6) | 75 (9.6) | <0.01 |
| Blood bank screening (n = 63) | 0 (0) | 4 (0.4) | 15 (2) | 44 (5.6) | |
| Patient’s request (n = 91) | 1 (0.1) | 11 (1.3) | 21 (2.8) | 58 (7.4) | |
| HIV contact (n = 268) | 43 (6.4) | 49 (5.6) | 97 (13.1) | 79 (10.1) | |
| HIV-related symptoms (n = 422) | 2 (0.3) | 22 (2.5) | 96 (13) | 302 (38.7) | |
| Not documented (n = 1834) | 614 (91.9) | 765 (87.7) | 403 (54.5) | 52 (6.7) | |
| Others (n = 270) | 7 (1.4) | 19 (2.2) | 74 (10) | 170 (21.8) | |
| Diagnosing health institution | |||||
| Public sector (n = 2966) | 663 (99.3) | 863 (99) | 730 (98.6) | 710 (91) | <0.01 |
| Private sector (n = 94) | 5 (0.7) | 9 (1) | 10 (1.4) | 70 (9) | |
Unless stated otherwise.
Characteristics of diagnosed people living with HIV/AIDS in Oman in 1984–2018, stratified by year of HIV diagnosis
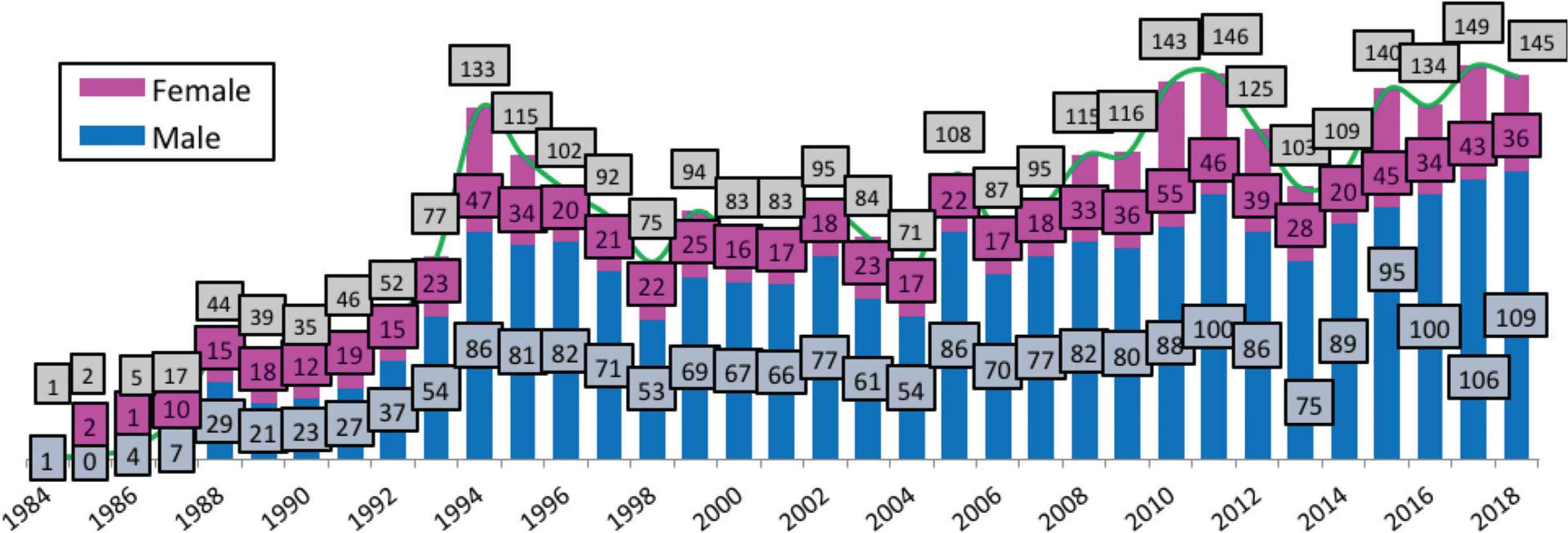
HIV cases by sex and year of diagnosis, 1984–2018.
3.2. Stage of HIV Diagnosis
Of 1417 patients with a documented CD4 count at entry of care, about two-thirds (69.9%) and 45.3% had a baseline CD4 count of <350 and <200 cells/mm3, respectively (Table 2). The proportions of female patients diagnosed in 1984–2018 with CD4 count <200 and <350 cells/mm3 were lower than males (34.3%; 150/437 vs 50.2%; 492/980 and 60.9%; 266/437 vs 73.9%; 724/980, respectively). Rates of advanced HIV disease and late HIV diagnosis rose consistently with age at diagnosis. Percentages of patients aged 15–24, 25–49, and ≥50 years with initial CD4 counts of <200 cells/mm3 were 39.9% (123/308), 46.8% (433/925), and 65% (76/117), respectively. Compared with heterosexuals, homosexuals had higher rates of advanced HIV disease [42.7% (388/908) vs 50.4% (136/270), respectively]. In contrast, patients who were diagnosed because of antenatal screening had a lower proportion (19.8%; 20/101) of advanced HIV disease.
| Characteristics (N = 1417) | Baseline CD4 count at diagnosis | p-value | ||
|---|---|---|---|---|
| CD4 <200, n (%)* | CD4 200–349, n (%) | CD4 ≥350, n (%) | ||
| Total (n = 1417) | 642 (45.3) | 348 (24.6) | 427 (30.1) | |
| Alive (n = 1245) | 507 (40.7) | 326 (26.2) | 412 (33.1) | <0.01 |
| Dead (n = 172) | 135 (78.5) | 22 (12.8) | 15 (8.7) | |
| Year of diagnosis | ||||
| 1984–1996 (n = 58) | 34 (58.6) | 15 (25.9) | 9 (15.5) | <0.01 |
| 1997–2006 (n = 240) | 128 (53.3) | 49 (20.4) | 63 (26.3) | |
| 2007–2012 (n = 483) | 190 (39.3) | 131 (27.1) | 162 (33.5) | |
| 2013–2018 (n = 636) | 290 (45.6) | 153 (24.1) | 193 (30.3) | |
| Gender | ||||
| Male (n = 980) | 492 (50.2) | 232 (23.7) | 256 (26.1) | <0.01 |
| Female (n = 437) | 150 (34.3) | 116 (26.5) | 171 (39.1) | |
| Residence | ||||
| Muscat (n = 436) | 198 (45.4) | 107 (24.5) | 131 (30.0) | 0.998 |
| Outside Muscat (n = 981) | 444 (45.3) | 241 (24.6) | 296 (30.2) | |
| Age at diagnosis (years) | ||||
| 0–4 (n = 35) | 4 (11.4) | 3 (8.6) | 28 (80.0) | <0.01 |
| 5–14 (n = 32) | 6 (18.8) | 9 (28.1) | 17 (53.1) | |
| 15–24 (n = 308) | 123 (39.9) | 80 (26.0) | 105 (34.1) | |
| 25–49 (n = 925) | 433 (46.8) | 233 (25.2) | 259 (28.0) | |
| ≥50 (n = 117) | 76 (65.0) | 23 (19.7) | 18 (15.4) | |
| Risk factor | ||||
| Heterosexual (n = 908) | 388 (42.7) | 243 (26.8) | 277 (30.5) | <0.01 |
| Homosexual/bisexual (n = 270) | 136 (50.4) | 67 (24.8) | 67 (24.8) | |
| Others (n = 107) | 28 (26.2) | 20 (18.7) | 59 (55.1) | |
| Unknown (n = 132) | 90 (68.2) | 18 (13.6) | 24 (18.2) | |
| Reason for testing | ||||
| Antenatal screening (n = 101) | 20 (19.8) | 22 (21.8) | 59 (58.4) | <0.01 |
| Blood bank screening (n = 53) | 17 (32.1) | 15 (28.3) | 21 (39.6) | |
| Client initiated testing (n = 77) | 24 (31.2) | 15 (19.5) | 38 (49.4) | |
| HIV contact (n = 184) | 50 (27.2) | 50 (27.2) | 84 (45.7) | |
| HIV-related symptoms (n = 361) | 226 (62.6) | 77 (21.3) | 58 (16.1) | |
| Not documented (n = 422) | 236 (55.9) | 105 (24.9) | 81 (19.2) | |
| Others (n = 219) | 69 (31.5) | 64 (29.2) | 86 (39.3) | |
Unless stated otherwise.
Characteristics of diagnosed people living with HIV/AIDS in Oman in 1984–2018, stratified by CD4 count at baseline
3.3. Mortality and Survival
Approximately half (48.8%; 1494/3060) of the patients diagnosed in 1984–2018 had deceased with an average survival of 5 years; 28.2% and 79.1% of them died within a year and 8 years of diagnosis, respectively. The average survival among living patients was 8.5 years, with 44% (689/1566) surviving for >8 years after diagnosis. Of the 848 female patients diagnosed in 1984–2018, 40.3% had died by December 2018 compared with 52.1% (1152/2212) of males (Table 3). However, there was no difference in the rate of death in the first year of diagnosis between females and males (27.5%; 94/342 vs 28.4%; 327/1152). A higher percentage (67.7%; 223/344) of patients aged ≥50 years at diagnosis had died by December 2018 compared with patients aged 15–24 years (36%; 202/561). The rate of death within a year of HIV diagnosis increased with age at diagnosis (Table 4). The proportions of patients died within a year of HIV diagnosis among those aged 15–24, 25–29, and ≥ 50 years at diagnosis were 17.8% (36/202), 26.4% (250/948), and 48.1% (112/233), respectively. Compared with patients residing outside Muscat, patients living in Muscat had higher overall mortality (47.3%; 953/2016 vs 51.8%; 541/1044) but lower short-term mortality (32.6%; 311/953 vs 20.3%; 110/541).
| Characteristics (N = 3060) | Alive (n = 1566), n (%)* | Dead (n = 1494), n (%) | p-value |
|---|---|---|---|
| Percentage from total cohort | 51.2 | 48.8 | |
| Sex | |||
| Male (n = 2212) | 1060 (47.9) | 1152 (52.1) | <0.01 |
| Female (n = 848) | 506 (59.7) | 342 (40.3) | |
| Age at diagnosis (years) | |||
| 0–4 (n = 77) | 43 (55.8) | 34 (44.2) | <0.01 |
| 5–14 (n = 120) | 43 (35.8) | 77 (64.2) | |
| 15–24 (n = 561) | 359 (64) | 202 (36) | |
| 25–49 (n = 1958) | 1010 (51.6) | 948 (48.4) | |
| ≥50 (n = 344) | 111 (32.3) | 233 (67.7) | |
| Year of diagnosis | |||
| 1984–1996 (n = 668) | 96 (14.4) | 572 (85.6) | <0.01 |
| 1997–2006 (n = 872) | 303 (34.7) | 569 (65.3) | |
| 2007–2012 (n = 740) | 486 (65.7) | 254 (34.3) | |
| 2013–2018 (n = 780) | 681 (87.3) | 99 (12.7) | |
| Residence | |||
| Muscat (n = 1044) | 503 (48.2) | 541 (51.8) | 0.017 |
| Outside Muscat (n = 2016) | 1063 (52.7) | 953 (47.3) | |
| Risk factor | |||
| Heterosexual (n = 1670) | 1011 (60.5) | 659 (39.5) | <0.01 |
| Homosexual/bisexual (n = 503) | 264 (52.5) | 239 (47.5) | |
| Others (n = 410) | 146 (35.6) | 264 (64.4) | |
| Not documented (n = 477) | 145 (32.4) | 332 (69.6) | |
| Reason for testing | |||
| Antenatal screening (n = 112) | 111 (99.1) | 1 (0.9) | <0.01 |
| Blood bank screening (n = 63) | 60 (95.2) | 3 (4.8) | |
| Patient’s request (n = 91) | 82 (90.1) | 9 (9.9) | |
| HIV contact (n = 268) | 206 (76.9) | 62 (23.1) | |
| HIV-related symptoms (n = 422) | 322 (76.3) | 100 (23.7) | |
| Not documented (n = 1834) | 530 (28.9) | 1304 (71.1) | |
| Others (n = 270) | 255 (94.4) | 15 (5.6) | |
| Baseline CD4 count, cells/mm3, median (IQR) | 252 (109–416) | 59 (21–161) | |
| Baseline HIV VL, copies/mL, median (IQR) | 64,621 (15,062–227,454) | 175,000 (56,810–544,489) | |
| ART (n = 1732) | |||
| NNRTI (n = 1102) | 881 (79.9) | 221 (20.1) | <0.01 |
| PI (n = 526) | 460 (87.5) | 66 (12.5) | |
| INIs (n = 41) | 38 (92.7) | 3 (7.3) | |
| Others (n = 63) | 55 (87.3) | 8 (12.7) | |
| Hepatitis B status | |||
| Positive | 47 (98) | 1 (2) | <0.01 |
| Negative | 1041 (94.6) | 59 (5.4) | |
| Not documented | 478 (25) | 1434 (75) | |
| Hepatitis C status | |||
| Positive | 67 (91.8) | 6 (8.2) | <0.01 |
| Negative | 1006 (95.1) | 52 (4.9) | |
| Not documented | 493 (25.6) | 1436 (74.4) | |
Unless stated otherwise.
ART, antiretroviral therapy; INIs, integrase inhibitors; IQR, interquartile range; HIV, human immunodeficiency virus; NRTIs, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; VL, viral load.
Characteristics of diagnosed people living with HIV/AIDS in Oman in 1984–2018, stratified by living status as of December 31, 2018
| Characteristics (N = 1494) | Survival after HIV diagnosis | p-value | ||
|---|---|---|---|---|
| <1 years, n (%)* | 1–4 years, n (%) | ≥4 years, n (%) | ||
| Percentage from total (n = 1494) | 421 (28.2) | 401 (26.8) | 672 (45) | |
| Sex | ||||
| Male (n = 1152) | 327 (28.4) | 311 (27) | 514 (44.6) | 0.875 |
| Female (n = 342) | 94 (27.5) | 90 (26.3) | 158 (46.2) | |
| Age at diagnosis (years) | ||||
| 0–4 (n = 34) | 9 (26.5) | 12 (35.3) | 13 (38.2) | <0.01 |
| 5–14 (n = 77) | 14 (18.2) | 31 (40.3) | 32 (41.6) | |
| 15–24 (n = 202) | 36 (17.8) | 58 (28.7) | 108 (53.5) | |
| 25–49 (n = 948) | 250 (26.4) | 253 (26.7) | 445 (46.9) | |
| ≥50 (n = 233) | 112 (48.1) | 47 (20.2) | 74 (31.8) | |
| Year of diagnosis | ||||
| 1984–1996 (n = 572) | 79 (13.8) | 150 (26.2) | 343 (60) | <0.01 |
| 1997–2006 (n = 569) | 168 (29.5) | 146 (25.7) | 255 (44.8) | |
| 2007–2012 (n = 254) | 100 (39.4) | 82 (32.3) | 72 (28.3) | |
| 2013–2018 (n = 99) | 74 (74.7) | 23 (23.2) | 2 (2) | |
| Residence | ||||
| Muscat (n = 541) | 110 (20.3) | 134 (24.8) | 297 (54.9) | <0.01 |
| Outside Muscat (n = 953) | 311 (32.6) | 267 (28) | 375 (39.3) | |
| Risk factor | ||||
| Heterosexual (n = 659) | 156 (23.7) | 186 (28.2) | 317 (48.1) | <0.01 |
| Homosexual/bisexual (n = 239) | 53 (22.2) | 67 (28) | 119 (49.8) | |
| Others (n = 264) | 57 (21.6) | 65 (24.6) | 142 (53.8) | |
| Not documented (n = 332) | 155 (46.7) | 83 (25) | 94 (28.3) | |
| Reason for testing | ||||
| Antenatal screening (n = 1) | 0 (0) | 0 (0) | 1 (100) | <0.01 |
| Blood bank screening (n = 3) | 0 (0) | 2 (66.7) | 1 (33.3) | |
| Patient’s request (n = 9) | 3 (33.3) | 5 (55.6) | 1 (11.1) | |
| HIV contact (n = 62) | 14 (22.6) | 21 (33.9) | 27 (43.5) | |
| HIV-related symptoms (n = 100) | 67 (67) | 15 (15) | 18 (18) | |
| Not documented (n = 1304) | 333 (25.5) | 354 (27.1) | 617 (47.3) | |
| Others (n = 15) | 4 (26.7) | 4 (26.7) | 7 (46.7) | |
Unless stated otherwise.
Characteristics of diagnosed people living with HIV/AIDS in Oman in 1984–2018 who had died by December 2018, stratified by survival after HIV diagnosis
3.4. Mother-to-Child Transmission of HIV
The number of HIV-infected children due to Mother-to-Child Transmission (MTCT) of HIV was 121 in 1984–2009 compared with 19 children born in 2010–2018. Only five of the latter group were born to women with known HIV-positive status before delivery; four women delivered in 2011–2015 with a documented history of poor adherence to ART during pregnancy. The fifth mother delivered in 2016 and had not received any intervention to prevent MTCT as, due to her complex social circumstances, the Primary Health Care (PHC) team failed to communicate the HIV-positive result to the patient and her obstetrical team. None of the 65 known HIV-positive pregnant women delivered in 2017 and 2018 had an HIV-positive child making the rate of MTCT of HIV in that period 0.
3.5. Coinfections
The rates of HIV/hepatitis B and C coinfection among PLWHA diagnosed in 1984–2018 were 4.2% (48/1148) and 6.5% (73/1131), respectively (Table 3).
In 1992–2018, the number of Tuberculosis (TB) cases in Oman was in the range of 172–268; the average rate of HIV/TB coinfection was 3.3%. In 2017 and 2018, 0.7% (2/268) and 2.1% (5/242) of people with TB were also infected with HIV, respectively. The incidence of TB (all forms) in the general population in Oman per 100,000 population) in 2017 and 2018 was 5.6 and 3.8, respectively.
3.6. Cascade of HIV Care
On December 31, 2018, the estimated number of adults PLWHA in Oman was 3030; 1532 (50.6%) were aware of their infection. Of the diagnosed patients, 95.9%, 85.8%, and 84.3% were linked to care, retained in care, and on ART, respectively. The proportions of patients with viral suppression (HIV VL <1000 copies/mL) out of the estimated PLWHA, the diagnosed PLWHA, and those on ART were 37.3%, 73.7%, and 87.5%, respectively. The ART coverage rate increased from 53.5% (888/1661) in 2015 to 84.3% (1291/1532) in 2018 (p < 0.001). In addition, the rate of viral suppression increased from 75.7% (672/888) to 87.5% (11210.1097/QAD.00000000000013359/1291) in the same period (p < 0.001).
4. DISCUSSION
These analyses show that the number of new HIV diagnoses in Oman has continued to fluctuate over the past decade, with the annual new cases exceeding 130 in the past 4 years. Sexual transmission has been the main driver of the epidemic. Males were disproportionately affected, with more than two-thirds of the annual new cases being males. Most (60.3–66.1%) of the new cases were among people aged between 25 and 49 years. The proportions of new cases who resided outside Muscat and those aged 15–24 years have steadily increased over time. In 2013–2018, 45.6% of new diagnoses had advanced HIV disease at the entry of care. We also observed a substantial increase in short-term mortality over the past 20 years, reaching 74.7% in 2013–2018, compared with 29.5% in 1997–2006. However, the overall all-cause mortality had remarkably fallen from 65.3% in 1997–2006 to 12.7% in 2013–2018. Male sex and increasing age at diagnosis were associated with advanced HIV disease at baseline. In contrast, female sex and decreasing age at diagnosis were associated with better overall survival. Compared with homosexuals, heterosexuals had higher rates of early HIV diagnosis and survival. In 2018, Oman attained a higher rate of viral suppression (87.5% vs 82%) and a higher percentage of ART coverage (84.3% vs 69%) than the average for the MENA region; however, the rates of diagnosed HIV were similar (50.6% vs 47%) [3].
The recent increase in the new diagnoses in Oman may be explained, in part, by an increase in HIV testing rather than new infections as suggested by the high rate of late HIV diagnosis and the increasing percentage of PLWHA who were tested due to HIV-related symptoms (13% in 2007–2012 and 38.7% in 2013–2018). The NAP has recommended HIV testing for patients presenting to health-care facilities with HIV indicator conditions since 2015 [5]. However, the rise in the number of new cases in persons aged 15–24 years suggests high risk-taking behavior in this cohort [7–9] that will require close study. Of note, the UNAIDS 2019 report shows that HIV incidence (0.07 per 1000 population) and prevalence (0.2 per 1000 population) in Oman were stable in 2010–2018 [3]. Future epidemiological and behavioral studies to determine the precise dynamic of HIV incidence and the key population in Oman are warranted. The observed steady reduction in the number of patients aged 0–14 years at diagnosis is best explained by the increase in ART coverage [10,11] and introduction of antenatal screening for HIV in 2009 [12]; indeed, the number of HIV pregnant women diagnosed through antenatal screening in 2007–2012 (n = 34) increased by more than twofold in 2013–2018 (n = 76). It is encouraging to note that the proportion of patients diagnosed at private clinics and hospitals had increased substantially from 1.4% in 2007–2012 to 9% in 2013–2018. The number of private hospitals and hospital beds in Oman has increased by more than twofold in the past 10 years [13]. Furthermore, the annual number of outpatient visits to private institutions among Omanis has been about 2 million over the same period [13]. This growth in access to private health-care underscores the critical need to expand HIV testing in the private sector; the NAP is currently working closely with private providers to achieve this.
The association between late HIV diagnosis and increased morbidity and mortality [14] is well established. Late HIV diagnosis is associated with a 10-fold increased risk of death within 12 months of diagnosis [15]. Our data, largely, concurred with this. People diagnosed in 2007–2012 had smaller proportions of advanced HIV disease (39.3% vs 45.6%) and short-term mortality (39.4% vs 74.7%) compared with persons diagnosed in 2013–2018. Similarly, we observed increasing rates of advanced HIV disease at presentation with age at diagnosis (39.9%, 46.8%, and 65% in patients aged 15–24, 25–49, and ≥50 years, respectively) and rising rates of death in the first 12 months after diagnosis with age at diagnosis (17.8%, 26.4%, and 48.1% in patients aged 15–24, 25–49, and ≥50 years, respectively). However, despite the higher rates of early HIV diagnosis in heterosexuals and females, we did not observe a reduction in short-term mortality in comparison with homosexuals and males. In addition, we are unable to explain our finding of the higher rate (32.6%) of short-term mortality among people living outside Muscat compared with Muscat region (20.3%), despite similar rates of late HIV diagnosis in both regions (69.8% vs 69.9%). Late-stage HIV diagnosis also increases the risk of onward transmission [16,17] and the cost of treatment and care [18]. To address the issue of late and undiagnosed HIV, the NAP has recently published an HIV manual for PHC intending to expand HIV testing in PHC [19]. Evidence shows that primary and secondary care providers often miss opportunities for earlier HIV diagnosis in patients diagnosed with late HIV diagnosis [20–23]. As seen in British [24,25] and Swiss [26] HIV cohorts, we observed a steady decline in all-cause mortality among PLWHA over the past 20 years. As of December 31, 2018, 85.6% of persons diagnosed in the pre-highly active antiretroviral therapy era had died compared with 65.3%, 34.3%, and 12.7% in 1997–2006, 2007–2012, and 2013–2018, respectively. This improvement in survival is, in large, due to the increase in ART coverage and improvement in the quality of HIV care in Oman [27].
The high proportion of undiagnosed people living with HIV in Oman and many other countries in the MENA region could be due to several reasons. A primary factor is the high level of HIV-related stigma and discrimination that prevent access to HIV testing facilities [28–30]. Second, HIV prevention services targeting key populations and their partners are lacking. Furthermore, social disapproval of and punitive laws against individuals with high-risk behaviors such as sex work, IDU, and men who have sex with men are prevalent in the MENA region [30]. To increase HIV testing rates in Oman, NAP has conducted several workshops and seminars to promote the current national HIV testing guidelines that recommend facility-based HIV indicator diseases guided testing and universal HIV screening of pregnant women. In addition, initiatives to assess feasibility, acceptability, and effectiveness routine HIV screening in accident and emergency departments and medical admission units are ongoing.
Strength of our study is that our data are comprehensive, including all Omani nationals diagnosed with HIV in Oman. Hence, the findings can inform future interventions targeted at various components of the HIV program in the country. Furthermore, to the authors’ knowledge, this is the first study with such a detailed national epidemiological data from the MENA region. One limitation worth noting is the inherent drawback of retrospective studies of missing data, which may lead to underestimation of the magnitude of point estimates. However, the NAP has made efforts to improve the central database over recent years. Indeed, the majority of missing data fields were among dead patients. Another limitation is that, due to data limitation, we did not include patients presenting with AIDS-defining illness, irrespective of CD4 count, in our definition of advanced HIV disease and late HIV diagnosis; this may result in underestimation of the scale of the problem of late HIV diagnosis in our cohort. Finally, the inability to compare our results to neighboring countries, due to the limited published epidemiological data from the region, makes our analyses merely descriptive.
5. CONCLUSION
The HIV epidemic in Oman has remained as a low-prevalence one with low rates of hepatitis and TB coinfections. Of concern, a large proportion of new HIV diagnoses continued to present late, which has resulted in a substantial increase in short-term mortality over the past 20 years. Furthermore, the 2018 cascade of care data showed that half of PLWHA in Oman remained unaware of their HIV diagnosis. Future epidemiological studies to determine the characteristics of undiagnosed PLWHA in Oman and inform targeted interventions, such as home HIV testing and community-based programs for key populations are warranted. We observed a remarkable decline in overall all-cause mortality over time due to the improvement in HIV care after diagnosis [27,31].
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
SS, ZA, MA, AA, RL, BA, HA and SA contributed in study conceptualization. AE, SS and MA contributed in data curation and formal analysis. AE contributed in writing (original draft) the manuscript. SS, ZA, MA, AA, RL, BA, HA and SA contributed in writing (review and editing) the manuscript.
REFERENCES
Cite this article
TY - JOUR AU - Ali Elgalib AU - Samir Shah AU - Adil Al-Wahaibi AU - Zeyana Al-Habsi AU - Maha Al-Fouri AU - Richard Lau AU - Hanan Al-Kindi AU - Bader Al-Rawahi AU - Seif Al-Abri PY - 2020 DA - 2020/01/30 TI - The Epidemiology of HIV in Oman, 1984–2018: A Nationwide Study from the Middle East JO - Journal of Epidemiology and Global Health SP - 222 EP - 229 VL - 10 IS - 3 SN - 2210-6014 UR - https://doi.org/10.2991/jegh.k.191208.001 DO - 10.2991/jegh.k.191208.001 ID - Elgalib2020 ER -
